Published online Mar 14, 2013. doi: 10.3748/wjg.v19.i10.1669
Revised: January 10, 2013
Accepted: January 18, 2013
Published online: March 14, 2013
Processing time: 77 Days and 22 Hours
This is a response letter to Verna E’s comments regarding our previous manuscript published last year in the World Journal of Gastroenterology entitled “Relationship between LYVE-1, VEGFR-3 and CD44 gene expressions and lymphatic metastasis in gastric cancer”, which evaluated the relationship between these expression levels and clinicopathological parameters (Ozmen F et al, World J Gastroenterology 2011; 17: 3220-3228). The mean values for lymphatic vessel endothelial hyaluronan receptor-1, CD44 and vascular endothelial growth factor receptor-3 expression (represented as 2-ΔΔCt) were 1.13, 1.24 and 1.17, respectively, suggesting an increase in gene expression in tumor tissue compared to normal tissue. Despite the increase in gene expression in the cancer tissues (2-ΔΔCt > 1), only some of the results reached statistical significance, which was thoroughly discussed in our paper. In the present letter, we report that his comments are flawed and result in confusion. Therefore, we herein provide more explanation regarding gene expression in gastric cancer. We hope that this letter will address Verna E’s misunderstandings.
- Citation: Ozmen F. Response letter regarding the interpretation of gene expression data. World J Gastroenterol 2013; 19(10): 1669-1670
- URL: https://www.wjgnet.com/1007-9327/full/v19/i10/1669.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i10.1669
I recently noticed that a Letter to the Editor written by Verna E, regarding our paper (Ozmen F et al, World J Gastroenterol 2011; 17: 3220-3228), had been published in the June issue of the Journal this year (Verna E, World J Gastroenterol 2012; 18: 3181-3182)[1,2].
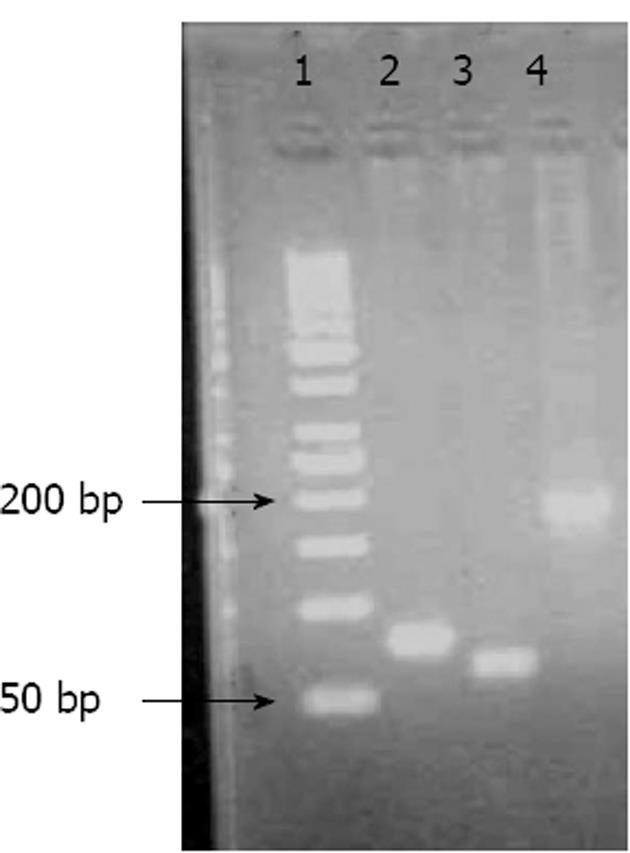
Although I would like to thank Dr. Verna for his interest and for providing us with this opportunity to address his concerns, I have to stress that it is very important to understand the paper in its entirety before coming to certain conclusions: (1) Our study investigated the expression levels of the lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), vascular endothelial growth factor receptor-3 (VEGFR-3), and CD44 genes in human tissues with or without a tumor using real-time polymerase chain reaction (RT-PCR) and evaluated the relationship in gastric cancer between these expression levels and clinicopathological parameters that included tumor type, stage, differentiation, and the presence of lymph node metastasis, vascular invasion, and neural/perineural invasion[1]; (2) Relative expression levels were calculated using the PCR cycle threshold (Ct) number for each tissue and control sample using the formula 2-(ΔCtsample-ΔCtcontrol). ΔCt represents the difference in Ct values between the target and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts. RT-PCR was performed in duplicate for each sample, and average Ct values were calculated according to the previous papers from Pfaffl[3] and Livak et al[4], which are references he cited in his letter; (3) It is not clear why he required an explanation of the interpretation of the gene expression data because everyone involved in this type of study is familiar with the analysis. As previously explained by Livak et al[4], the choice of the calibrator for the 2-ΔΔCt method depends on the type of gene expression experiment that one has planned. The simplest design is to use the untreated control (in our study, the patient’s own normal tissue) as the calibrator and GAPDH as an internal control. Using the 2-ΔΔCt method, the data are presented as the fold change in gene expression normalized to an endogenous reference gene and relative to the normal control. For the control sample, ΔΔCt equals zero, and 20 equals one; as a result, the fold change in gene expression relative to the untreated control equals one, by definition. For the treated samples (tumor tissue), an evaluation of 2-ΔΔCt indicates the fold change in gene expression relative to the untreated control. The gene expression levels in tumor tissues represent the difference from normal controls in our study, and all values were shown in Fig. 2[1]. There were some values over 1, representing increased expression, and there were also values less than 1, representing decreased expression; (4) The mean values for LYVE1, CD44 and VEGFR3 expression (represented as 2-ΔΔCt and shown in Fig. 2) were 1.13, 1.24 and 1.17, respectively, suggesting increased gene expression in tumor tissues compared to normal tissue. Therefore, we believe it is natural to conclude from these results that the expression levels were increased. Despite the increase in gene expression in the cancer tissues (2-ΔΔCt > 1), only some of the results reached statistical significance, which was thoroughly discussed in our paper[1]; (5) In our study, we did not only report the gene expression data, but also presented data obtained using immunohistochemistry, pathology, and other clinical features of the tumors. Although we are commenting on our results, we used all of these data to reach a logical conclusion. As shown in Fig. 3, 4 and 5, gene expression was increased (2-ΔΔCt > 1) with increased T-stage, a PLN/TLN ratio > 0.4 and the presence of perineural invasion[1]; and (6) In our manuscript, we used Figure 1 to show the PCR products of the genes. Dr. Verna is correct to note that there are some non-specific amplicons of the VEGFR-3 gene in that Figure. However, we optimized both the conventional PCR and real-time PCR reactions for the VEGFR-3 gene and also all others, as shown in the lower part of Figure 1. It is easy to note that no nonspecific band is present in this reaction for VEGFR-3.
Once again, I would like to thank Dr. Verna for his interest in our study and hope that this letter will resolve any misunderstandings.
P- Reviewer Morise Z S- Editor Wen LL L- Editor Cant MR E- Editor Xiong L
| 1. | Ozmen F, Ozmen MM, Ozdemir E, Moran M, Seçkin S, Guc D, Karaagaoglu E, Kansu E. Relationship between LYVE-1, VEGFR-3 and CD44 gene expressions and lymphatic metastasis in gastric cancer. World J Gastroenterol. 2011;17:3220-3228. [PubMed] |
| 2. | Verna E. More attention should be paid on the interpretation of gene expression data. World J Gastroenterol. 2012;18:3181-3182. [PubMed] |
| 3. | Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24144] [Cited by in RCA: 25972] [Article Influence: 1082.2] [Reference Citation Analysis (0)] |
| 4. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 133472] [Article Influence: 5561.3] [Reference Citation Analysis (1)] |