Published online Mar 14, 2013. doi: 10.3748/wjg.v19.i10.1602
Revised: December 7, 2012
Accepted: January 23, 2013
Published online: March 14, 2013
Processing time: 198 Days and 0.6 Hours
AIM: To determine the rates and impact of hepatitis B virus (HBV) and hepatitis C virus (HCV) infections on response to long-term highly active antiretroviral therapy (HAART) in a large human immunodeficiency virus (HIV) population in Nigeria.
METHODS: HBV and HCV as well as HIV infections are endemic in sub Saharan Africa. This was a retrospective cohort study of 19 408 adults who were recruited between June 2004 and December 2010 in the AIDS Prevention Initiative in Nigeria in Nigeria programme at Jos University Teaching Hospital. Serological assays, including HBV surface antigen (HBsAg) and hepatitis C antibody were used to categorise hepatitis status of the patients. HBsAg was determined using enzyme immunoassay (EIA) (Monolisa HBsAg Ultra3; Bio-Rad). HCV antibody was tested using third generation EIA (DIA.PRO Diagnostic, Bioprobes srl, Milan, Italy). HIV RNA levels were measured using Roche COBAS Amplicor HIV-1 monitor test version 1.5 (Roche Diagnostics, GmbH, Mannheim, Germany) with a detection limit of 400 copies/mL. Flow cytometry was used to determine CD4+ cell count (Partec, GmbH Munster, Germany). Comparison of categorical and continuous variables were achieved using Pearson’s χ2 and Kruskal Wallis tests respectively, on MedCalc for Windows, version 9.5.0.0 (MedCalc Software, Mariakerke, Belgium).
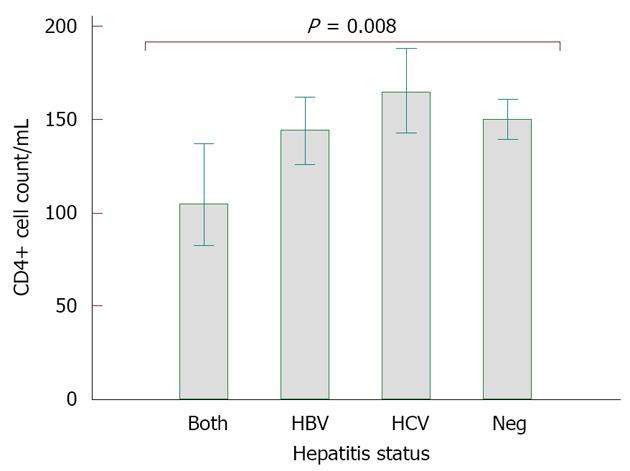
RESULTS: With an overall hepatitis screening rate of over 90% for each virus; HBV, HCV and HBV/HCV were detected in 3162 (17.8%), 1983 (11.3%) and 453 (2.5%) HIV infected adults respectively. The rate of liver disease was low, but highest among HIV mono-infected patients (29, 0.11%), followed by HBV co-infected patients (15, 0.08%). Patients with HBV co-infection and triple infection had higher log10 HIV RNA loads (HBV: 4.6 copies/mL vs HIV only: 4.5 copies/mL, P < 0.0001) and more severe immune suppression (HBV: 645, 55.4%; HBV/HCV: 97, 56.7%) prior to initiation of HAART compared to HIV mono-infected patients (1852, 48.6%) (P < 0.0001). Of 3025 patients who were 4.4 years on HAART and whose CD4 cell counts results at baseline and end of follow up were available for analyses, CD4 increase was significantly lower in those with HBV co-infection (HBV: 144 cells/mm3; HBV/HCV: 105 cells/mm3) than in those with HCV co-infection (165 cells/mm3) and HIV mono-infection (150 cells/mm3) (P = 0.0008).
CONCLUSION: High rates of HBV and HCV infections were found in this HIV cohort. CD4 recovery was significantly diminished in patients with HBV co-infection.
- Citation: Ladep NG, Agaba PA, Agbaji O, Muazu A, Ugoagwu P, Imade G, Cooke G, McCormack S, Taylor-Robinson SD, Idoko J, Kanki P. Rates and impact of hepatitis on human immunodeficiency virus infection in a large African cohort. World J Gastroenterol 2013; 19(10): 1602-1610
- URL: https://www.wjgnet.com/1007-9327/full/v19/i10/1602.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i10.1602
Studies of the prevalence of hepatitis in human immunodeficiency virus (HIV) infected individuals confirm that the rates of hepatitis B virus (HBV) in HIV-infected patients vary widely with tendency towards higher values compared to HBV prevalence in the general population[1,2]. In the north central region of Nigeria, the prevalence of HBV and hepatitis C virus (HCV) in HIV infected people were 27.8% and 18.3% respectively, and triple infection (HBV/HCV/HIV) was found in 7.2% of 180 HIV infected patients[3]. HBV prevalence in the Nigeria general population ranges between 10% and 20%[4].
Although it is widely recommended that HIV infected patients be screened for hepatitis before antiretroviral therapy (ART), no data are accessible to ascertain adherence to this guideline in Nigeria. Reports from Thailand confirm that compliance to hepatitis screening in HIV patients prior to initiating antiretrovirals (ARVs) was poor (55%-69%)[5,6]. Inadequate epidemiological information on hepatitis in HIV patients may underpin one reason for national health schemes in many developing countries not offering integrated hepatitis services in HIV infected persons. As a consequence, patients co-infected with HBV and HCV are being ignored in regards to timing of antiretroviral therapy, screening for cirrhosis of the liver and hepatocellular carcinoma (HCC), as well as in the choice of ARV regimens that have the potential to optimise their care.
Rising trends in the prevalence of HBV and HCV among HIV-infected individuals during the last decade have been reported in a United States study. That study, involving about 30 000 HIV-infected patients recorded a low, but significantly increasing proportion of patients being screened for hepatitis; from 20% in 1998 to 60% in 2004[7]. The researchers found that the rate of HBV and HCV increased from 7% to 8.5% and 9% to 24% respectively. To date, no report of trends in the rate of hepatitis in patients infected with HIV from sub Saharan countries has been published.
The choice of ART regimen can be critical in achieving good treatment outcomes; and knowledge of hepatitis co-infection is vital in this regard. Lamivudine resistance in HIV/HBV co-infected patients on ART has been described in some studies within the West African sub region[8,9]. A French study has recently demonstrated the advantage of treating HBV-HIV co-infected patients with Tenofovir-containing ARV regimen, particularly for wild type precore mutant and lamivudine-resistant HBV[10]. Guidelines for the choice of ART regimens generally recommend screening for hepatitis, but this is not routinely undertaken and/or largely depends on availability of resources. Even where screening for hepatitis takes place, a large number of HIV physicians base the choice of ART on available drugs rather than on informed co-morbid conditions. However, changes in treatment guidelines have advocated administration of HBV active ART to co-infected patients in Nigeria.
The importance of well-designed research to answer these questions cannot be overemphasised in order to inform adequate provision of resources for the optimisation of care for HIV/hepatitis co-infected individuals in Africa. We thus aimed to determine the rate of hepatitis screening in HIV infected patients, magnitude of hepatitis co-infection in this large cohort, impact of hepatitis co-infection on baseline HIV parameters, HIV suppression and CD4+ cell increase following HAART.
The AIDS Prevention Initiative in Nigeria (APIN) and Harvard School of Public Health HIV program, supported by a grant from the United States President’s Emergency Plan for AIDS Relief have been providing ART, at no cost to patients in Nigeria from 2004 till date. This programme is run on a community-based model (although the major sites in Nigeria are located within tertiary health centres), in which integrated community prevention outreaches, on-site HIV screening, counselling, provision of medications, follow up, monitoring and evaluation of all activities are embarked upon. Jos University Teaching Hospital (JUTH) site is one of several centres in Nigeria, with latest HIV prevalence of 4.4%[11]. JUTH has a specialised centre of care for HIV infection where patients are seen at the outpatient facility at planned intervals of 4-12 wk.
The initial first line ARVs in this population included Stavudine/Zidovudine, Lamivudine and Efavirenz/Nevirapine. However, from 2006, Truvada® (Tenofovir plus Emtricitabine) started to be administered to HIV patients initiating ART in the programme. From June 2004 to December 2010, approximately 19 408 HIV-infected individuals had been recruited in the JUTH/APIN/Harvard programme and were initiated on ARVs and anti-tuberculosis drugs if indicated.
For the first part of the study, we included all the subjects whose HIV status was confirmed by Western blot assay and enrolled in the programme between 2004 and 2010. Information on age, gender, educational attainment, status of HBV and HCV were obtained. HIV RNA levels and CD4+ cell counts at baseline and most recent assays were also included as were information on the last day of follow up, death or discontinuation of therapy.
Subjects were defined as having HBV and HCV infection if they tested positive for HBV surface antigen (HBsAg) and hepatitis C antibody (HCV Ab) respectively on baseline blood samples. HIV RNA levels and CD4+ cell count were determined at baseline for patients and at 3 monthly intervals until the end point of the study. Hepatotoxicity was defined as alanine aminotransferase (ALT) values ≥ 5 folds over upper limit of normal (ULN) (41 IU/mL for JUTH) or if ≥ 3.5 folds over ULN if baseline ALT was above ULN.
Recruited patients gave written informed consents approved by the ethical committee at JUTH and the institutional review board at the Harvard School of Public Health. For the present work, we obtained a further approval for secondary use of data to study liver-related morbidities in this cohort.
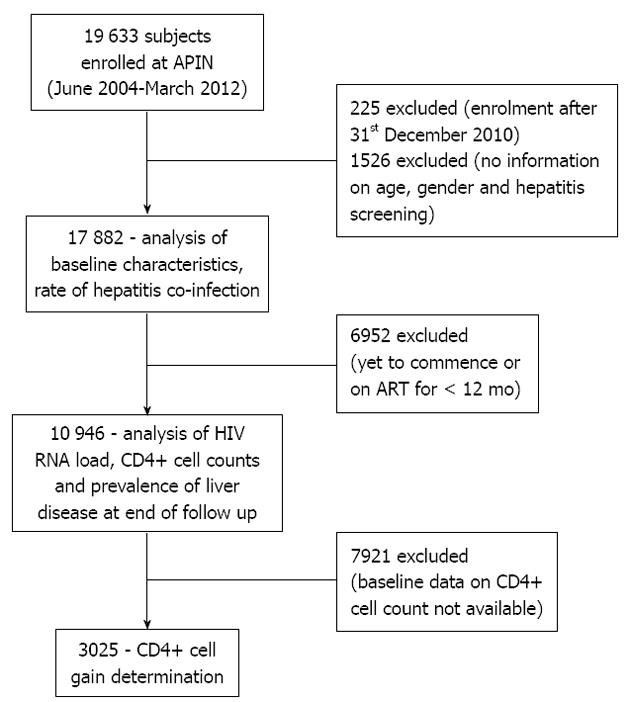
This was a retrospective cohort study. The pro-forma utilised in the analyses is summarised in Figure 1. The number of HIV infected individuals that were screened for HBV and HCV were divided by the total number of patients recruited to ascertain the proportion of hepatitis screening. We then calculated the prevalence of HBV and HCV from the numbers that underwent serological testing. We categorised all patients who had hepatotoxicity, liver cirrhosis and hepatocellular carcinoma to a single group (liver disease). As there was overlap of the morbidities, we categorised the cumulative rates of liver related morbidities.
Case-controlled studies of the impact of hepatitis co-infections on baseline HIV viral load and CD4+ cell counts were also embarked upon. HBV, identified by HBsAg, HCV (anti-HCV) and both infections (triple infection) were categorised as cases; which were compared to HIV-only (controls).
Before recruitment into the APIN programme, subjects were screened for HIV, using enzyme linked immunoassay and subsequently confirmed by Western blot assay. HBsAg was determined using enzyme immunoassay (EIA) (Monolisa HBsAg Ultra3; Bio-Rad). HCV antibody was tested using third generation EIA (DIA.PRO Diagnostic, Bioprobes srl, Milan, Italy). HIV RNA levels were measured using Roche COBAS Amplicor HIV-1 monitor test version 1.5 (Roche Diagnostics, GmbH, Mannheim, Germany) with a detection limit of 400 copies/mL (Figure 1). Flow cytometry was used to determine CD4+ cell count (Partec, GmbH Munster, Germany).
As the diagnoses of HBV, HCV and liver diseases were likely to overlap, we calculated the cumulative prevalence of liver morbidities by overlapping diagnoses; categorised into HBV only, HCV only, HBV/HCV, liver disease only and liver disease with any of HBV, HCV and HBV/HCV. We determined relationships in the demographics of the patients and liver morbidities as well as baseline HIV parameters. The obtained characteristics of HBV, HCV, HBV/HCV and HIV only subjects were compared against each other at baseline using Spearman’s chi square and Kruskal Wallis tests for categorical and continuous variables respectively. Analyses were accomplished using MedCalc for Windows, version 9.5.0.0 (MedCalc Software, Mariakerke, Belgium). P values of < 0.05 were considered statistically significant.
Between June 2004 and December 2010, 19 408 HIV individuals were enrolled and followed for a median of 53 mo (interquartile range: 31-72 mo). Table 1 presents a summary of the main characteristics of the cohort at baseline. Subjects diagnosed with HBV were more likely to be young (median age: 32 years; P < 0.001), male and to have had high HIV RNA loads and CD4+ cell count below 200/mm3. HCV co-infected individuals were more likely to be males, older (median age: 36 years), have had low level of education and high HIV RNA in their plasma.
| Characteristic | Total | HBV | HCV | Triple infection | HIV only |
| Gender | |||||
| Male | 6222 (34.8) | 1214 (38.1)b | 764 (37.9)b | 178 (39.3)b | 4066 (33.2) |
| Female | 11 660 (65.2) | 1971 (61.9)b | 1250 (62.1)b | 275 (60.7)b | 8164 (66.8) |
| Age group (yr) | |||||
| 15-29 | 5870 (32.8) | 1097 (34.4)b | 463 (23.0)b | 120 (26.5) | 4190 (34.3) |
| 30-39 | 7106 (39.7) | 1309 (41.1)b | 786 (39.0)b | 206 (45.5) | 4805 (39.4) |
| 40-49 | 3619 (20.2) | 615 (19.3)b | 533 (26.5)b | 94 (20.8) | 2377 (19.5) |
| ≥ 50 | 1287 (7.2) | 164 (5.1)b | 232 (11.5)b | 33 (7.3) | 828 (6.8) |
| Tuberculosis diagnosis | |||||
| Present | 2552 (14.3) | 470 (14.8) | 300 (14.9) | 68 (15.0) | 1714 (14.0) |
| Absent | 15 330 (85.7) | 2715 (85.2) | 1714 (85.0) | 385 (85.0) | 10516 (86.0) |
| Education status | |||||
| None | 3230 (18.8) | 527 (17.2) | 471 (24.3) | 87 (19.8) | 2145 (18.3) |
| Primary | 3487 (20.3) | 623 (20.3) | 440 (22.7) | 96 (21.8) | 2328 (19.9) |
| Secondary | 5208 (30.4) | 962 (31.4) | 536 (27.6) | 146 (33.2) | 3564 (30.5) |
| Tertiary | 5219 (30.4) | 954 (31.1) | 494 (25.4) | 111 (25.2) | 3660 (31.3) |
| CD4 (cells/mm3) | |||||
| < 200 | 2937 (50.7) | 645 (55.4)b | 343 (52.5) | 97 (56.7)b | 1852 (48.6) |
| 200-499 | 2214 (38.2) | 410 (35.3)b | 249 (38.1) | 57 (33.3)b | 1498 (39.3) |
| ≥ 500 | 646 (11.1) | 108 (9.3)b | 61 (9.3) | 17 (9.9)b | 460 (12.1) |
| HIV (copies/mL) | |||||
| Undetect (< 400) | 2194 (12.4) | 351 (11.2)b | 221 (11.1)b | 29 (6.7)b | 1583 (13.1) |
| Low (400-9999) | 3581 (20.3) | 562 (17.9)b | 364 (18.2)b | 99 (22.8)b | 2556 (21.1) |
| Interm (10 000-29 999) | 2774 (15.7) | 523 (16.7)b | 321 (16.1)b | 72 (16.6)b | 1858 (15.4) |
| High (≥ 30 000) | 9115 (51.6) | 1700 (54.2)b | 1090 (53.9)b | 234 (53.9)b | 6091 (50.4) |
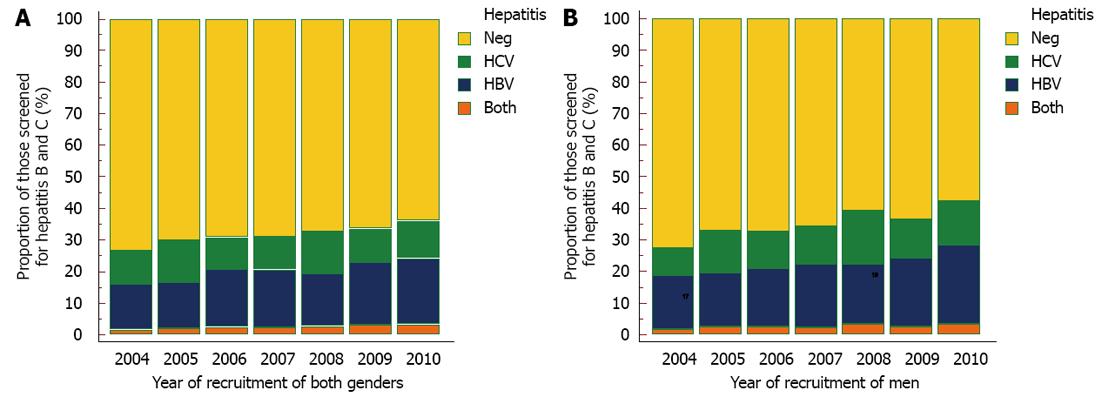
At the beginning of the study period, 99.3% and 99.5% of recruited HIV infected individuals underwent HBV and HCV screening respectively. There has been a significant decline in the rates of screening for HBV and HCV during approximately 7 year study period to 73.0% and 87.6% respectively in 2010 (P < 0001, Figure 2). Overall, the prevalence of HBsAg was 20.7%. A significant increase in the rate of HBV from 14.4% in 2004 to 21.0% in 2010 was observed (P < 0.001) and although, fluctuating rates of HCV Ab was recorded among those that were screened, an increasing pattern was noted. The prevalence of HCV Ab was 10.6% in 2004, increasing to 11.7% in 2010. Higher rates of HBV infection was found in men than women rising from 17% in 2004 to 25% in 2010.
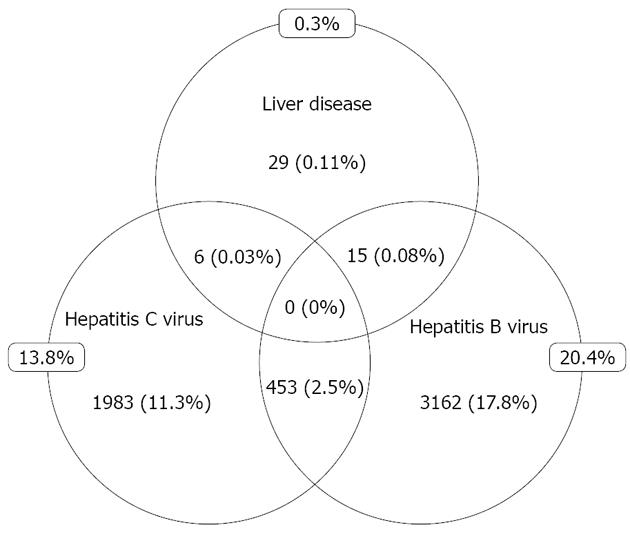
Cumulatively, 3185 (17.8%) patients were positive to HBsAg and 2014 (11.3%) patients had HCV Ab. 453 (2.5%) patients had evidence of combined HBV and HCV infections (Figure 3). Liver disease was diagnosed in 50 (0.3%) patients. Of these, 15 had HBV, 6 had HCV and 29 had no evidence of hepatitis co-infection. None of those with triple infection had a diagnosis of liver disease. Diagnoses of liver disease were achieved via conventional means, including: assessment of sequential liver enzymes, liver ultrasound, and alpha fetoprotein.
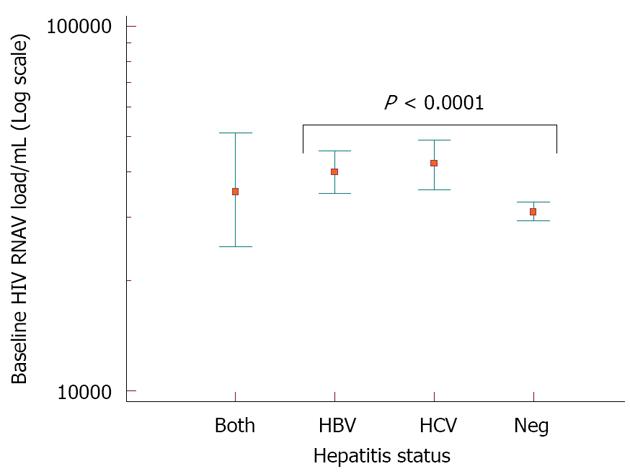
Higher proportion of HBV (645, 55.4%) and HBV/HCV (97, 56.7%) co-infected patients had CD4+ cell counts below 200 cells/mm3 at baseline compared with HCV (343, 52.5%) and HIV (1852, 48.6%) patients (P < 0.0001) (Table 2). The median HIV RNA at baseline was Log10 4.6 copies/mL each for HBV and HCV patients; and 4.5 copies/mL for HIV mono-infected patients (P < 0.0001) (Figure 4). At the end of follow up on ART [median duration: 4.4 years (interquartile range: 2.6-6 years)], no significant difference in HIV RNA load suppression was observed in all study groups. However, there was a significantly lower CD4+ cell increase among those individuals co-infected by HBV/HCV (105 cells/mm3) and HBV (144 cells/mm3) than in HCV (165 cells/mm3) and HIV-only (150 cells/mm3) patient groups (P = 0.008, Figure 5).
| Variable | Total | HBV | HCV | Both | HIV only |
| CD4 (cells/mm3) | † | ||||
| < 200 | 2028 (18.5) | 396 (21.3)b | 218 (17.5) | 50 (20.2)b | 1364 (17.9) |
| 200-499 | 5549 (50.7) | 953 (51.3)b | 625 (50.3) | 129 (52.2)b | 3842 (50.6) |
| ≥ 500 | 3369 (30.8) | 508 (27.4)b | 400 (32.2) | 68 (27.5)b | 2393 (31.4) |
| HIV RNA (copies/mL) | |||||
| Undetectable (< 400) | 7155 (65.4) | 1186 (63.9) | 809 (65.1) | 155 (62.8) | 5005 (65.9) |
| Detectable (≥ 400) | 3792 (34.6) | 671 (36.1) | 434 (34.9) | 92 (37.2) | 2595 (34.1) |
| Liver disease | |||||
| Present | 31 (2.8) | 8 (4.3) | 4 (3.2) | 0 (0.0) | 19 (2.5) |
| None diagnosed | 10 916 (97.2) | 1849 (95.7) | 1239 (96.8) | 247 (100) | 7581 (97.5) |
In this large cohort of HIV infected sub-Sahara African patients, we found that whereas chronic HBV and HCV were frequent diagnoses, liver disease was not common; although investigated only when there were overt clinical symptoms. Overall, one out of every five patients had HBV and more than a tenth had HCV. Almost every patient that was recruited at the beginning of study was screened for HBV and HCV. This is rather remarkable for a resource limited setting and much higher than obtainable in some cohorts in Thailand[5,6]. The fact that our study site benefited from grants for research and involved the services of specialists may explain the high hepatitis screening rate. However, this initial enthusiasm was not sustained, as there has been a significant decline in the rates of screening for hepatitis during the study period. Interestingly, diagnoses of HBV and HCV showed significant rising trends. We note that there may be a selection bias in this regard as people tend to go to tertiary care centres to seek treatment.
Our findings corroborate the reports of other researchers who had observed higher prevalence of HBV and HCV infection among HIV-infected patients than in the general population[12]. Studies of prevalence of HBV in the general population of people living within the study area during the period between 2002 and 2007 had found rates of between 10.3% and 15.1%[13-16]. These confirm that the rates of hepatitides are higher in the HIV patients than in the general population of Nigeria. The fact that HIV and hepatitis viruses share the same routes of transmission supports this explanation. However, it remains unknown whether hepatitis occurs at the same time as HIV infection or predates it.
The precise modes of transmission of HBV and HCV in our cohort are not known. However, it has been reported that transmission of HBV most commonly occurs in early childhood among African populations[17], compared to high transmission rates among adults in industrialised countries[18]. Whereas intravenous drug use is the major route of transmission of HIV and indeed hepatitis viruses in western industrialised countries, heterosexual and horizontal routes, as well as indiscriminate injections (unsterile needles) are thought to be the prevalent modes of transmission of these viral infections in African communities[19]. Many patients may have iatrogenic transmission from poor sterilisation during routine medical, obstetric, dental and surgical procedures. Most HIV/hepatitis co-infected patients in Nigeria are postulated to have become infected by hepatitis viruses before HIV[17]. Longitudinal studies will be required to appropriately determine patients that may have acquired hepatitis before, at the same time or after HIV infection. Such a study has the advantage of providing additional information for reinforcing prevention methods, for example HBV vaccination not only for the present cohort but for HIV infected patients in HBV endemic regions.
The incidence of liver disease in the present study was not assiduously documented, although observed to be common. Only 11 patients were documented to have had primary liver cancer in the present study. Eight of these patients were screened for hepatitis. While four were HBsAg positive, 4 were negative to both HBV and HCV. As population-based cancer registries are not routinely available and/or reliable in Nigeria owing to poor registration of diseases and deaths, we did not compare the incidence of primary liver cancer in the present cohort with those from the general population. However, studies in United States have confirmed that primary liver cancer occurs about 6 times more commonly in HIV infected individuals than in the general population[20,21]. With such a high rate of HBV and HCV infection in this African cohort, there is a chance that a large number of primary liver cancer cases were missed or will yet manifest. It should be noted that prior to free provision of ART to HIV patients in Nigeria from 2004, the cost of these medications was prohibitive and the incidence of HIV mirrored its mortality[22]. It is thus likely that most patients would have died earlier than they could present with HCC. Furthermore, with prolonged ART, many of these patients will survive longer and HCC could become more frequently diagnosed.
Unfortunately, the study design did not allow for prospective evaluation of hepatitis status. In addition, the patients were treated with ARVs (e.g.,Truvada) that in some cases may have been both active on hepatitis and HIV infection. The baseline evaluation and hepatitis status seems to indicate that other non-infectious causes of hepatic disease may need to be considered. It will be anticipated that HIV hepatitis co-infected patients would have higher incidence of liver disease. This would have been the case if we restricted the definition of liver disease to end stage liver disease (fibrosis, cirrhosis and liver cancer). However, we included hepatotoxicity of ARVs in the definition. This would explain, in part the higher incidence of liver disease in HIV mono-infected patients. Chronic liver disease was small in the cohort, perhaps due to under reporting, or perhaps a high threshold for recording cases in the database. Reasons for the apparent rarity of liver disease in HIV/hepatitis co-infected patients and higher cases of liver disease in HIV mono-infected than hepatitis co-infected patients require further studies.
At baseline, patients with hepatitis co-infection had higher HIV RNA than patients with HIV mono-infection. Correspondingly, a higher proportion of patients co-infected with HBV had CD4+ cell counts below 200/mL compared to HIV mono-infected individuals. The finding of higher HIV RNA at baseline corroborates earlier findings in a study of a small number of patients (1564) from the same study site[23], as well as another study from China[24]. Following HAART, the gain in CD4+ cell count was significantly diminished in those patients who had HBV co-infection compared to those with HIV mono-infection. In contrast to our findings, two studies that assessed the impact of HBV on response to HAART found no difference in CD4+ cell gain[25,26]. Reasons for the differential outcomes are not obvious. Differences in environment are unlikely, as our findings contrast the observation of a study of South African patients[27]. We note however, that whereas the South Africa study had a shorter duration of follow up (1.5 years), the present cohort was followed for a longer duration on ART (4.4 years). Nevertheless, results of HBV and long-term HIV outcomes (7 years) in the US found no difference in the HIV load suppression and CD4+ cell gain. Be that as it may, as the natural history of HBV is likely to differ between US and African populations, owing to differential age at acquisition of hepatitis infections, studies comparing African patients on long-term ART would provide a better assessment.
Our findings suggest that CD4+ cell loss by HIV is accentuated by HBV, despite on-going HIV treatment. A few studies have highlighted that active HBV infection is associated with T-lymphocyte exhaustion[28,29]. This has been further strengthened by the fact that inhibition of HBV DNA replication using anti-HBV drugs resulted in immune restoration[30,31]. One would expect such an effect to be universal. However, variable outcomes of HAART in regards to HIV load suppression and/or increases in CD4+ cell count in HBV co-infected versus HIV mono-infected patients have been reported. While some studies found no differences between HBV and HIV mono-infected groups[27,32], others found non-sustained differences in CD4+ cell increases[25,33]. Studies of HIV treatment outcomes of HBV co-infected and HIV mono-infected African patients comparing HBV suppressive agents versus regimens that are non HBV suppressing will be required to adequately characterise the importance of HBV in the era of HAART.
Our study was not without limitations. First, the use of HBsAg positivity as the sole indicator of chronic HBV infection may be misleading. Definition of chronic HBV would require a positive HBsAg assay consecutively carried out at least 6 mo apart. As single HBsAg was utilised to define cases of HBV in this current study, cases of misclassification might have occurred. Also, delineating HBV cases by their HBV DNA loads and HBeAg status would have provided more meaningful analyses. However, as it is generally known that HBV infection in Africans occur more commonly in childhood, the chance of falsely misclassifying HBV is low. For want of resources, we could not perform HBeAg and HBV DNA. Another issue that could have led to misclassification to hepatitis status is reliance on HCV Ab result to define active HCV infection. Earlier data (unpublished) from a sub group of the current cohort had found HCV viraemia of 33% in those that were HCV Ab positive. It is thus possible that of the patients that were classified “HCV”; only a third may actually be HCV viraemic. More studies with better characterisation of HCV status in this cohort would be required. Missing data was another issue we encountered. In some of the patients, HBsAg and HCV Ab and baseline CD4+ data results were unavailable. Also, we relied on diagnosis of liver diseases (hepatotoxicity, cirrhosis and primary liver cancer) on clinical notes of the patients, where available. As a result of these, we only analysed the rates of hepatitis among those that had hepatitis results. Also, CD4 cell gain was analysed for 3012 patients for whom there were baseline and follow up results and who were on HAART.
In conclusion, high and increasing rates of HBV, HCV and HBV/HCV co-infections were found in this large HIV infected cohort of Africans. The prevalence of liver disease, particularly liver cancer was low; mostly reported among HBV/HIV and HIV-only individuals. HBV co-infection was associated with high HIV RNA load and decreased CD4+ cell counts at baseline and attenuated immunological recovery after a median follow up duration on HAART of 4.4 years. Our findings underscore the urgent need to maintain a strict hepatitis screening policy among HIV infected patients undergoing HAART as well as inclusion of ART regimens with potent anti-HBV activities in HBV endemic regions of the world. Longitudinal studies in African patients to ascertain super-infection of HIV by hepatitis, assessments of impact of HBV-suppressive versus HBV non-suppressive HAART regimens and predictive value of hepatitis on the mortality of the present cohort will form a significant contribution to future research.
The authors acknowledge the unwavering contribution of all the clinic staff of APIN, JUTH; particularly members of the Hepatitis co-infection group, Hope Support group and the Tracking Team as well as the patients without which there would be no manuscript.
Owing to shared routes of transmission, co-infection of human immunodeficiency virus (HIV) by hepatitis B (HBV) and hepatitis C viruses (HCV) is common. National guidelines recommend that commencement of antiretroviral therapy be preceded by hepatitis screening. Untill the writing of this paper, no data is accessible from Africa to ascertain adherence to this guideline. Perhaps, more interesting is the fact that the impact of HBV (most prevalent hepatitis infection in Africa) on HIV therapy is yet to be determined in a large HIV infected population.
Studies from Asia have reported a modest hepatitis screening rate among HIV patients. A landmark study in France led to the recommendation of Tenofovir-based antiretroviral regimen for HBV/HIV co-infected patients going on treatment. It will be helpful to lay a foundational study in African patients to ascertain whether the effects of such treatment will be the same; especially as the mode and natural history of chronic viral hepatitis vary between developed and developing countries.
In the present study, the authors characterised the patients based on hepatitis status, comparing them with HIV mono-infected cohort and have shown that HBV in particular was associated with decreased CD4 cell increase following long-term highly active antiretroviral therapy. Additionally, the fact that hepatitis screening rate was about 90% in this Nigeria cohort suggests that the recommendation of pre-highly active antiretroviral therapy (HAART) screening for hepatitis is achievable in resource-limited healthcare settings.
This study suggests that HBV/HCV infection among HIV population is higher than in the general population of Nigeria and portends considerations for HBV preventive programmes, including vaccination. As HBV co-infection negatively impacted CD4 increase during HAART, individualised treatment algorithms, which had been advocated by international specialists needs to be followed assiduously in order to reduce morbidity in HIV patients.
This article delves into a topic that we have few data, coming from sub-Saharan Africa grappling the scourge of hepatitis and HIV and looks on the impact of the former on HIV care. However, it fails to address the impact of the latter on the course of hepatitis. The surprising revelation is the rarity of liver disease in those with co-infection; this is deserving of further studies.
P- Reviewer Onyekwere CA S- Editor Zhai HH L- Editor A E- Editor Xiong L
| 1. | Adekunle AE, Oladimeji AA, Temi AP, Adeseye AI, Akinyeye OA, Taiwo RH. Baseline CD4+ T lymphocyte cell counts, hepatitis B and C viruses seropositivity in adults with Human Immunodeficiency Virus infection at a tertiary hospital in Nigeria. Pan Afr Med J. 2011;9:6. [PubMed] |
| 2. | Adesina O, Oladokun A, Akinyemi O, Adedokun B, Awolude O, Odaibo G, Olaleye D, Adewole I. Human immuno-deficiency virus and hepatitis B virus coinfection in pregnancy at the University College Hospital, Ibadan. Afr J Med Med Sci. 2010;39:305-310. [PubMed] |
| 3. | Forbi JC, Gabadi S, Alabi R, Iperepolu HO, Pam CR, Entonu PE, Agwale SM. The role of triple infection with hepatitis B virus, hepatitis C virus, and human immunodeficiency virus (HIV) type-1 on CD4+ lymphocyte levels in the highly HIV infected population of North-Central Nigeria. Mem Inst Oswaldo Cruz. 2007;102:535-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Ladep NG, Taylor-Robinson SD. Management of liver disease in Nigeria. Clin Med. 2007;7:439-441. [PubMed] |
| 5. | Kiertiburanakul S, Chotiprasitsakul D, Atamasirikul K, Sungkanuparph S. Late and low compliance with hepatitis B serology screening among HIV-infected patients in a resource-limited setting: an issue to improve HIV care. Curr HIV Res. 2011;9:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Sungkanuparph S, Wongprasit P, Manosuthi W, Atamasirikul K. Compliance with hepatitis B and hepatitis C virus infection screening among HIV-1 infected patients in a resource-limited setting. Southeast Asian J Trop Med Public Health. 2008;39:863-866. [PubMed] |
| 7. | Buskin SE, Barash EA, Scott JD, Aboulafia DM, Wood RW. Hepatitis B and C infection and liver disease trends among human immunodeficiency virus-infected individuals. World J Gastroenterol. 2011;17:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Kouanfack C, Aghokeng AF, Mondain AM, Bourgeois A, Kenfack A, Mpoudi-Ngolé E, Ducos J, Delaporte E, Laurent C. Lamivudine-resistant HBV infection in HIV-positive patients receiving antiretroviral therapy in a public routine clinic in Cameroon. Antivir Ther. 2012;17:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Stewart B, Jobarteh ML, Sarge-Njie R, Alabi A, de Silva T, Peterson K, Peterson I, Whittle H, Rowland-Jones S, Jaye A. Emergence of HBV resistance to lamivudine (3TC) in HIV/HBV co-infected patients in The Gambia, West Africa. BMC Res Notes. 2011;4:561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Benhamou Y, Fleury H, Trimoulet P, Pellegrin I, Urbinelli R, Katlama C, Rozenbaum W, Le Teuff G, Trylesinski A, Piketty C. Anti-hepatitis B virus efficacy of tenofovir disoproxil fumarate in HIV-infected patients. Hepatology. 2006;43:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 118] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Agaba PA, Digin E, Makai R, Apena L, Agbaji OO, Idoko JA, Murphy R, Kanki P. Clinical characteristics and predictors of mortality in hospitalized HIV-infected Nigerians. J Infect Dev Ctries. 2011;5:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Chun HM, Fieberg AM, Hullsiek KH, Lifson AR, Crum-Cianflone NF, Weintrob AC, Ganesan A, Barthel RV, Bradley WP, Agan BK. Epidemiology of Hepatitis B virus infection in a US cohort of HIV-infected individuals during the past 20 years. Clin Infect Dis. 2010;50:426-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Sirisena ND, Njoku MO, Idoko JA, Isamade E, Barau C, Jelpe D, Zamani A, Otowo S. Carriage rate of hepatitis-B surface antigen (HBsAg) in an urban community in Jos, Plateau State, Nigeria. Niger Postgrad Med J. 2002;9:7-10. [PubMed] |
| 14. | Uneke CJ, Ogbu O, Inyama PU, Anyanwu GI, Njoku MO, Idoko JH. Prevalence of hepatitis-B surface antigen among blood donors and human immunodeficiency virus-infected patients in Jos, Nigeria. Mem Inst Oswaldo Cruz. 2005;100:13-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Jombo GT, Egah DZ, Banwat EB. Hepatitis B virus infection in a rural settlement of northern Nigeria. Niger J Med. 2005;14:425-428. [PubMed] |
| 16. | Egah DZ, Banwat EB, Audu ES, Iya D, Mandong BM, Anele AA, Gomwalk NE. Hepatitis B surface antigen, hepatitis C and HIV antibodies in a low-risk blood donor group, Nigeria. East Mediterr Health J. 2007;13:961-966. [PubMed] |
| 17. | Lesi OA, Kehinde MO, Oguh DN, Amira CO. Hepatitis B and C virus infection in Nigerian patients with HIV/AIDS. Niger Postgrad Med J. 2007;14:129-133. [PubMed] |
| 18. | Puoti M, Manno D, Nasta P, Carosi G. Hepatitis B virus and HIV coinfection in low-income countries: unmet needs. Clin Infect Dis. 2008;46:367-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Simonsen L, Kane A, Lloyd J, Zaffran M, Kane M. Unsafe injections in the developing world and transmission of bloodborne pathogens: a review. Bull World Health Organ. 1999;77:789-800. [PubMed] |
| 20. | Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, Grigg R, Hylton T, Pawlish KS, McNeel TS. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 553] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 21. | Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 396] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 22. | Idoko J. A clinician’s experience with the President’s Emergency Plan for AIDS Relief in Nigeria: a transformative decade of hope. Health Aff (Millwood). 2012;31:1422-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Idoko J, Meloni S, Muazu M, Nimzing L, Badung B, Hawkins C, Sankalé JL, Ekong E, Murphy R, Kanki P. Impact of hepatitis B virus infection on human immunodeficiency virus response to antiretroviral therapy in Nigeria. Clin Infect Dis. 2009;49:1268-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Sheng WH, Chen MY, Hsieh SM, Hsiao CF, Wang JT, Hung CC, Chang SC. Impact of chronic hepatitis B virus (HBV) infection on outcomes of patients infected with HIV in an area where HBV infection is hyperendemic. Clin Infect Dis. 2004;38:1471-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Law WP, Duncombe CJ, Mahanontharit A, Boyd MA, Ruxrungtham K, Lange JM, Phanuphak P, Cooper DA, Dore GJ. Impact of viral hepatitis co-infection on response to antiretroviral therapy and HIV disease progression in the HIV-NAT cohort. AIDS. 2004;18:1169-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Konopnicki D, Mocroft A, de Wit S, Antunes F, Ledergerber B, Katlama C, Zilmer K, Vella S, Kirk O, Lundgren JD. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS. 2005;19:593-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 375] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 27. | Hoffmann CJ, Charalambous S, Martin DJ, Innes C, Churchyard GJ, Chaisson RE, Grant AD, Fielding KL, Thio CL. Hepatitis B virus infection and response to antiretroviral therapy (ART) in a South African ART program. Clin Infect Dis. 2008;47:1479-1485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Iser DM, Avihingsanon A, Wisedopas N, Thompson AJ, Boyd A, Matthews GV, Locarnini SA, Slavin J, Desmond PV, Lewin SR. Increased intrahepatic apoptosis but reduced immune activation in HIV-HBV co-infected patients with advanced immunosuppression. AIDS. 2011;25:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Dunn C, Peppa D, Khanna P, Nebbia G, Jones M, Brendish N, Lascar RM, Brown D, Gilson RJ, Tedder RJ. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology. 2009;137:1289-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 281] [Article Influence: 17.6] [Reference Citation Analysis (1)] |
| 30. | Zoutendijk R, Zaaijer HL, de Vries-Sluijs TE, Reijnders JG, Mulder JW, Kroon FP, Richter C, van der Eijk AA, Sonneveld MJ, Hansen BE. Hepatitis B surface antigen declines and clearance during long-term tenofovir therapy in patients coinfected with HBV and HIV. J Infect Dis. 2012;206:974-980. [PubMed] |
| 31. | You J, Sriplung H, Geater A, Chongsuvivatwong V, Zhuang L, Li YL, Lei H, Liu J, Chen HY, Tang BZ. Impact of viral replication inhibition by entecavir on peripheral T lymphocyte subpopulations in chronic hepatitis B patients. BMC Infect Dis. 2008;8:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Hoffmann CJ, Seaberg EC, Young S, Witt MD, D’Acunto K, Phair J, Thio CL. Hepatitis B and long-term HIV outcomes in coinfected HAART recipients. AIDS. 2009;23:1881-1889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Sagoe KW, Agyei AA, Ziga F, Lartey M, Adiku TK, Seshi M, Arens MQ, Mingle JA. Prevalence and impact of hepatitis B and C virus co-infections in antiretroviral treatment naïve patients with HIV infection at a major treatment center in Ghana. J Med Virol. 2012;84:6-10. [PubMed] |