Published online Jan 7, 2013. doi: 10.3748/wjg.v19.i1.125
Revised: September 26, 2012
Accepted: October 16, 2012
Published online: January 7, 2013
Russell bodies are globular and eosinophilic inclusion bodies in the cytoplasm of mature plasma cells. Plasma cells whose cytoplasm is filled with Russell bodies are designated as Mott cells. Russell body duodenitis (RBD) is a unique form of chronic duodenitis that is characterized by infiltration of numerous Mott cells. RBD is very rare; only two cases have been reported to date. In this paper, we report a case of RBD in a patient with retroperitoneal metastasis of ureteral cancer. A 77-year-old man was admitted to our hospital complaining of appetite loss, vomiting, and upper abdominal distension. He had undergone left nephroureterectomy for ureteral cancer 4 years earlier. Upper digestive tract endoscopy revealed edema, stenosis, and punctate redness of the mucosa of the duodenum, and a biopsy was performed. Histological analysis showed that numerous Mott cells had infiltrated the lamina propria mucosae, and the condition was diagnosed as RBD. A mass lesion in the retroperitoneum adjacent to the duodenum was detected by abdominal computed tomography, and was diagnosed as metastatic urothelial carcinoma by biopsy. It is possible that chemokines produced by tumor cells caused RBD in this case.
- Citation: Takahashi Y, Shimizu S, Uraushihara K, Fukusato T. Russell body duodenitis in a patient with retroperitoneal metastasis of ureteral cancer. World J Gastroenterol 2013; 19(1): 125-128
- URL: https://www.wjgnet.com/1007-9327/full/v19/i1/125.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i1.125
Russell bodies are globular and eosinophilic inclusion bodies in the cytoplasm of mature plasma cells, and were first described by Russell[1] in 1890. They correspond to markedly dilated rough endoplasmic reticulum (rER) filled with produced proteins. Plasma cells whose cytoplasm is filled with Russell bodies are designated as Mott cells. Although the appearance of small numbers of Mott cells in chronic inflammation lesions is not rare, the appearance of numerous Mott cells in these lesions is very rare.
In 1998, Tazawa et al[2] reported a case of chronic gastritis with the appearance of numerous Mott cells and proposed that such a lesion be designated as “Russell body gastritis (RBG)”. Since then, there has been a gradual increase in reported cases of RBG[3-9], and association with Helicobacter pylori (H. pylori) infection[2,4,6,7], human immunodeficiency virus (HIV) infection[5], and alcohol abuse[2,3,5,8] has been suspected. However, Russell body duodenitis (RBD), which has a similar histological appearance to RBG, is very rare, and only two cases have been reported to date[10,11]. A previously reported case of RBD occurred in an HIV-positive patient[10]; however, the pathogenesis and clinicopathological characteristics of RBD remain to be determined. In this paper, we report a case of RBD that occurred in a patient with retroperitoneal metastasis of ureteral cancer and discuss the pathogenesis of RBD.
