Published online Mar 7, 2012. doi: 10.3748/wjg.v18.i9.896
Revised: December 21, 2011
Accepted: December 31, 2011
Published online: March 7, 2012
AIM: To test the efficiency of a recently proposed histotype-based grading system in a consecutive series of gastric cancers.
METHODS: Two hundred advanced gastric cancers operated upon in 1980-1987 and followed for a median 159 mo were investigated on hematoxylin-eosin-stained sections to identify low-grade [muconodular, well differentiated tubular, diffuse desmoplastic and high lymphoid response (HLR)], high-grade (anaplastic and mucinous invasive) and intermediate-grade (ordinary cohesive, diffuse and mucinous) cancers, in parallel with a previously investigated series of 292 cases. In addition, immunohistochemical analyses for CD8, CD11 and HLA-DR antigens, pancytokeratin and podoplanin, as well as immunohistochemical and molecular tests for microsatellite DNA instability and in situ hybridization for the Epstein-Barr virus (EBV) EBER1 gene were performed. Patient survival was assessed with death rates per 100 person-years and with Kaplan-Meier or Cox model estimates.
RESULTS: Collectively, the four low-grade histotypes accounted for 22% and the two high-grade histotypes for 7% of the consecutive cancers investigated, while the remaining 71% of cases were intermediate-grade cancers, with highly significant, stage-independent, survival differences among the three tumor grades (P = 0.004 for grade 1 vs 2 and P = 0.0019 for grade 2 vs grade 3), thus confirming the results in the original series. A combined analysis of 492 cases showed an improved prognostic value of histotype-based grading compared with the Lauren classification. In addition, it allowed better characterization of rare histotypes, particularly the three subsets of prognostically different mucinous neoplasms, of which 10 ordinary mucinous cancers showed stage-inclusive survival worse than that of 20 muconodular (P = 0.037) and better than that of 21 high-grade (P < 0.001) cases. Tumors with high-level microsatellite DNA instability (MSI-H) or EBV infection, together with a third subset negative for both conditions, formed the T8 cell-rich HLR group, the largest group among low-grade histotypes. Coexisting HLR proved to be a factor in improved prognosis in tumors with microsatellite instability (P = 0.0015 vs HLR-/MSI-H tumors) or DR type human leukocyte antigen expression (P = 0.033 vs HLR-/HLA-DR+ tumors).
CONCLUSION: Identification of low- and high-grade histotypes can improve the prognostic assessment of a substantial proportion of gastric cancers in routine diagnostic practice.
- Citation: Chiaravalli AM, Klersy C, Vanoli A, Ferretti A, Capella C, Solcia E. Histotype-based prognostic classification of gastric cancer. World J Gastroenterol 2012; 18(9): 896-904
- URL: https://www.wjgnet.com/1007-9327/full/v18/i9/896.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i9.896
The difficulty of assessing the prognosis of gastric cancer using histological methods is well known and this is also reflected in the essentially descriptive character of presently used classifications[1-4]. However, several histotypes characterized by lower malignant potential have been identified and separated from more common cohesive (so-called “intestinal”) or diffuse tumors. They include lymphocyte-rich cancer[5-7], muconodular cancer[8], very-well-differentiated tubular cancer with an intestinal[9] or gastric[10] phenotype, and a low-grade subtype of diffuse desmoplastic cancer[11]. Similarly, various kinds of cancer with poor outcome have been identified, from poorly differentiated neuroendocrine carcinoma, small to large cell[12,13], to anaplastic diffuse cancer[11] or hepatoid[14], chorioncarcinomatous[15] and adenosquamous carcinoma[16]. In addition, comparative genomic hybridization analysis has shown a clear relationship between the number and severity of genomic alterations and tumor histotype and prognosis[17,18].
The different behavior of these histotypes offered an opportunity to develop a three-grade system of prognostic evaluation, which, when applied to a large tumor series, was highly predictive of patient outcome[19]. However, the tumor series used in that study was substantially selected (1) to be representative of all main stages (intramucosal cases apart) and histological types of the disease; and (2) to include uncommon histological subtypes or variants, as well as earlier invasive stages (submucosal or confined to muscularis propria). Therefore, in order to ascertain the effectiveness of the system in routine diagnostic work, a continuous, homogeneous series of advanced cancers needs to be evaluated.
In this study, we retrospectively identified prognostic histotypes according to previously reported criteria[19] in a consecutive series of advanced (muscularis propria invasion or beyond) gastric cancers collected at Varese General Hospital during 1980-1987, and we tested such histotypes as potential predictors of patient outcome and compared the results with those of the original Pavia series. During the study, we realized that we needed to investigate further mucinous and lymphocyte-rich cancers, due to discrepancies concerning: (1) the impact of histology or stage on mucinous cancer prognosis[20-23]; and (2) the contribution of tumor microsatellite instability, Epstein-Barr virus (EBV) infection and DR type human leukocyte antigen (HLA-DR) expression, rather than lymphoid cell response per se, to the natural history of lymphocyte-rich neoplasms[5-7,24-27]. Therefore, mucinous and lymphocyte-rich tumors from both series were combined to obtain tumor groups large enough to allow appropriate investigation.
A consecutive series of 200 invasive (T2-T4) gastric cancers were retrieved from the files of the Anatomic Pathology Service, for patients who had undergone potentially curative surgery at Varese General Hospital during 1980-1987. The clinicopathological and follow-up data of all the patients were carefully collected from hospital records, interviews with family doctors, and the Varese Province Tumor Registry. One hundred and eighty-five of the cases had already been the subject of a previous investigation[2]. Eight cases were operated on during January 1980-April 1987 and were not considered in the previous study because the available clinical or follow-up documentation was incomplete. This information was retraced, appropriately documented and added to the present study to ensure the continuous pattern of the patient series, together with seven more cases operated on in May-June 1987. The original tumor node metastasis (TNM) stage assessment of each tumor was revised according to the criteria of the 2002, 6th Edition American Joint Committee on Cancer system[28]. No antiblastic therapy had been given to the patients. For survivors, the follow-up period was prolonged until 2008; a median follow-up of 159 mo was recorded.
Archival and newly cut paraffin sections were stained with hematoxylin-eosin, Alcian blue-periodic acid Schiff or the immunoperoxidase procedure using antibodies directed against h-MLH1 (G-168.15 clone; Pharmingen, San Diego, CA, United States), hMSH2 (Fe11 clone; Oncogene, Cambridge, MA, United States), hPMS2 (clone A16-4; BD Pharmingen), hMSH6 (clone 44; BD Transduction Laboratories, Lexington, KY, United States), CD8 antigen (C8/144B clone; Dako, Glostrup, Denmark), CD11c antigen (5D11 clone; Novocastra Laboratories, Newcastle, United Kingdom), pancytokeratin (AE1/AE3 clone; Novocastra Laboratories), HLA-DR (LN3 clone; Biotest, Dreieich, Germany) and podoplanin (D2-40 clone; Biocare Medical, Concord, CA, United States) as previously reported[7,19,29].
In situ hybridization for the EBER1 gene of EBV was performed as described previously[3,7]. Microsatellite instability was assessed at Bat 25, Bat 26, BAT40, D5S346 and D2S123 loci. Tumors with instability involving at least two of the five loci were classified as highly instable (MSI-H), while those with only one instable locus were classified as low instable (MSI-L) and included in the MSI negative tumor group together with microsatellite stable cases[7,19,30].
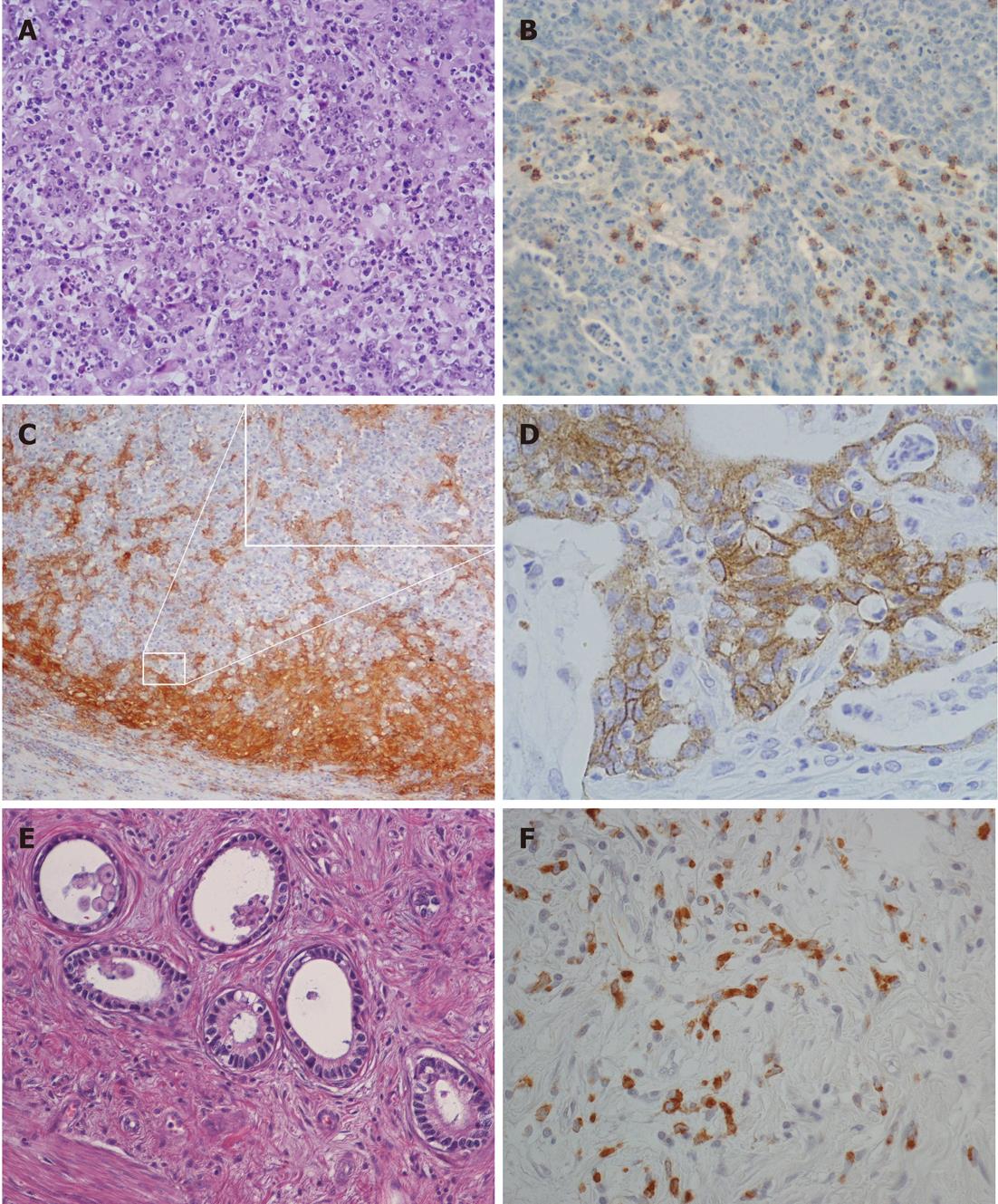
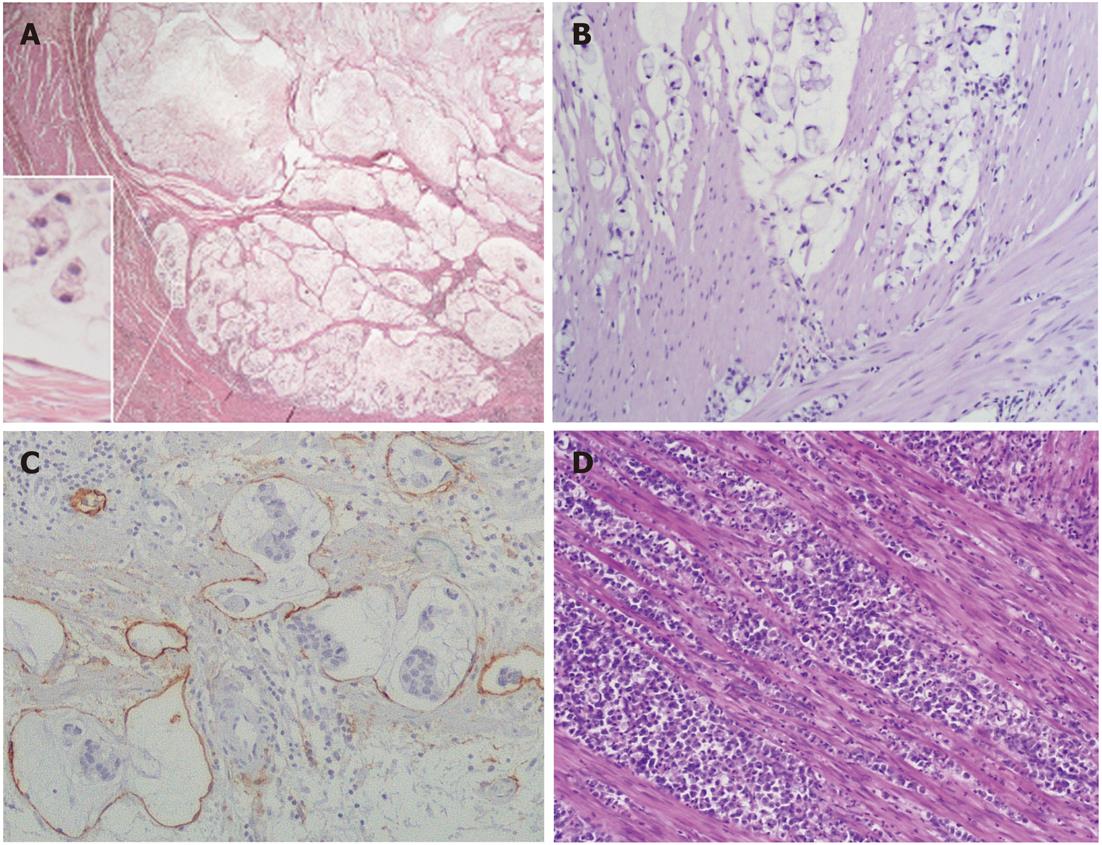
Tumor histotypes were identified as described previously[8-11,19]. In particular, very-well-differentiated tubular (VWDT) cancer is characterized by glands with moderately atypical, polarized cells arranged in a monostratified epithelium, low-grade diffuse desmoplastic cancer shows fibroblast-rich desmoplasia surrounding individual (or minute groups of) moderately atypical tumor cells, while muconodular cancer forms extracellular mucin lakes with expansile borders in which isolated signet ring cells or cords of mucin-producing tumor cells are freely floating.
To increase the diagnostic accuracy of lymphocyte-rich tumors, as well as intratumor CD8+ T cell counts, intraepithelial T8 cells (i.e., cells infiltrating tumor aggregates so as to contact neoplastic cells directly, with the exclusion of purely stromal T8 cells) were also counted[7,31], and an evaluation of dendritic cells was added[27]. Thus, in this study, classification of a lymphocyte-rich tumor as high lymphoid response (HLR) required one of the following: (1) a lymphoepithelial type histological pattern with an overwhelming lymphocyte infiltrate dissecting tumor cells; or (2) > 400 intratumor and/or >200 intraepithelial CD8-positive cells in 10 high-power fields (HPFs), coupled with a band of lymphoid cells rich in CD8+ T cells and CD11c+ dendritic cells surrounding expansile tumor nodules.
Anaplastic cancers were characterized by small to large, cytokeratin-positive cells with highly atypical nuclei, with or without prominent nucleoli and with or without signs of poor neuroendocrine differentiation, high cellularity, scarce stroma, and high proliferative rates (> 20 mitoses/10 HPFs)[11,12]. During characterization of the mucinous infiltrative tumors, it was found that those showing local infiltration of peritumoral tissues in the absence of prominent lymphoinvasion or angioinvasion had a less severe prognosis. Therefore, in this study, infiltrative tumors lacking vascular invasion or with only sporadic lymphoinvasion were added to the grade 2 group, together with ordinary cohesive and diffuse cancers, while only prominently lymphoinvasive (two or more foci per microscopic tumor sections) or angioinvasive cases remained in the grade 3 group together with anaplastic cancers, as in the original classification[19]. Cases showing a coexistence of two or more histological patterns were classified according to their prevalent histotype, provided that all the components were low-grade; otherwise, they were classified according to their higher grade component.
A reproducibility test involving two senior pathologists (Solcia E and Capella C) gave a κ value of 0.84 concerning interobserver agreement for five main histotypes (cohesive, diffuse, mucinous, anaplastic and HLR), a κ of 0.81 agreement for nine subtypes (HLR, VWDT, ordinary cohesive, low-grade diffuse desmoplastic, ordinary diffuse, muconodular, ordinary mucinous, invasive mucinous and anaplastic), and a κ of 0.79 agreement for the three histotype-based grades (low, intermediate and high).
From the previously investigated Pavia series of 294 cases[19], 292 cases (two tumors had to be excluded because there was no remaining tumor tissue) were considered for comparative analysis with the Varese series, as well as for a joint reinvestigation of both series looking at mucinous and HLR tumors according to the above criteria. In addition, the prognostic value of the histotype-based grading system was compared with that of the commonly used Lauren classification[1].
Statistical analysis was performed using Stata version 11 (Stata Corporation, College Station, TX, United States). All tests were two-sided. Categorical variables were described with counts and percentages and compared with the Fisher exact test. Continuous variables were described with median and quartiles, and compared with the Kruskal-Wallis test. Death rates per 100 person-years, with 95% confidence intervals (CIs), and Kaplan-Meier estimates were computed to describe survival. The Cox model was used to assess the prognostic role of the considered variables; both univariate and bivariate models (inclusive of stage) were fitted. The hazard ratio (HR) and 95% CI were reported. Proportional hazard assumptions were satisfied in all cases. The Harrell c statistic was computed to assess model performance (discrimination ability); a value of 0.5 indicating no discrimination and a value of 1 indicating perfect discrimination.
The Varese consecutive, non-selected series of 200 advanced (T2 or above) gastric cancers is described in Table 1 according to stage and patient survival. Compared with the original Pavia selected series, which also included a substantial number (44 cases) of deeply submucosal (penetrating T1b) tumors, the present series showed more advanced tumors (Stages III + IV: 49.5% vs 39% of the original series; Stage II: 30.5% vs 25%). A clear step-wise, stage-dependent behavior emerges from the survival analysis.
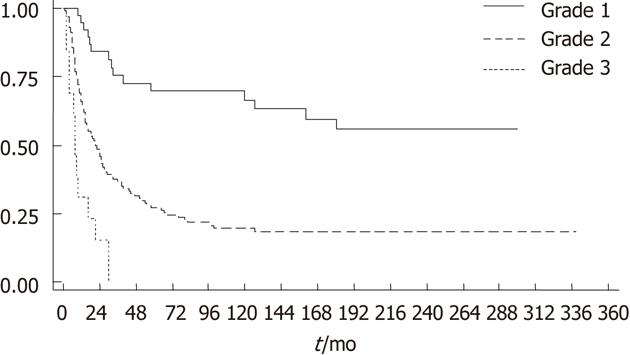
Of the 200 tumors, 44 (22%) had low-grade, 14 (7%) high-grade and 142 (71%) intermediate-grade histotypes (Figures 1 and 2). Survival analysis according to histotype-based grade is outlined in Table 2 and Figure 3. The more favorable behavior of grade 1 compared to grade 2 and of grade 2 compared to grade 3 tumors is evident.
| Cox survival analysis | |||||||
| P value | |||||||
| n (%) | Death rate | 95% CI | HR | 95% CI | Univariate | With stage | |
| Grade | |||||||
| 1 | 44 (22) | 3.35 | 2.02-5.55 | 1 | |||
| 2 | 142 (71) | 17.24 | 14.16-21.90 | 3.5 | 2.02-6.05 | < 0.001 | 0.004 |
| 3 | 14 (7) | 95.66 | 54.33-168.45 | 9.64 | 4.41-21.06 | < 0.001a | < 0.001b |
| Lauren type1 | |||||||
| Intestinal | 116 (58) | 8.86 | 6.93-11.31 | 1 | |||
| Diffuse | 50 (25) | 21.08 | 15.20-29.22 | 1.71 | 1.13-2.57 | 0.011 | 0.362 |
| Unclassified | 34 (17) | 18.4 | 12.53-27.03 | 1.7 | 1.07-2.68 | 0.023 | 0.165 |
In Table 2, univariate analysis of the Varese series after reclassification according to Lauren[1] shows a significantly worse prognosis for the diffuse compared to intestinal and unclassified types [model: χ2 (2γ) = 8.67, P = 0.013]. However no significant difference was observed among the same cases in the stage-inclusive bivariate analysis (model: P = 0.341 for Lauren classification), while no difference was found by either univariate (model: χ2 (2γ) = 4.87, P = 0.087) or bivariate (model: P = 0.342) analysis among the 292 tumors in the Pavia series. In both the Varese and Pavia series, the Harrell’s concordance (c) test showed a higher efficiency of the histotype-based grading (c = 0.63 and 0.73, respectively) compared to the Lauren classification (c = 0.57 and 0.55).
Individual histotypes in the three grades are detailed in Table 3, first column. It appears that, while ordinary cohesive or diffuse and HLR tumors form a substantial group, the number of other histotypes is too low to allow appropriate statistical analysis.
| Cox survival analysis | ||||||||
| n (%) | P value | |||||||
| Varese series | Joint series | Death rate | 95% CI | HR1 | 95% CI | Univariate | With stage | |
| Grade 1 | 44 (22) | 132 (26.8) | 2.54 | 1.80-3.57 | 1 | |||
| HLR | 38 (19) | 82 (16.6) | 3.67 | 2.50-5.39 | 1 | |||
| WD tubular | 2 (1) | 13 (2.6) | 0 | |||||
| Lg diff.desm | 2 (1) | 17 (3.5) | 1.50 | 0.48-4.64 | 0.43 | 0.13-1.42 | 0.167 | 0.109 |
| muconodular | 2 (1) | 20 (4.1) | 1.67 | 0.63-4.44 | 0.51 | 0.18-1.46 | 0.211 | 0.271 |
| Grade 2 | 142 (71) | 307 (62.4) | 15.97 | 13.96-18.28 | 4.91 | 3.40-7.11 | < 0.001 | < 0.001 |
| Mucinous ord. | 4 (2) | 10 (2.0) | 11.50 | 5.16-25.59 | 2.16 | 0.89-5.24 | 0.090a | 0.987b |
| Cohesive ord. | 95 (47.5) | 196 (39.8) | 15.28 | 12.89-18.11 | 3.43 | 2.25-5.23 | < 0.001 | 0.001 |
| Diffuse ord. | 43 (21.5) | 101 (20.6) | 18.09 | 14.36-22.79 | 3.96 | 2.53-6.22 | < 0.001 | < 0.001 |
| Grade 3 | 14 (7) | 53 (10.8) | 108.84 | 82.02-144.43 | 18.47 | 11.56-29.50 | < 0.001c | < 0.001c |
| Mucinous Hg. | 3 (1.5) | 21 (4.3) | 100.0 | 64.07-160.0 | 11.54 | 6.32-21.48 | < 0.001 | < 0.001 |
| Anaplastic | 11 (5.5) | 32 (6.5) | 120.0 | 80.01-170.0 | 14.11 | 8.17-24.39 | < 0.001 | < 0.001 |
When corresponding tumors of the two series were analyzed jointly (Table 3), the more favorable behavior of grade 1 compared to grade 2 tumors and of the latter compared to grade 3 cases was confirmed. In addition, the resulting number of the rare histotypes was sufficient to allow survival analysis of each histotype. Thus, the prognostic similarity of types belonging to the same grade and their significant difference from those of other grades was assessed.
It also appears from Table 3 that mucinous neoplasms, when appropriately reclassified as muconodular, ordinary mucinous and highly invasive mucinous cancers, may form three prognostically different histological subsets, as confirmed by separate Cox univariate and stage-inclusive survival analyses, where ordinary mucinous cancers proved significantly worse than muconodular and better than highly invasive cancers (Table 3). Significant differences were also found between the three groups in terms of TNM stage, T level invasion and lymph node involvement (for all: P < 0.001, Fisher’s exact test) and even diameter (P < 0.001, Kruskal-Wallis test). In contrast, only a nonsignificant trend (Cox univariate P = 0.126 and stage-inclusive P = 0.102) for better survival was noted among mucinous cancers as a whole; for those with cohesive vs diffuse or mixed histological patterns.
No survival difference was found between HLR and the three other low-grade histotypes or, among the HLR cases, between MSI-H and the EBV-/MSI- subset, while a trend for worse behavior of the EBV+ compared to the other subsets was noted by univariate analysis, which disappeared with stage-inclusive bivariate analysis (Table 4). EBV+ tumors also differed from the other two HLR subsets in showing significantly higher proportions of lymphoepithelioid histology (17/24, 71%, vs 5/57, 9%, P < 0.001, Fisher’s exact test) and median intratumor T8 (107.5 vs 58.5 per HPF, P < 0.001, Kruskal-Wallis test). In contrast, 40 HLR MSI-H cases obviously showed more favorable behavior than their 38 non-HLR MSI-H counterparts (22 cohesive, 10 mucinous, three diffuse and anaplastic cancers), of which six were low-, 24 intermediate- and eight high-grade.
The proportion of mucinous neoplasms showing MSI-H (10/51; 19.6%) did not differ significantly from that of the whole tumor population (78/492; 15.9%) while remaining significantly lower than that of HLR cases (41/81; 50.6%). Notably, the 10 MSI-H cases were equally distributed among the three grades of mucinous cancers, being grade 1 [14/20 (20%)], grade 2 [1/10 (10%)], and grade 3 [5/21 (23.8%)].
For the combined analysis of HLR and HLA-DR status, 77 of the total 82 HLR tumors from both series had sufficient histological material left to allow reinvestigation, together with 202 randomly selected non-HLR tumors representative of all histotypes and stages. HLA-DR positivity in > 10% of tumor cells was found in 100/279 (35.8%) cases. Positive tumors showed a trend for lower death rate (5.97, 4.39-8.19 vs 9.41, 7.75-11.44) and improved survival (HR: 0.63, 0.44-0.91, P = 0.014) compared with HLA-DR- cases, a behavior probably accounted for by the HLR+/HLA-DR+ subset, in which 57 tumors showed a significantly lower death rate (3.21, 1.93-5.32 vs 11.84, 8.06-17.39) and better survival (HR: 0.35, 0.18-0.66, P = 0.001) than their HLR-/HLA-DR+ counterparts.
The present study confirms, in an independent patient series, the effectiveness of a recently proposed histotype-based grading system for the prognostic evaluation of gastric cancer[19], despite substantial differences between the present series and the original one. Indeed, the present series differed in being consecutive rather than selected for uncommon histotypes and in lacking submucosal (T1b) cancers, while including more advanced cases, diagnosed and operated on about a decade earlier in another hospital serving a different territory. All these differences may help to explain the lower prevalence of low-grade cases (22% vs 31% in the original series), known to be more frequent in lower stages[19]. This is especially true for rare histotypes like muconodular[8], VWDT[9,10] or low-grade diffuse desmoplastic[11] cancers, which were specifically selected in the original series. However, the HLR histotype was confirmed to represent a fairly large (19%) population of low-grade gastric cancers, even after introducing more stringent diagnostic criteria. Thus, our consecutive Varese Hospital series suggests that only about 20% of all invasive (T2 or beyond) gastric cancers may be of low-grade, while < 10% may fit into the high-grade category. However, within these quantitative limits, the histotype-based three-grade system was confirmed to be highly predictive of patient outcome. This conclusion seems especially interesting considering the limited prognostic value of commonly used histological classifications[1-4]. Indeed, in this study the histotype-based classification and grading system showed superior Harrell’s discriminative power when compared with the Lauren classification and, unlike the latter, outlined stage-independent prognostic differences.
Reinvestigation of the two series taken together increased the number of rare tumor subsets and allowed better characterization of their clinicopathological profile, with special reference to the HLR and mucinous tumors. The presence of three etiological subtypes among HLR tumors[19] was also confirmed in the new series. Joint analysis of the 82 HLR cases obtained from the two series allowed us to confirm the distinct clinicopathological pattern of EBV+ (preferred proximal location in the stomach, higher frequency of lymphoepithelioid pattern, higher intratumor T8 cell counts, and a trend toward worse survival), as already illustrated in previous studies[6,7,32], compared to both the MSI-H and the MSI-/EBV- subsets, whose behavior was remarkably similar to each other. Among MSI-H tumors, the better prognosis of those associated with HLR compared with those lacking this association is of interest, because it suggests that cytotoxic T8 cell response, more than the MSI status itself, is a crucial factor in defining the behavior of this tumor subset, with potential implications for appropriate therapy[26].
The cause of the T8-cell-rich lymphoid response in the EBV-/MSI- subset remains to be ascertained. Tumor cell overexpression of highly antigenic molecules like HLA-DR, as suggested in the present investigation, or of mutated p53 protein, as found in a previous study[19], might be among its driving factors. Whatever its origin, it should be pointed out that the favorable prognostic implication of HLR deserves attention in clinical studies because, besides accounting for the better prognosis of MSI-H+[5,7,19,26] and HLA-DR+ cancers[24], it might interfere with therapies potentially affecting antitumor immune response[26].
In our cumulative series of 492 tumors, the proportion of mucinous cancers showing high MSI was not significantly higher than in the remaining neoplasms; a finding confirming recent observations[22], although at variance with the behavior of mucinous cancers in the colon, where they represent in some series a dominant histotype among MSI-H cases[31,33]. Moreover, the few MSI+ mucinous cancers that we found in the stomach lacked an HLR, and failed to show a preferential concentration in the low-grade, muconodular histotype, or evidence for more favorable survival compared to their MSI-counterpart. This further outlines the limited prognostic value of MSI status in the absence of HLR, at least in the stomach. It should also be recalled that, at variance with gut cancers, in other cancers (e.g., breast and lung), MSI has more often been linked to adverse rather than favorable postoperative survival[25,34].
Several studies have shown that mucinous gastric cancers have a worse prognosis[20-22]. However, it is uncertain whether a higher stage at diagnosis[23], rather than mucinous type histology, may account for this. In this study we reinvestigated the issue with the help of an improved histological classification separating low-grade muconodular tumors from infiltrative mucinous cancers, among which grade 3 cases showing prominent vascular (especially lympho-) invasion were distinguished from grade 2 “ordinary” cases lacking it. Survival analysis showed that these may represent three prognostically distinct, stage-independent cancer subsets, despite the fact that they also show highly significant differences in stage and size. Thus, for the prognostic assessment of mucinous cancers both stage and histology should be carefully investigated.
In conclusion, our histotype-based grading system for gastric cancers proved to be an effective tool, at least for a minority (about 30%) of neoplasms. For the evaluation of grade 2 ordinary cancers, which form a very large, histologically heterogeneous group with a wide prognostic spectrum (e.g., a 95% CI 14-187 in our joint series), we should rely on common histological parameters (invasive pattern, proliferative rate, structural or cytological atypia, tumor cell phenotype)[4] or a variety of promising molecular tools[17,18,35], as well as on carefully assessed stage.
Gastric carcinoma is a very heterogeneous tumor, often characterized by the coexistence of two or more distinct histological components within the same tumor, and by different host responses in terms of stromal response. A detailed histopathological classification should enable us to identify tumor subtypes that could provide useful prognostic and therapeutic information. The efficacy of a recently proposed classification system in predicting survival needs to be tested in a non-selected series of gastric carcinomas.
This new histological classification takes advantage of the cytological, biological and architectural features of tumor cells to identify histological types (histotypes) of tumors with low, intermediate or high malignancy. Lymphoid and stromal reactions, which seem to play an important role in contrasting or favoring tumor growth, and consequently, resulting in a better or worse prognosis, also need to be taken into consideration.
The proposed three-grade system proved to be highly predictive of patient outcome. It identified low-grade (muconodular, well-differentiated tubular, diffuse desmoplastic and high lymphoid response), intermediate-grade (ordinary cohesive, diffuse and mucinous) and high-grade (anaplastic and mucinous invasive) gastric cancers, with highly significant stage-independent survival differences and had a better prognostic value compared to the Lauren classification.
A careful histological examination of gastric cancers with the criteria proposed by the histotype-based prognostic classification was shown to be an effective tool in everyday diagnostic practice. Additional studies are necessary to identify histological and molecular parameters that could better characterize the large population of intermediate-grade cancers.
The manuscript fits well with the scope of the journal, and is well written. It addresses a relevant aspect of gastric cancer, the exact histopathological assessment of the tumor and correlation of these data to clinical and molecular alterations.
Peer reviewers: Liang-Shun Wang, Professor, Shuang-Ho Hospital-Taipei Medical University, Jhonghe City, New Taipei City 235, Taiwan, China; Dr. Thomas Wex, Clinic of Gastroenterology and Hepatology, Otto-von-Guericke University, Magdeburg 39120, Germany; Dr. Takaaki Arigami, Department of Surgical Oncology and Digestive, Kagoshima University Graduate School of Medical and Dental Sciences, 8-35-1 Sakuragaoka, Kagoshima 890-8520, Japan; Ki Baik Hahm, Professor, Gastroenterology, Gachon Graduate School of Medicine, 7-45 Songdo-dong Yeonsu-gu, Lee Gil Ya Cancer and Diabetes Institute, Incheon 406-840, South Korea
S- Editor Gou SX L- Editor Kerr C E- Editor Xiong L
| 1. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 2. | Ribeiro MM, Seixas M, Sobrinho-Simoes M. Prognosis in gastric carcinoma. The preeminence of staging and futility of histological classification. Dig Dis Pathol. 1988;1:51-68. |
| 3. | Chiaravalli AM, Cornaggia M, Furlan D, Capella C, Fiocca R, Tagliabue G, Klersy C, Solcia E. The role of histological investigation in prognostic evaluation of advanced gastric cancer. Analysis of histological structure and molecular changes compared with invasive pattern and stage. Virchows Arch. 2001;439:158-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Lauwers GY, Carneiro F, Graham DY, Curado MP, Franceschi S, Montgomery E, Tatematsu M, Hattori T. Gastric carcinoma. WHO Classification of Tumors of the Digestive System. 4th Edition. Lyon: JARC 2010; 48-68. |
| 5. | Minamoto T, Mai M, Watanabe K, Ooi A, Kitamura T, Takahashi Y, Ueda H, Ogino T, Nakanishi I. Medullary carcinoma with lymphocytic infiltration of the stomach. Clinicopathologic study of 27 cases and immunohistochemical analysis of the subpopulations of infiltrating lymphocytes in the tumor. Cancer. 1990;66:945-952. [PubMed] |
| 6. | Grogg KL, Lohse CM, Pankratz VS, Halling KC, Smyrk TC. Lymphocyte-rich gastric cancer: associations with Epstein-Barr virus, microsatellite instability, histology, and survival. Mod Pathol. 2003;16:641-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Chiaravalli AM, Feltri M, Bertolini V, Bagnoli E, Furlan D, Cerutti R, Novario R, Capella C. Intratumour T cells, their activation status and survival in gastric carcinomas characterised for microsatellite instability and Epstein-Barr virus infection. Virchows Arch. 2006;448:344-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Solcia E, Luinetti O, Tava F, Klersy C, Grillo F, Pandolfo N, Fiocca R. Identification of a lower grade muconodular subtype of gastric mucinous cancer. Virchows Arch. 2004;445:572-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Endoh Y, Tamura G, Motoyama T, Ajioka Y, Watanabe H. Well-differentiated adenocarcinoma mimicking complete-type intestinal metaplasia in the stomach. Hum Pathol. 1999;30:826-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Yao T, Utsunomiya T, Oya M, Nishiyama K, Tsuneyoshi M. Extremely well-differentiated adenocarcinoma of the stomach: clinicopathological and immunohistochemical features. World J Gastroenterol. 2006;12:2510-2516. [PubMed] |
| 11. | Chiaravalli AM, Klersy C, Tava F, Manca R, Fiocca R, Capella C, Solcia E. Lower- and higher-grade subtypes of diffuse gastric cancer. Hum Pathol. 2009;40:1591-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Rindi G, Azzoni C, La Rosa S, Klersy C, Paolotti D, Rappel S, Stolte M, Capella C, Bordi C, Solcia E. ECL cell tumor and poorly differentiated endocrine carcinoma of the stomach: prognostic evaluation by pathological analysis. Gastroenterology. 1999;116:532-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 225] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Jiang SX, Mikami T, Umezawa A, Saegusa M, Kameya T, Okayasu I. Gastric large cell neuroendocrine carcinomas: a distinct clinicopathologic entity. Am J Surg Pathol. 2006;30:945-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Nagai E, Ueyama T, Yao T, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach. A clinicopathologic and immunohistochemical analysis. Cancer. 1993;72:1827-1835. [PubMed] |
| 15. | Krulewski T, Cohen LB. Choriocarcinoma of the stomach: pathogenesis and clinical characteristics. Am J Gastroenterol. 1988;83:1172-1175. [PubMed] |
| 16. | Mori M, Iwashita A, Enjoji M. Adenosquamous carcinoma of the stomach. A clinicopathologic analysis of 28 cases. Cancer. 1986;57:333-339. [PubMed] |
| 17. | Weiss MM, Kuipers EJ, Postma C, Snijders AM, Pinkel D, Meuwissen SG, Albertson D, Meijer GA. Genomic alterations in primary gastric adenocarcinomas correlate with clinicopathological characteristics and survival. Cell Oncol. 2004;26:307-317. [PubMed] |
| 18. | Rossi E, Klersy C, Manca R, Zuffardi O, Solcia E. Correlation between genomic alterations assessed by array comparative genomic hybridization, prognostically informative histologic subtype, stage, and patient survival in gastric cancer. Hum Pathol. 2011;42:1937-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Solcia E, Klersy C, Mastracci L, Alberizzi P, Candusso ME, Diegoli M, Tava F, Riboni R, Manca R, Luinetti O. A combined histologic and molecular approach identifies three groups of gastric cancer with different prognosis. Virchows Arch. 2009;455:197-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Yasuda K, Adachi Y, Shiraishi N, Yamaguchi K, Shiromizu A, Kitano S. Pathology and prognosis of mucinous gastric carcinoma. J Surg Oncol. 2001;76:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Kunisaki C, Akiyama H, Nomura M, Matsuda G, Otsuka Y, Ono HA, Shimada H. Clinicopathologic characteristics and surgical outcomes of mucinous gastric carcinoma. Ann Surg Oncol. 2006;13:836-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Choi JS, Kim MA, Lee HE, Lee HS, Kim WH. Mucinous gastric carcinomas: clinicopathologic and molecular analyses. Cancer. 2009;115:3581-3590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Choi MG, Sung CO, Noh JH, Kim KM, Sohn TS, Kim S, Bae JM. Mucinous gastric cancer presents with more advanced tumor stage and weaker β-catenin expression than nonmucinous cancer. Ann Surg Oncol. 2010;17:3053-3058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Ma XC, Hattori T, Kushima R, Terata N, Kodama M. Expression of HLA-class II antigen in gastric carcinomas. Its relationship to histopathological grade, lymphocyte infiltration and five-year survival rate. Acta Oncol. 1994;33:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Paulson TG, Wright FA, Parker BA, Russack V, Wahl GM. Microsatellite instability correlates with reduced survival and poor disease prognosis in breast cancer. Cancer Res. 1996;56:4021-4026. [PubMed] |
| 26. | Guidoboni M, Gafà R, Viel A, Doglioni C, Russo A, Santini A, Del Tin L, Macrì E, Lanza G, Boiocchi M. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol. 2001;159:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 249] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | van Beek J, zur Hausen A, Snel SN, Berkhof J, Kranenbarg EK, van de Velde CJ, van den Brule AJ, Middeldorp JM, Meijer CJ, Bloemena E. Morphological evidence of an activated cytotoxic T-cell infiltrate in EBV-positive gastric carcinoma preventing lymph node metastases. Am J Surg Pathol. 2006;30:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC Cancer staging handbook. TNM Classification of malignant tumors, 6th edition. New York: Springer 2002; . |
| 29. | Yonemura Y, Endou Y, Tabachi K, Kawamura T, Yun HY, Kameya T, Hayashi I, Bandou E, Sasaki T, Miura M. Evaluation of lymphatic invasion in primary gastric cancer by a new monoclonal antibody, D2-40. Hum Pathol. 2006;37:1193-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248-5257. [PubMed] |
| 31. | Jass JR, Do KA, Simms LA, Iino H, Wynter C, Pillay SP, Searle J, Radford-Smith G, Young J, Leggett B. Morphology of sporadic colorectal cancer with DNA replication errors. Gut. 1998;42:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 335] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 32. | Kijima Y, Ishigami S, Hokita S, Koriyama C, Akiba S, Eizuru Y, Aikou T. The comparison of the prognosis between Epstein-Barr virus (EBV)-positive gastric carcinomas and EBV-negative ones. Cancer Lett. 2003;200:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Leopoldo S, Lorena B, Cinzia A, Gabriella DC, Angela Luciana B, Renato C, Antonio M, Carlo S, Cristina P, Stefano C. Two subtypes of mucinous adenocarcinoma of the colorectum: clinicopathological and genetic features. Ann Surg Oncol. 2008;15:1429-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Rosell R, Pifarré A, Monzó M, Astudillo J, López-Cabrerizo MP, Calvo R, Moreno I, Sanchez-Céspedes M, Font A, Navas-Palacios JJ. Reduced survival in patients with stage-I non-small-cell lung cancer associated with DNA-replication errors. Int J Cancer. 1997;74:330-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Carneiro F, Oliveira C, Leite M, Seruca R. Molecular targets and biological modifiers in gastric cancer. Semin Diagn Pathol. 2008;25:274-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |