Published online Feb 21, 2012. doi: 10.3748/wjg.v18.i7.704
Revised: July 10, 2011
Accepted: July 17, 2011
Published online: February 21, 2012
AIM: To investigate the effects of ESC-3 isolated from crocodile bile on the growth and apoptosis induction of human cholangiocarcinoma cells.
METHODS: ESC-3 was isolated from crocodile bile by Sephadex LH-20 and RP-18 reversed-phase column. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay was conducted to determine the effects of ESC-3 on the proliferation of human cholangiocarcinoma cell lines (QBC939, Sk-ChA-1 and MZ-ChA-1). Giemsa staining, Hoechst 33258 and acridine orange/ethidium bromide staining showed the morphological changes of Mz-ChA-1 cells exposed to ESC-3 at different concentrations. Flow cytometry with regular propidium iodide (PI) staining was performed to analyze the cell cycle distribution of Mz-ChA-1 cells and to assess apoptosis by annexin v-fluorescein isothiocyanate (V-FITC)/PI staining. Rh123 staining was used to detect the alteration of mitochondrial membrane potential (ΔΨm). The protein levels of Bax, Bcl-2, Cdk2, cytochrome c and caspase-3 were further confirmed by Western blotting.
RESULTS: ESC-3 significantly inhibited the growth of three human cholangiocarcinoma cell lines and arrested Mz-ChA-1 cell cycle at G0/G1 phase. Mz-ChA-1 cells showed typical apoptotic morphological changes after treated with ESC-3 (10 μg/mL) for 48 h. Cell death assay indicated that Mz-ChA-1 cells underwent apoptosis in a dose-dependent manner induced by ESC-3. In addition, ESC-3 treatment could downregulate the protein level of Bcl-2 and upregulate the Bax, leading to the increase in the ratio of Bax to Bcl-2 in Mz-ChA-1 cells. Meanwhile, cytochrome c was released from the mitochondria into the cytosol, which subsequently initiated the activation of caspase-3. All these events were associated with the collapse of the mitochondrial membrane potential.
CONCLUSION: ESC-3, the active ingredient of crocodile bile, induced apoptosis in Mz-ChA-1 cells through the mitochondria-dependent pathway and may be a potential chemotherapeutic drug for the treatment of cholangiocarcinoma.
-
Citation: Song W, Shen DY, Kang JH, Li SS, Zhan HW, Shi Y, Xiong YX, Liang G, Chen QX. Apoptosis of human cholangiocarcinoma cells induced by ESC-3 from
Crocodylus siamensis bile. World J Gastroenterol 2012; 18(7): 704-711 - URL: https://www.wjgnet.com/1007-9327/full/v18/i7/704.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i7.704
Cholangiocarcinoma is the second most common primary hepatic tumor and currently accounts for 3% of all gastrointestinal cancers; its incidence has recently been increasing globally[1-3]. Clinically, cholangiocarcinoma patients are treated with surgical resection, radiation and chemotherapy. Although progress has been made in the diagnosis and treatment of cholangiocarcinoma in recent years, the prognosis is still unsatisfactory due to the lack of efficient anticancer drugs[4]. Currently, more than 30 compounds of natural origin are in various phases of clinical study for the treatment of several types of cancer[5]. Hence, it is a potential strategy to discover effective compounds from natural products for cancer treatment[6].
With the progress in research on traditional Chinese medicine (TCM), more natural products have been used in medical treatment, especially in the treatment of cancers. Bile is a fluid that consists of several acids, including cholic acid (CA) and chenodeoxycholic acid (CDCA) and has potential anticancer effects; CA and CDCA are the predominant active components in snakes[7]. Previous reports showed that bile acid played a key role in regulating cholangiocyte growth and secretion[8,9]. In addition, deoxycholic acid (DCA) has been shown to rapidly induce apoptosis in HCT116 cells, a colon tumor cell line[10]. The effects of synthetic derivatives of ursodeoxycholic acid (UDCA), such as HS-1183, and CDCA, such as HS-1199 and HS-1200, on the proliferation of human prostate carcinoma PC-3 cells have been investigated[11]. Based on these previous reports, it was suggested that components of crocodile bile could inhibit cell proliferation and induce apoptosis and may be a source of potential anti-tumor agent. In this study, we isolated anti-tumor component ESC-3 from crocodile bile, demonstrated the effects of ESC-3 on Mz-ChA-1 cells and elucidated the mechanism by which ESC-3 induces apoptosis.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), bisbenzimide (Hoechst 33258), acridine orange (AO), ethidium bromide (EB), propidium iodide (PI) and proteinase K were purchased from Sigma-Aldrich Co. (St. Louis, MO, United States). RPMI-1640 medium and fetal calf serum were purchased from Gibco (Grand Island, NY, United States). Mouse monoclonal antibodies against human p53, Bax, Bcl-2, cytochrome c, CDK2 and caspase-3 were obtained from Santa Cruz Biotechnology, Inc. (CA, United States). Crocodylus siamensis bile was obtained from the Sriracha Tiger Zoo Thailand Co. Ltd.
The gallbladder containing bile was directly deposited after dried. The whole gallbladder was homogenized and extracted with phosphate buffered saline (PBS) for 4 h at 4 °C in triplicate and then centrifuged at 20 000 ×g for 30 min at 4 °C. The supernatant was pooled and lyophilized by vacuum freeze-drying, and the powder was stored at -20 °C until purification. The powder was redissolved in methanol and loaded onto a Sephadex LH-20 column (Pharmacia, Sweden) with a flow rate of 2 mL/min, and 1 mL per tube was collected. The anti-cancer compound-containing fraction was then loaded onto a RP-18 reversed-phase column (25 cm × 0.3 cm). The solvent system was methanol-0.01 M NaH2PO4 (70:30, v/v), which was adjusted to pH 4.0 by the addition of H3PO4. The solvent was filtered through a 0.22 μm filter (Millipore) prior to use. The detection wavelength was 215 nm.
The QBC939, Sk-ChA-1 and MZ-ChA-1 cell lines were maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in a humidified atmosphere containing 50 mL/L CO2. Twenty-four hours after seeding, the cells were treated with culture medium containing various concentrations of ESC-3.
Cell viability was determined using the MTT assay. Briefly, cells were seeded in a 96-well plate at a density of 1 × 105/mL. After overnight growth, the cells were treated with various concentrations of ESC-3 for 12 h, 24 h, 36 h, 48 h or 72 h. After the ESC-3 treatment, 20 mL MTT (5 mg/mL) was added to each well, and the cells were cultured for another 4 h at 37 °C. The medium was then removed, and 150 mL dimethyl sulfoxide (DMSO) was added to each well. The absorbance of each well was recorded at 490 nm.
Mz-ChA-1 cells from the control group and the group treated with 10 μg/mL ESC-3 for 48 h were seeded onto coverslips and grown for 24 h. After washing with PBS three times, the cells were stained with Giemsa staining solution for 10 min and observed under a light microscope (Olympus BH-2).
Cells treated with 10 μg/mL ESC-3 for 48 h were harvested and fixed with a mixture of glacial acetic acid and methanol (1:3, v/v) for 5 min and then washed twice with PBS. The cells were resuspended in Hoechst 33258 solution (5 μg/mL) and incubated at room temperature for 10 min. After three washes with PBS, the cells were dried thoroughly and observed under a fluorescence microscope. For AO/EB staining, the cells were harvested and washed twice with PBS. The cells were then incubated with 100 μL PBS plus 4 μL AO/EB solution (100 μg/mL AO and 100 μg/mL EB in PBS) for 3 min at room temperature in the dark and immediately observed under a fluorescence microscope.
After treatment with ESC-3 at different concentrations (0, 5, 10 and 15 μg/mL) for 72 h, the Mz-ChA-1 cells were harvested, washed twice with PBS and fixed with 70% ethanol at 4 °C overnight. After centrifugation, the cells were resuspended in 100 μg/mL RNase A at 37 °C for 30 min and subsequently stained with 50 μg/mL propidium iodide at 4 °C for 30 min in the dark. The cells were analyzed by flow cytometry at 488 nm, and the data were analyzed with the CellFit software.
For quantifying cell apoptosis, an annexin v-fluorescein isothiocyanate (V-FITC)/PI double staining assay was performed according to the manufacturer’s instructions. Briefly, the cells were harvested and stained with annexin V-FITC and PI for 20 min at room temperature. The cells were then washed twice with PBS, and the fluorescence of the cells was measured by flow cytometry.
After treatment with different concentrations of ESC-3 (0 μg/mL, 5 μg/mL, 10 μg/mL and 15 μg/mL) for 48 h, the Mz-ChA-1 cells were incubated with Rh123 (1 mg/mL in dimethyl sulfoxide) at 37 °C for 30 min and washed three times with PBS. The cells were harvested and analyzed by flow cytometry at an excitation wavelength of 488 nm and an emission wavelength of 530 nm.
Western blotting analysis was performed as previously described. Briefly, cell lysates were prepared, separated on 12% sodium dodecyl sulfate polyacrylamide gels and transferred onto polyvinylidene fluoride (PVDF) membrane (Amersham Biosciences, Piscataway, NJ). Non-specific reactivity was blocked by incubating the membranes for 1 h in 5% nonfat milk at room temperature. The membranes were incubated with primary antibody overnight at 4 °C. After three washes for 10 min with phosphate buffered saline tween-20 (PBST), the membranes were incubated at 37 °C for 1 h with the appropriate secondary antibody (1:5000 dilution, Sigma) and washed three times with PBST. Reactive proteins were detected with the enhanced chemiluminescence (ECL) detection system (Pierce). β-actin was used as an internal control.
To investigate caspase-3 activation after treatment with ESC-3, a caspase-3 colorimetric assay kit (Kaiji Bio Co., Nanjing, China) was used according to the manufacturer’s instructions. Briefly, cells were harvested and lysed by incubation with cell lysis buffer on ice for 1 h, followed by centrifugation at 10 000 ×g for 1 min. Enzymatic reactions were performed in a 96-well microplate, and 50 μL cell lysate was incubated with the substrate for 4 h at 37 °C. The absorbance was measured at 405 nm. The total protein concentration was determined using the Coomassie brilliant blue method.
All results obtained from ESC-3-treated Mz-ChA-1 cells were analyzed using the SPSS software. Data were expressed as the means ± SD error of the mean (SEM) of separate experiments (n≥ 3). Differences between two treatments were considered significant at P < 0.05.
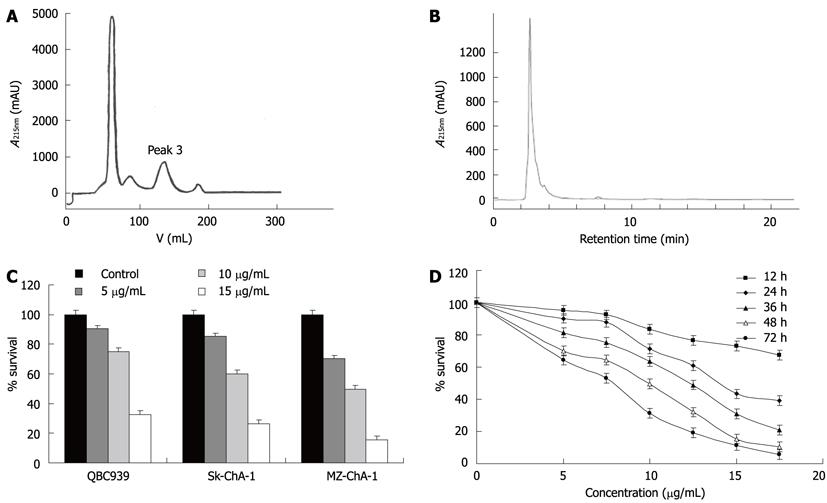
The crude extract was subjected to separation on a Sephade × LH-20 column. As shown in Figure 1A, four peaks were obtained. The effects of each pooled peak on Mz-ChA-1 cells were tested. Peak 3 had the strongest anticancer activity (data not shown). Peak 3 was further analyzed by high performance liquid chromatography (HPLC) with methanol elution. Figure 1B shows that the elution profile had only one main peak, which was pooled and named ESC-3.
To evaluate the cytotoxicity of ESC-3 on the proliferation of human cholangiocarcinoma cells, QBC939, Sk-ChA-1 and MZ-ChA-1 cells were treated with 5 μg/mL, 10 μg/mL and 15 μg/mL ESC-3 for 48 h, as shown in Figure 1C, the cell survivals of the three cholangiocarcinoma cell lines were all sensitive to ESC-3 in a dose-dependent manner and MZ-ChA-1 cells are more sensitive to ESC-3 than are the other human cholangiocarcinoma cell lines.
With ESC-3 concentrations of 5 μg/mL, 7.5 μg/mL, 10 μg/mL, 12.5 μg/mL, 15 μg/mL and 17.5 μg/mL, the proliferation of Mz-ChA-1 cells was inhibited significantly. After treatment with various concentrations of ESC-3 for 72 h, the survival rates were 64.3%, 53.2%, 31.3%, 19%, 11.2% and 5.8%, respectively (Figure 1D).
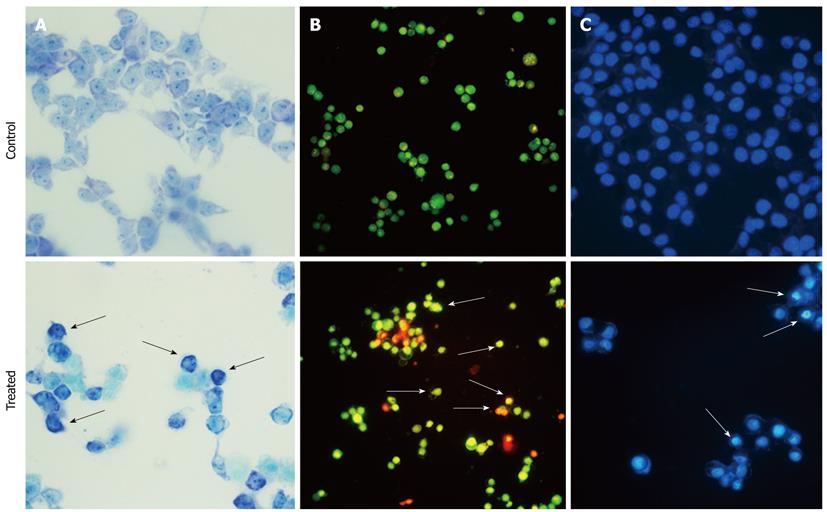
Giemsa staining was used to visualize morphological changes under an optical inverted microscope. After exposure to 10 μg/mL ESC-3 for 48 h, the Mz-ChA-1 cells showed typical apoptotic morphology. When compared with the untreated cells, the chromatin of the ESC-3-treated cells was condensed, and the size of the cells decreased. As shown in Figure 2, after AO/EB staining, the untreated Mz-ChA-1 cells displayed green fluorescence, while the ESC-3-treated cells emitted orange and red fluorescence and were smaller in size. The results of the Hoechst 33258 staining showed that the nuclei of untreated Mz-ChA-1 cells emitted a low fluorescence intensity in a homogeneous manner, and the nuclear structure was intact. Our data indicated that the ESC-3-treated Mz-ChA-1 cells displayed typical morphological features of apoptosis: condensed chromatin, gradual disintegration of the nuclear membrane, and pyknotic (shrunken and dark) nuclei.
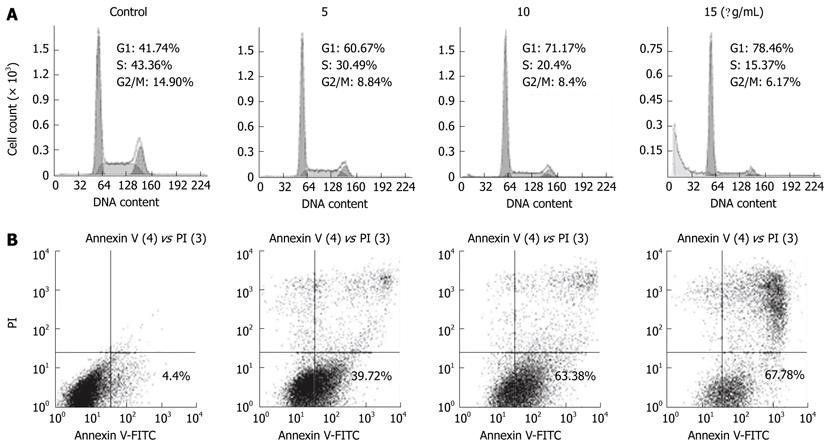
To confirm that ESC-3 inhibited cellular proliferation by affecting the cell cycle distribution of the cells, we performed a cell cycle analysis after treatment with various concentrations of ESC-3 (0 μg/mL, 5 μg/mL, 10 μg/mL and 15 μg/mL). Forty-eight h after ESC-3 treatment, the cell cycle distribution of Mz-ChA-1 cells was altered in a dose-dependent manner. The percentage of cells in G0/G1 phase was 41.74% in the control group and increased to 78.46% after treatment with 15 μg/mL ESC-3, while the percentage of cells in G2/M phase decreased from 14.9% to 6.17% (Figure 3A). These results suggested that ESC-3 arrested Mz-ChA-1 cells at G0/G1 phase and suppressed cellular proliferation.
To further verify the apoptotic effect of ESC-3 on Mz-ChA-1 cells, flow cytometric analysis was conducted using dual staining with annexin V and PI, which was used to distinguish between viable, early apoptotic, late apoptotic or necrotic cells. As shown in Figure 3B, the population of early apoptotic cells increased with treatment with increasing ESC-3 concentrations (4.4%, 39.72%, 63.38% and 67.78% for 0 μg/mL, 5 μg/mL, 10 μg/mL and 15 μg/mL ESC-3, respectively). We, therefore, demonstrated that ESC-3 induced apoptosis.
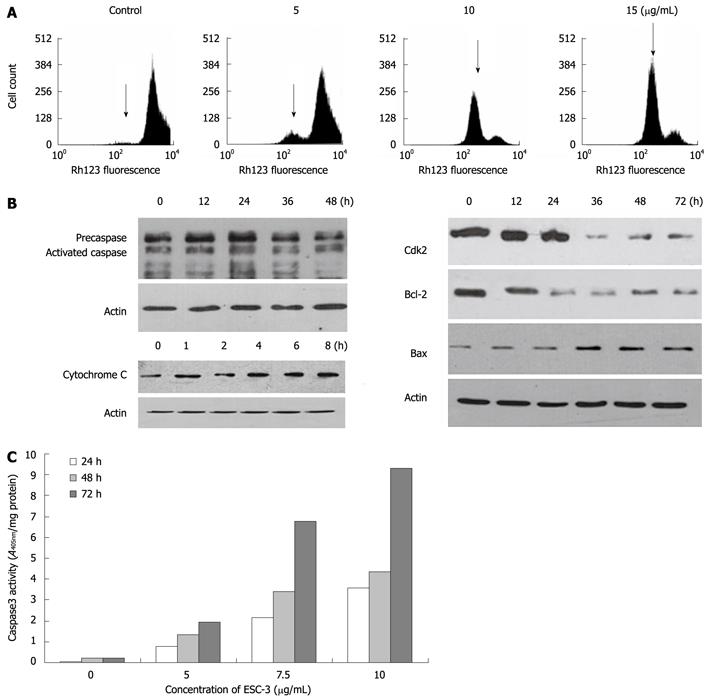
Rhodamine 123 staining was used to measure the change in the mean fluorescence intensity as a measure of the mitochondrial membrane potential (ΔΨm), which drives the uptake and accumulation of Rh123 in the mitochondria. The hypofluorescence peak observed was indicative of a collapse in the ΔΨm and depolarization of the mitochondrial membrane. As shown in Figure 4A, significant changes in the mean fluorescent intensity of the cells and a collapse of the ΔΨm were observed in the Mz-ChA-1 cells 48 h after treatment with ESC-3 in a dose-dependent manner.
To investigate how ESC-3 induced apoptosis in Mz-ChA-1 cells, we examined the expression levels of apoptosis-related proteins, including Bax, Bcl-2, CDK2 and caspase-3, which are generally activated by the caspase-dependent apoptotic signal transduction pathways[12]. As shown in Figure 4B and C, after exposure to ESC-3 for 48 h, the procaspase-3 expression level was lowered, while the activity of caspase-3 increased significantly in dose- and time-dependent manners in Mz-ChA-1 cells. Moreover, the expression of Bax was increased, while the levels of the Bcl-2 and Cdk2 proteins significantly decreased; therefore, the ratio of Bax to Bcl-2 also increased in a time-dependent manner (Figure 4B). Furthermore, we observed that the levels of cytochrome c in the cytosol of ESC-3-treated Mz-ChA-1 cells increased in a dose-dependent manner. This suggests that the mitochondrial release of cytochrome c into the cytosol may play a role in induction of cell apoptosis by ESC-3.
Apoptosis is a process by which cells undergo programmed cell death under certain physiological or pathological conditions[13]. Apoptosis and its related signaling pathways have a profound effect on the progression of cancer[14]; therefore, the induction of apoptosis is a desirable goal for the prevention of cancer[15]. Recently, researchers have focused on screening novel anticancer drugs from organisms to identify compounds that could induce apoptosis.
In the present study, we isolated an anticancer compound, ESC-3, from the components of the Crocodylus siamensis bile. Our results demonstrated that ESC-3 significantly inhibited the proliferation of QBC939, Sk-ChA-1 and MZ-ChA-1 cells in a dose-dependent manner. Cell cycle arrest is one of the typical responses displayed by proliferating eukaryotic cells after exposure to DNA damaging agents, such as UV light and inhibitors[16]. To determine whether ESC-3 arrested the cell cycle in Mz-ChA-1 cells, we examined the cell cycle distribution of ESC-3-treated cells using flow cytometry. Our data indicated that ESC-3 induced a cell cycle arrest at G0/G1 phase.
Changes in cell morphology are the primary indicators of apoptosis[17-19]. There were significant morphological changes in Mz-ChA-1 cells after exposure to ESC-3, including cell shrinkage, chromatin agglutination, marginalization, nuclear fragmentation, and apoptotic body formation. Early apoptotic cells exhibited bright annexin V-FITC fluorescence (annexin V-FITC positive only), and the proportion of apoptotic cells increased in a dose-dependent manner. It is reported that CDCA and DCA presented significant cytotoxic activities in ovarian cancer cells via inducing apoptosis[20], which is consistent with our results, suggesting that ESC-3 is capable of inducing apoptosis in Mz-ChA-1 cells.
The mechanisms of apoptosis mainly involve two signaling pathways: the mitochondrial pathway and the cell death receptor pathway[21]. Upregulation of Bax is often associated with the mitochondrial release of cytochrome c, which is a key element of the mitochondrial pathway[22,23]. Cytochrome c is regulated by the Bcl-2 family and has been shown to initiate the activation of caspase-3[24]. The Bcl-2 family consists of many important regulators of apoptosis, including Bcl-2, which prevents cells from entering apoptosis, and Bax and Bak, which induce cell death[25]. The increase in the ratio of Bax to Bcl-2 usually triggers cell death[22]. In the present study, we found that ESC-3 downregulated Bcl-2 expression while upregulating Bax expression, which resulted in an elevation of the ratio of Bax to Bcl-2 in Mz-ChA-1 cells. Hydrophobic bile acids have been reported to cause oxidative stress, DNA damage, and mitochondrial membrane instability in several cancer cells[26,27]. Mitochondrial dysfunction is correlated with DNA damage and oxidative stress[28]. ESC-3 also induced a loss of the mitochondrial membrane potential and the release of cytochrome c into the cytosol. Depolarization of the mitochondrial membrane was also detected. All these data suggest that DNA damage or oxidative stress may induce mitochondrial damage and dysfunction which finally triggers apoptosis in cancer cells. Thus, our data indicates that ESC-3-induced apoptosis of Mz-ChA-1 cells occurs through the mitochondrial pathway.
All of the typical signs of apoptosis result from a complex biochemical cascade of events[21]. A family of cysteine-dependent aspartate-directed proteases propagates death signaling by cleaving key cellular proteins. Caspase-3 is a well-known key executioner of apoptosis. Our results showed that ESC-3 activated caspase-3 and enhanced the levels of cleaved caspase-3 in a dose-dependent manner.
In summary, ESC-3 is a novel cytotoxic compound that blocked the proliferation of three cholangiocarcinoma cell lines and arrested the Mz-ChA-1 cell cycle at G0/G1 phase. ESC-3 induced apoptosis in Mz-ChA-1 cells in a dose-dependent manner via the mitochondria-dependent pathway. Understanding how ESC-3 regulates cell death would provide insight into the potential anticancer mechanisms and help screen novel active natural compounds for cancer treatment.
Cholangiocarcinoma is the second most common primary hepatic tumor and currently accounts for 3% of all gastrointestinal cancers; its incidence has recently been increasing globally. Progress in the diagnosis and treatment of cholangiocarcinoma has been achieved in recent years, but the prognosis is still unsatisfactory due to the lack of efficient anticancer drugs.
Anti-tumor component ESC-3 isolated from crocodile bile could inhibit cell proliferation and induce apoptosis, and it may be a source of potential anti-tumor agent.
ESC-3 induced apoptosis in Mz-ChA-1 cells in a dose-dependent manner via the mitochondria-dependent pathway. Understanding how ESC-3 regulates cell death would provide insight into potential anticancer mechanisms and help screen novel active natural compounds for cancer therapy.
ESC-3, the active ingredient of crocodile bile may be a potential chemotherapeutic drug for the treatment of cholangiocarcinoma.
This paper reports the effects of some extracts from crocodile’s bile (ESC-3) on cholangiocarcinoma cells. In particular, these authors showed that ESC-3 inhibits cell proliferation, induces apoptosis and cell death. In addition, they provided evidences of Bax/Bcl-2 ratio increase and changes of the mitochondrial membrane potential in these cell lines. It is of interest to identify the natural products with anti-cancer activities in traditional Chinese medicine. Indeed, one component of the bear bile, i.e., the ursodeoxycholic acid, was found to have anti-cholestatic effect and it is now the only accepted therapy for many cholestatic disorders.
Peer reviewer: Pietro Invernizzi, MD, PhD, Division of Internal Medicine and Hepatobiliary Immunopathology Unit, IRCCS Istituto Clinico Humanitas, Via A. Manzoni 113, 20089 Rozzano, Milan, Italy
S- Editor Tian L L- Editor Ma JY E- Editor Zhang DN
| 1. | Taylor-Robinson SD, Toledano MB, Arora S, Keegan TJ, Hargreaves S, Beck A, Khan SA, Elliott P, Thomas HC. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968-1998. Gut. 2001;48:816-820. [PubMed] |
| 2. | Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353-1357. [PubMed] |
| 3. | Nagino M, Kamiya J, Arai T, Nishio H, Ebata T, Nimura Y. One hundred consecutive hepatobiliary resections for biliary hilar malignancy: preoperative blood donation, blood loss, transfusion, and outcome. Surgery. 2005;137:148-155. [PubMed] |
| 4. | Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology. 2005;128:1655-1667. [PubMed] |
| 5. | Gordaliza M. Natural products as leads to anticancer drugs. Clin and Transl Oncol. 2008;9:767-776. [RCA] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 433] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 6. | Zhang HT, Luo H, Wu J, Lan LB, Fan DH, Zhu KD, Chen XY, Wen M, Liu HM. Galangin induces apoptosis of hepatocellular carcinoma cells via the mitochondrial pathway. World J Gastroenterol. 2010;16:3377-3384. [PubMed] |
| 7. | Yeh Y. Bile acid composition in snake bile juice and toxicity of snake bile acids to rats. Comparative Biochemistry and Physiology Part C. Toxic & Pharmac. 2003;136:277-284. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Alpini G, Baiocchi L, Glaser S, Ueno Y, Marzioni M, Francis H, Phinizy JL, Angelico M, Lesage G. Ursodeoxycholate and tauroursodeoxycholate inhibit cholangiocyte growth and secretion of BDL rats through activation of PKC alpha. Hepatology. 2002;35:1041-1052. [PubMed] |
| 9. | Alpini G, Glaser S, Alvaro D, Ueno Y, Marzioni M, Francis H, Baiocchi L, Stati T, Barbaro B, Phinizy JL. Bile acid depletion and repletion regulate cholangiocyte growth and secretion by a phosphatidylinositol 3-kinase-dependent pathway in rats. Gastroenterology. 2002;123:1226-1237. [PubMed] |
| 10. | LaRue JM, Stratagoules ED, Martinez JD. Deoxycholic acid-induced apoptosis is switched to necrosis by bcl-2 and calphostin C. Cancer Lett. 2000;152:107-113. [PubMed] |
| 11. | Choi YH, Im EO, Suh H, Jin Y, Yoo YH, Kim ND. Apoptosis and modulation of cell cycle control by synthetic derivatives of ursodeoxycholic acid and chenodeoxycholic acid in human prostate cancer cells. Cancer Lett. 2003;199:157-167. [PubMed] |
| 12. | Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2026] [Cited by in RCA: 2002] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 13. | Qin F, Song Y, Li Z, Zhao L, Zhang Y, Geng L. S100A8/A9 induces apoptosis and inhibits metastasis of CasKi human cervical cancer cells. Pathol Oncol Res. 2010;16:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485-495. [PubMed] |
| 15. | Reed JC, Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood. 2005;106:408-418. [PubMed] |
| 16. | Kim YH, Shin KJ, Lee TG, Kim E, Lee MS, Ryu SH, Suh PG. G2 arrest and apoptosis by 2-amino-N-quinoline-8-yl-benzenesulfonamide (QBS), a novel cytotoxic compound. Biochem Pharmacol. 2005;69:1333-1341. [PubMed] |
| 19. | Han P, Kang JH, Li HL, Hu SX, Lian HH, Qiu PP, Zhang J, Li WG, Chen QX. Antiproliferation and apoptosis induced by tamoxifen in human bile duct carcinoma QBC939 cells via upregulated p53 expression. Biochem Biophys Res Commun. 2009;385:251-256. [PubMed] |
| 20. | Horowitz NS, Hua J, Powell MA, Gibb RK, Mutch DG, Herzog TJ. Novel cytotoxic agents from an unexpected source: bile acids and ovarian tumor apoptosis. Gynecol Oncol. 2007;107:344-349. [PubMed] |
| 21. | Jegham H, Roy J, Maltais R, Desnoyers S, Poirier D. A novel aminosteroid of the 5?-androstane-3?,17?-diol family induces cell cycle arrest and apoptosis in human promyelocytic leukemia HL-60 cells. Invest New Drugs. 2010;30:176-185. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Karmakar S, Banik NL, Ray SK. Curcumin suppressed anti-apoptotic signals and activated cysteine proteases for apoptosis in human malignant glioblastoma U87MG cells. Neurochem Res. 2007;32:2103-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Huang RH, Chai J, Tarnawski AS. Identification of specific genes and pathways involved in NSAIDs-induced apoptosis of human colon cancer cells. World J Gastroenterol. 2006;12:6446-6452. [PubMed] |
| 24. | Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147-157. [PubMed] |
| 25. | Wang TT, Phang JM. Effects of estrogen on apoptotic pathways in human breast cancer cell line MCF-7. Cancer Res. 1995;55:2487-2489. [PubMed] |
| 26. | Lechner S, Müller-Ladner U, Schlottmann K, Jung B, McClelland M, Rüschoff J, Welsh J, Schölmerich J, Kullmann F. Bile acids mimic oxidative stress induced upregulation of thioredoxin reductase in colon cancer cell lines. Carcinogenesis. 2002;23:1281-1288. [PubMed] |
| 27. | Glinghammar B, Inoue H, Rafter JJ. Deoxycholic acid causes DNA damage in colonic cells with subsequent induction of caspases, COX-2 promoter activity and the transcription factors NF-kB and AP-1. Carcinogenesis. 2002;23:839-845. [PubMed] |
| 28. | Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003;76:397-403. [PubMed] |