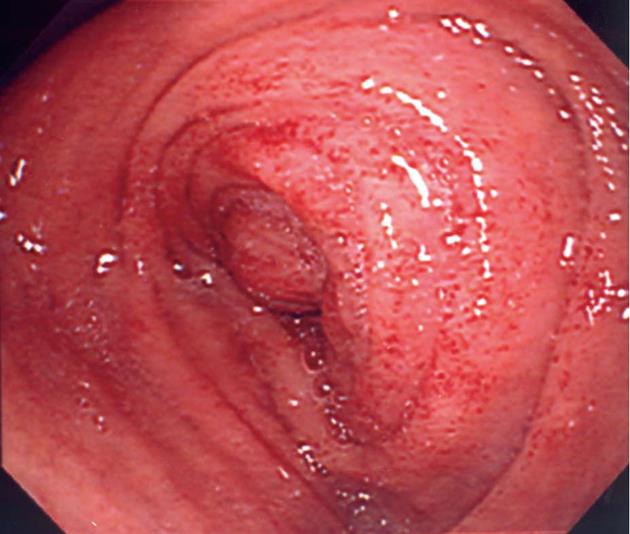
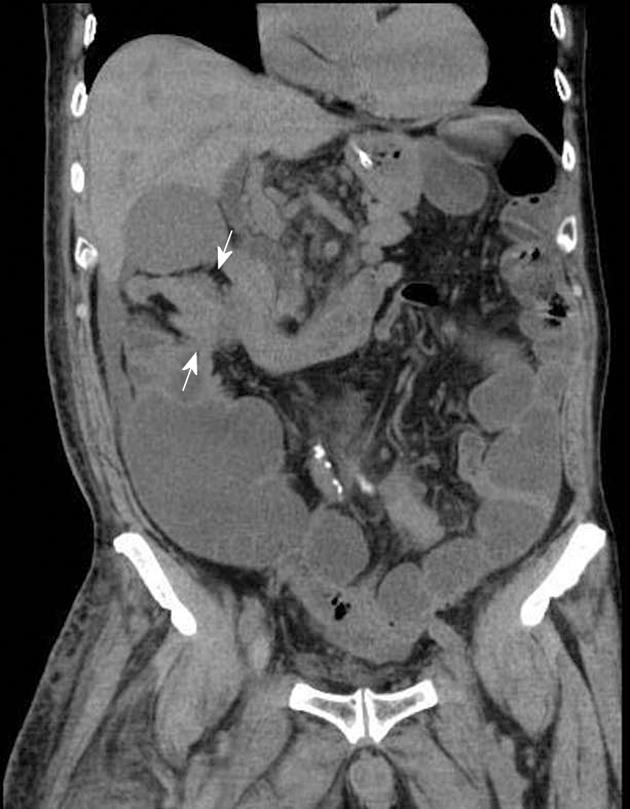
A 77-year-old man was admitted to our hospital complaining of appetite loss, vomiting, and upper abdominal distension. He had undergone left nephroureterectomy for ureteral cancer 4 years earlier, and 2 years later, he had undergone chemotherapy (methotrexate + vinblastine + doxorubicin + cisplatin) and radiation therapy (39.6 Gy in total) for paraaortic lymph node metastasis. He did not abuse alcohol, and was HIV-negative. He had not undergone any treatment for H. pylori. Edema, stenosis, and punctate redness of the mucosa were observed in the second portion of the duodenum by upper digestive tract endoscopy (Figure 1), and a biopsy was performed. Endoscopic visualization revealed no tumorous lesions in the upper digestive tract mucosa. Computed tomography showed a mass lesion in the retroperitoneum adjacent to the duodenum (Figure 2), and the clinical symptoms were attributed to duodenal obstruction by the mass. Gastrojejunal bypass surgery was performed 2 wk after admission. Biopsy of the retroperitoneal mass was performed during surgery, and the lesion was diagnosed as a metastasis of ureteral cancer. The retroperitoneal metastatic mass subsequently increased in size, and the patient died 2 mo after the surgery. Autopsy was not permitted.
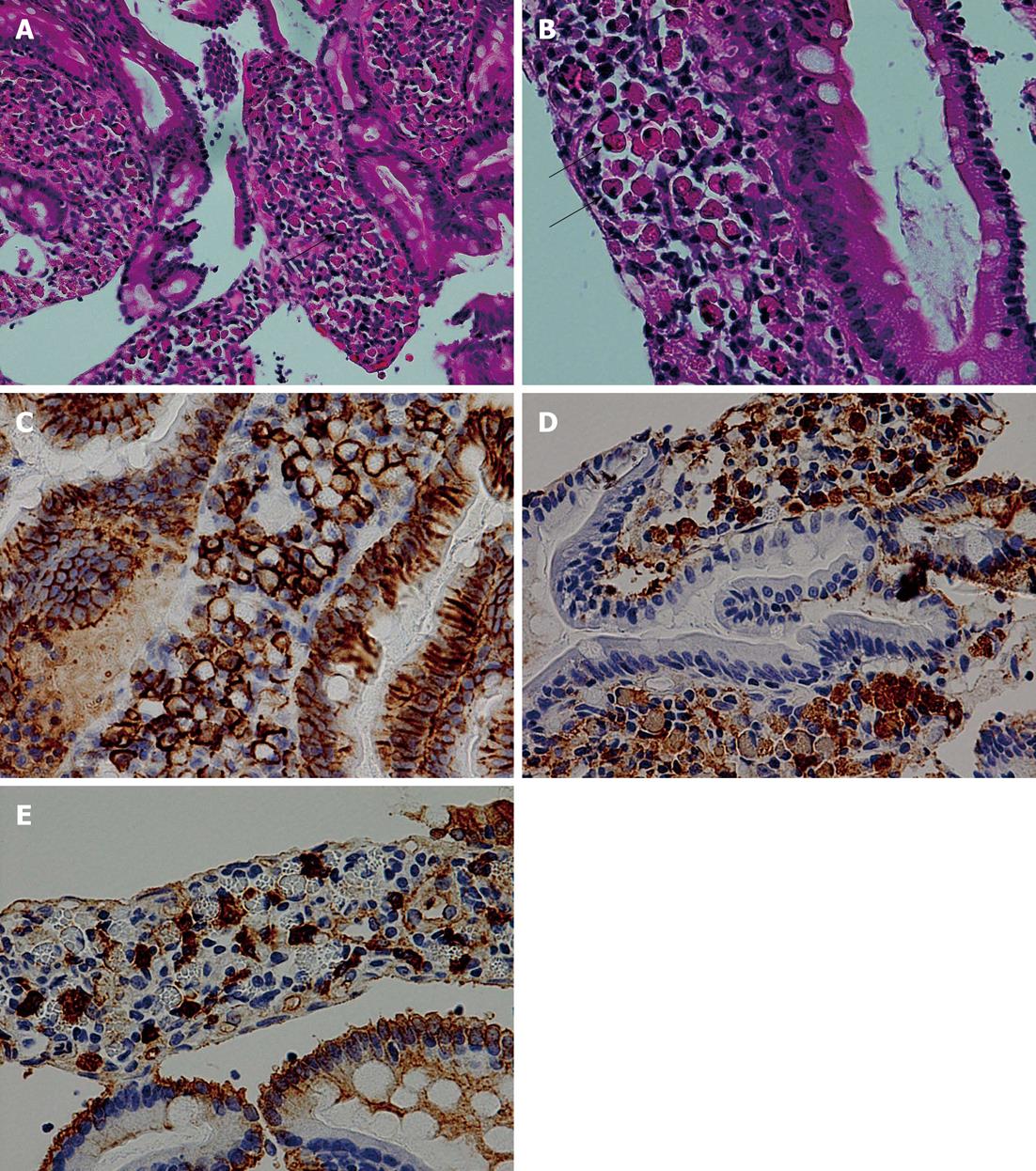
Three biopsy fragments were taken from the second portion of the duodenum. Numerous plasmacytoid cells, whose cytoplasm was filled with eosinophilic inclusion bodies and whose nuclei were eccentrically located, had infiltrated the lamina propria mucosae of one biopsy fragment (Figure 3A and B). Neither nuclear atypia nor mitosis was observed in these cells. These inclusion bodies were positive for periodic acid-Schiff (PAS) staining. Immunohistochemically, the cells were positive for CD138 (Figure 3C), CD38, and CD79a and negative for cytokeratin, confirming that they were plasma cells containing Russell bodies (Mott cells). Although scattered lymphocytes and plasma cells without Russell bodies were also present, these cells showed no nuclear atypia, and no lymphoepithelial lesions were observed. Immunohistochemical staining for κ and λ chains revealed that the infiltrating plasma cells were of polyclonal origin (Figure 3D and E), although Russell bodies showed no obvious positive staining for either antibody. No gastric metaplasia was observed in the epithelium, and no H. pylori infection was detected by immunohistochemical staining. The other two duodenal biopsy fragments showed only non-specific chronic inflammation. No cancer invasion was observed in the biopsy fragments. The histological appearance of the biopsy specimen from the retroperitoneal mass was compatible with that of metastatic urothelial carcinoma.
The duodenal biopsy in this case helped us to reach a diagnosis of RBD. Differential diagnoses included B-cell lymphomas with plasmacytic differentiation, plasmacytoma, celiac disease, Whipple’s disease, and lymphocytic gastroenteritis. Mott cells occasionally appear in B-cell lymphomas with plasmacytic differentiation (particularly mucosa-associated lymphoid tissue lymphoma) and plasmacytoma. However, RBD can be differentiated from B-cell lymphomas and plasmacytoma because no atypical lymphoid or plasmacytoid cells and lymphoepithelial lesions are present and the infiltrating plasma cells show a polyclonal pattern in immunohistochemical staining for κ and λ chains in RBD. In celiac disease, the endoscopy results indicate reduced or absent folds of the small intestinal mucosa, and the histological analysis indicates the accumulation of large fat globules in the surface epithelium. These features of celiac disease were not observed in the present case. In Whipple’s disease, the inflammatory cells that aggregate in the lamina propria mucosae of the small intestine are not Mott cells, but large macrophages whose cytoplasm contains large amounts of a diastase-resistant, PAS-positive material. Lymphocytic gastroenteritis is characterized histologically by increased numbers of intraepithelial lymphocytes (mostly cytotoxic T-cells), which were not observed in the present case.
Russell bodies are eosinophilic inclusions in the cytoplasm of plasma cells. They are situated within the cisternae of the rER and contain non-immunoglobulin molecules, by-products of immunoglobulin synthesis, or some altered form of immunoglobulins that can no longer be recognized by an anti-immunoglobulin antibody[12,13]. They may be formed by excessive production of, or failure in, the secretion of immunoglobulin molecules[12]. The appearance of numerous Mott cells in the lesion in cases of chronic inflammation is very rare, and the diagnosis of RBG/RBD is made when numerous Mott cells appear in a chronic gastritis/duodenitis lesion. RBG/RBD is thought to be a focal condition[2]; the fact that RBD was found in only one of three duodenal biopsy fragments in the present case is consistent with this hypothesis.
Although reported cases of RBG are gradually increasing in number[2-9], RBD is very rare, and only two cases have been reported to date[10,11]. The first reported case of RBD[10] was that of a 55-year-old HIV-positive man whose chief complaint was abdominal pain. The upper digestive tract endoscopy revealed non-specific gastritis and duodenitis. Histologically, gastric metaplasia was observed in the duodenal epithelium, although H. pylori infection was not detected. The second reported RBD case[11] was that of a 69-year-old woman. She complained of refractory dysphagia; however, this symptom was thought to be unrelated to the duodenal lesion. Using endoscopy, the RBD lesion was recognized as a nodule within the duodenal bulb. Histological analysis indicated gastric metaplasia in the duodenal epithelium, although neither H. pylori nor other discernible microorganisms were identified. The patient in our study was a 77-year-old HIV-negative man, and the clinical symptoms were appetite loss, vomiting, and upper abdominal distension; however, these symptoms were attributed to obstruction of the duodenum by retroperitoneal metastasis of ureteral cancer. Endoscopic findings were non-specific inflammation, and no H. pylori infection was detected. No gastric metaplasia was detected in the duodenal epithelium in the present case.
H. pylori infection was detected in more than half of the reported cases of RBG[2,4,6,7], and it is estimated that antigenic stimulation by H. pylori is an important factor in the etiology of RBG. Alcohol[3,5,8] or analgesic[3] abuse and/or HIV infection[5,10] were reported in the H. pylori-negative RBG/RBD cases, and stimulation or immunological abnormality caused by these factors may also be important in the pathogenesis of RBG/RBD. The present patient did not have any of these suggested etiological factors for RBG/RBD. It is noteworthy that metastatic urothelial carcinoma was present in the vicinity of the duodenum in the present case. Several cancer cases with prominent Mott cell proliferation have been reported[14,15], and it is speculated that chemokine production by tumor cells may be the cause of Mott cell accumulation in these cases[14]. Thus, chemokine production by tumor cells may be the cause of RBD in the present case, although cancer invasion was not present in the duodenal mucosa. It is possible that H. pylori infection is not important in the RBD pathogenesis, because no H. pylori infection has been detected in any of the reported RBD cases, including the present case. However, the pathogenesis of RBG/RBD should be further investigated using larger number of cases and molecular techniques.
In summary, this paper has described a case of RBD occurring in a patient with retroperitoneal metastasis of ureteral cancer. This is the third RBD case to be reported. It is possible that chemokines produced by tumor cells caused RBD in the present patient. It is important that pathologists maintain enough knowledge of the disease to avoid misdiagnosis of the condition as malignant diseases, such as B-cell lymphomas and plasmacytoma. The clinicopathological features of RBD should be further elucidated by continually accumulating and studying reported cases.
P- Reviewers Rodriguez DC, Gupta C S- Editor Gou SX L- Editor Stewart GJ E- Editor Xiong L
| 1. | Russell W. An Address on a Characteristic Organism of Cancer. Br Med J. 1890;2:1356-1360. [PubMed] |
| 2. | Tazawa K, Tsutsumi Y. Localized accumulation of Russell body-containing plasma cells in gastric mucosa with Helicobacter pylori infection: ‘Russell body gastritis’. Pathol Int. 1998;48:242-244. [PubMed] |
| 3. | Erbersdobler A, Petri S, Lock G. Russell body gastritis: an unusual, tumor-like lesion of the gastric mucosa. Arch Pathol Lab Med. 2004;128:915-917. [PubMed] |
| 4. | Ensari A, Savas B, Okcu Heper A, Kuzu I, Idilman R. An unusual presentation of Helicobacter pylori infection: so-called “Russell body gastritis”. Virchows Arch. 2005;446:463-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Drut R, Olenchuk AB. Images in pathology. Russell body gastritis in an HIV-positive patient. Int J Surg Pathol. 2006;14:141-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Paik S, Kim SH, Kim JH, Yang WI, Lee YC. Russell body gastritis associated with Helicobacter pylori infection: a case report. J Clin Pathol. 2006;59:1316-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Pizzolitto S, Camilot D, DeMaglio G, Falconieri G. Russell body gastritis: expanding the spectrum of Helicobacter pylori - related diseases? Pathol Res Pract. 2007;203:457-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Habib C, Gang DL, Ghaoui R, Pantanowitz L. Russell body gastritis. Am J Hematol. 2010;85:951-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Del Gobbo A, Elli L, Braidotti P, Di Nuovo F, Bosari S, Romagnoli S. Helicobacter pylori-negative Russell body gastritis: case report. World J Gastroenterol. 2011;17:1234-1236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Savage NM, Fortson T, Schubert M, Chamberlain S, Lee J, Ramalingam P. Isolated Russell body duodenitis. Dig Dis Sci. 2011;56:2202-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Paniz Mondolfi A, Samuel M, Kikhney J, Moter A, Feldman D, Slova D, Filatov A, Theise N. Russell body duodenitis: a histopathological and molecular approach to a rare clinical entity. Pathol Res Pract. 2012;208:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Blom J, Mansa B, Wilk A. A study of Russell bodies in human monoclonal plasma cells by means of immunofluorescence and electron microscopy. Acta Pathol Microbiol Scand A. 1976;84:335-349. [PubMed] |
| 13. | Hsu SM, Hsu PL, McMillan PN, Fanger H. Russell bodies: a light and electron microscopic immunoperoxidase study. Am J Clin Pathol. 1982;77:26-31. [PubMed] |
| 14. | Shinozaki A, Ushiku T, Fukayama M. Prominent Mott cell proliferation in Epstein-Barr virus-associated gastric carcinoma. Hum Pathol. 2010;41:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Wolf EM, Mrak K, Tschmelitsch J, Langner C. Signet ring cell cancer in a patient with Russell body gastritis--a possible diagnostic pitfall. Histopathology. 2011;58:1178-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |