Published online Dec 14, 2012. doi: 10.3748/wjg.v18.i46.6801
Revised: August 13, 2012
Accepted: August 25, 2012
Published online: December 14, 2012
AIM: To explore whether patients with a defective ileocecal valve (ICV)/cecal distension reflex have small intestinal bacterial overgrowth.
METHODS: Using a colonoscope, under conscious sedation, the ICV was intubated and the colonoscope was placed within the terminal ileum (TI). A manometry catheter with 4 pressure channels, spaced 1 cm apart, was passed through the biopsy channel of the colonoscope into the TI. The colonoscope was slowly withdrawn from the TI while the manometry catheter was advanced. The catheter was placed across the ICV so that at least one pressure port was within the TI, ICV and the cecum respectively. Pressures were continuously measured during air insufflation into the cecum, under direct endoscopic visualization, in 19 volunteers. Air was insufflated to a maximum of 40 mmHg to prevent barotrauma. All subjects underwent lactulose breath testing one month after the colonoscopy. The results of the breath tests were compared with the results of the pressures within the ICV during air insufflation.
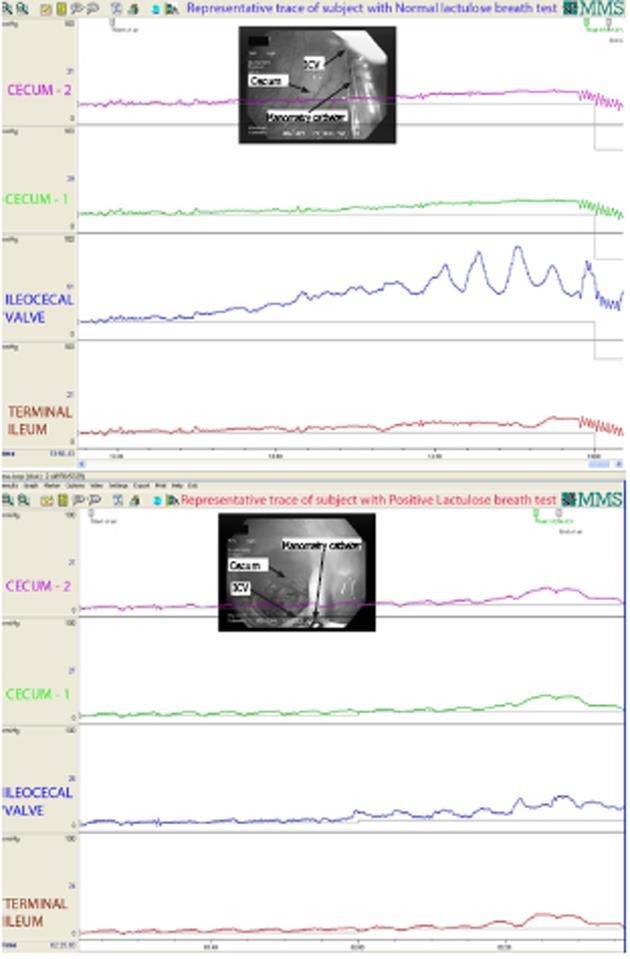
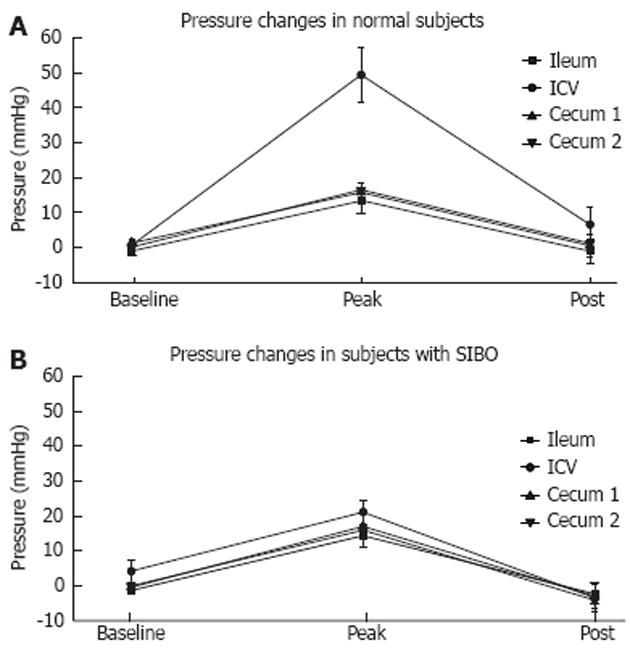
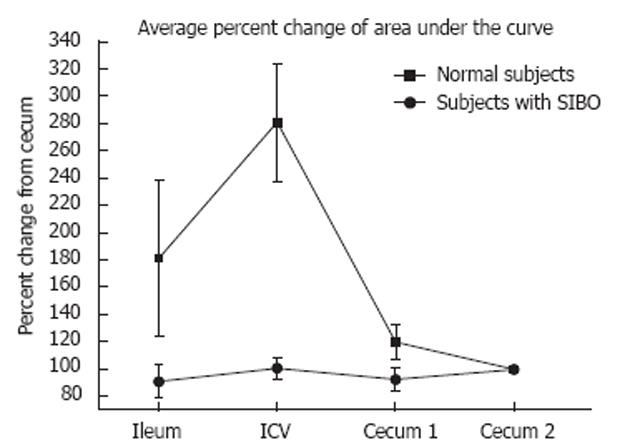
RESULTS: Nineteen subjects underwent colonoscopy with measurements of the ICV pressures after intubation of the ICV with a colonoscope. Initial baseline readings showed no statistical difference in the pressures of the TI and ICV, between subjects with positive lactulose breath tests and normal lactulose breath tests. The average peak ICV pressure during air insufflation into the cecum in subjects with normal lactulose breath tests was significantly higher than cecal pressures during air insufflation (49.33 ± 7.99 mmHg vs 16.40 ± 2.14 mmHg, P = 0.0011). The average percentage difference of the area under the pressure curve of the ICV from the cecum during air insufflations in subjects with normal lactulose breath tests was significantly higher (280.72% ± 43.29% vs 100% ± 0%, P = 0.0006). The average peak ICV pressure during air insufflation into the cecum in subjects with positive lactulose breath tests was not significantly different than cecal pressures during air insufflation 21.23 ± 3.52 mmHg vs 16.10 ± 3.39 mmHg. The average percentage difference of the area under the pressure curve of the ICV from the cecum during air insufflation was not significantly different 101.08% ± 7.96% vs 100% ± 0%. The total symptom score for subjects with normal lactulose breath tests and subjects with positive lactulose breath tests was not statistically different (13.30 ± 4.09 vs 24.14 ± 6.58). The ICV peak pressures during air insufflations were significantly higher in subjects with normal lactulose breath tests than in subjects with positive lactulose breath tests (P = 0.005). The average percent difference of the area under the pressure curve in the ICV from cecum was significantly higher in subjects with normal lactulose breath tests than in subjects with positive lactulose breath tests (P = 0.0012). Individuals with positive lactulose breath tests demonstrated symptom scores which were significantly higher for the following symptoms: not able to finish normal sized meal, feeling excessively full after meals, loss of appetite and bloating.
CONCLUSION: Compared to normal, subjects with a positive lactulose breath test have a defective ICV cecal distension reflex. These subjects also more commonly have higher symptom scores.
- Citation: Miller LS, Vegesna AK, Sampath AM, Prabhu S, Kotapati SK, Makipour K. Ileocecal valve dysfunction in small intestinal bacterial overgrowth: A pilot study. World J Gastroenterol 2012; 18(46): 6801-6808
- URL: https://www.wjgnet.com/1007-9327/full/v18/i46/6801.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i46.6801
The literature regarding the ileocecal valve (ICV) and its relationship to small intestinal bacterial overgrowth (SIBO) is limited[1-3]. Surgical section of the ileocecal ligament in dog, a procedure that suppresses the ileocolonic angle and reduces sphincter competence, increases the amount of cecoileal reflux[4,5]. Surgical removal of the ICV maximizes reflux ultimately leading to bacterial overgrowth[6].
Dinning et al[1] using temporary, side diverting, defunctioning ileostomies, recorded ICV pressures in a configuration close to that surgically produced by Quigley et al[7] in dogs, hence allowing precise positioning of the manometric assembly across the ICV for prolonged periods. In these patients, a sustained pressure of about 10 mmHg is observed either using a pull-through or sleeve recording over a 4.8 cm distance.
In fasted humans, phasic activity of the ICV, unrelated to motor activity of more oral or aboral zones, is observed for 35% of the recording time[1-3]. During these waves that occur at about four to eight waves per minute, the basal tone is doubled. On the contrary, ICV tone is reduced in humans and in dogs while prolonged propagated contractions are observed on the distal ileum[1,8]. In dogs and to a lesser extent in humans, ileal motor events propagate across the ICV into the colon. For instance, Quigley et al[8] show that 50% of ileal discrete clustered contractions and 76% of ileal prolonged propagated contractions continued propagating in the proximal canine colon. Colonic distension is followed consistently by contraction of the ICV in dogs and in humans. This enhanced motility of the ICV comprises simultaneously an increase in tone together with larger amplitude ICV phasic pressure waves[1-3] while the ileal contractions are unaffected[7].
Using a technique in which radioactive tracer is instilled in the cecum it was found that backward flow from the cecum to the ileum is episodic. In dog, the volume of the refluxate is low accounting for about 7% of the total radioactivity injected to the cecum[9]. This minimal reflux rate can be explained by the competence of the canine ICV towards reflux. Surgical section of the ileocecal ligament in dog, a procedure that suppresses the ileocolonic angle and reduces sphincter competence[4,5], increases the amount of cecoileal reflux to 44% of the total radioactivity[9].
In the current study, we aimed to explore whether patients with a positive lactulose breath test (indicative of SIBO) may have an incompetent ICV leading to reflux of colonic contents into the small intestine[10]. To test this hypothesis we measured pressures within the ICV during cecal distension and compared these pressures with the results of lactulose breath tests.
This research study was approved by the Temple University Institutional review board on 06/27/2007. This study is registered with clinicaltrials.gov ID: NCT01413945. Written Informed consent was given by all the participants prior to their inclusion in this study. All subjects filled out a symptom questionnaire on a scale of 0 to 5 with 0 being no symptom and 5 being very severe symptoms.
Subjects undergoing screening colonoscopy were included in the study. Subjects who were currently on medications such as prokinetics, antibiotics and anticholinergics were excluded from the study. Subjects with history of gastroparesis, Crohn’s disease, ulcerative colitis, diseases causing diarrhea, and long standing uncontrolled diabetes were also excluded from the study.
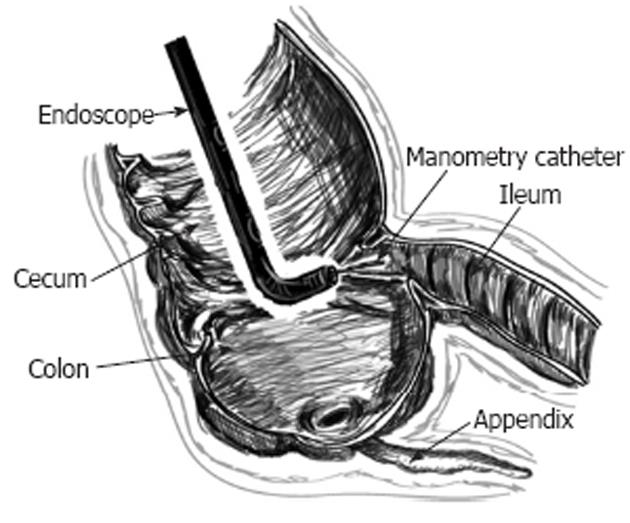
All subjects were sedated with Midazolam and Fentanyl. Colonic preparation was used in all subjects (polyethylene glycol preparation). A custom make water perfused manometry catheter (Mui Scientific, Mississauga, Canada) with 4 pressure measurement ports spaced one cm apart was passed through the biopsy channel of the colonoscope and placed across the ICV with at least one port in the terminal ileum and one port in the cecum (Figure 1). The simultaneous video endoscopy and pressure readings (30 Hz) were continuously recorded on a Medical Measurement Solar System (MMS, Dover, NH) (Figure 2). Video endoscopy was used to make sure that the catheter remained in the correct position during the entire study. If the catheter position changed during the study, the catheter was repositioned according to markings on the catheter. All the air was removed from the cecum by suction through the biopsy channel of the colonoscope and baseline measurements were taken. After the baseline measurements, air was slowly and continuously instilled into the cecum through the air/water channel of the colonoscope with the air insufflation setting, set at low on endoscopy processor (Evis, Exera II, CLV-180, Olympus America Inc, Center Valley, PA). The peak pressures were measured in all the channels at a time when the pressure in ICV reached its peak between the start of air insufflation and the end of air insufflation. Only studies in which the video playback showed the catheter to be in the correct position were used for analysis. A threshold cecal pressure of 40 mmHg was used to avoid barotrauma. If the pressure exceeded 40 mmHg the air flow into the cecum was stopped. In vivo and cadaveric animal experiments have yielded data regarding intraluminal pressure that can lead to rupture of the colon[11-16]. An adult human cadaveric cecum exposed to less than 40 mmHg of intraluminal pressure generally does not rupture, but cecum exposed to more than 150 mmHg of pressure always ruptures[11-16]. The pressures needed for perforation of the right colon and cecum are lower than those needed for perforation of the sigmoid and descending colon[12,13]. It is estimated that the upper limit of safe intraluminal human colonic pressure is 80 mmHg, because perforation can occur at pressures greater than 140 mmHg[17]. The area under the pressure curve was measured within all the channels, from the time of the beginning of the upslope of the pressures within the cecum, after the start of air insufflation, to the time when the ICV reached its peak pressure, before the end of air insufflation.
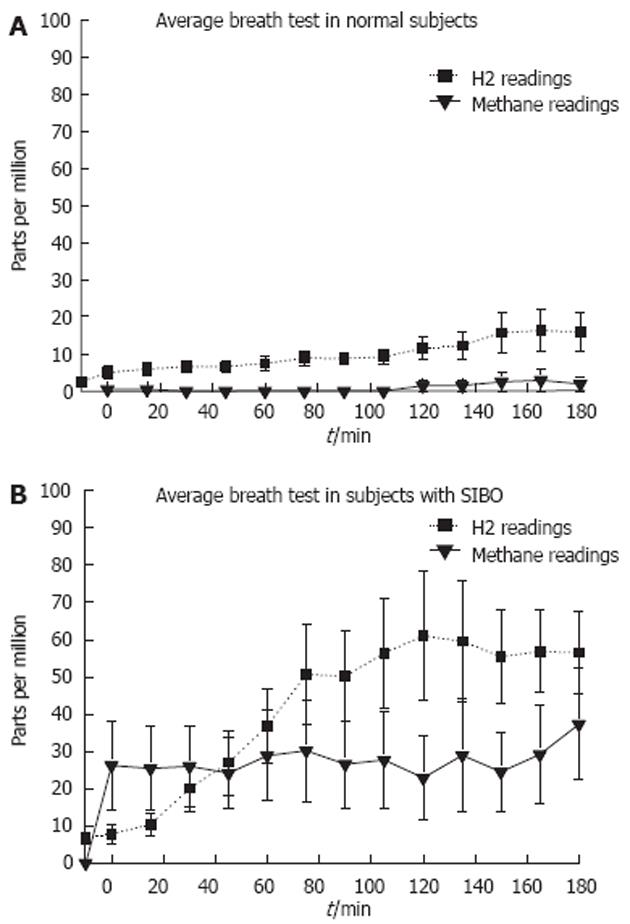
All patients returned one month after the colonoscopy for a lactulose breath test using “Breath Tracker Digital Microlyzer”, QuinTron Instrument Company, Milwaukee, WI, United States. All subjects presented for the lactulose breath test after an overnight fast. The subjects were given 10 g of lactulose in 120 mL of water to drink and instructed to breathe into a collection bag every 15 min for 3 h after a sample of their breath was collected at baseline. All breath samples were end-expiratory and analyzed immediately. The concentrations of breath hydrogen and methane were measured in parts per million (ppm). The measurements were then graphed and analyzed[18-21].
Graphs of methane and hydrogen concentration were plotted against time. A positive lactulose breath test was defined as a double peak of hydrogen or a combination of both hydrogen and methane above 20 ppm within the first 2 h.
All subjects were given a gastrointestinal questionnaire which evaluated the following habitual symptoms: nausea, vomiting, stomach fullness, not able to finish normal sized meal, feeling excessively full after meals, loss of appetite, bloating, stomach or belly visibly larger, upper abdominal pain, upper abdominal discomfort, lower abdominal pain, lower abdominal discomfort, diarrhea, gas from above, and gas from below.
All pressures were calculated relative to baseline colonic pressures. The analysis of pressures was performed using an unpaired Student’s t-test, with a two tail distribution and equal variance. The questionnaires were evaluated using non-parametric tests (Mann Whitney U-test). The analysis was performed in a blinded manner. A Spearman correlation was used to compare ICV pressures to hydrogen and methane excretion.
Nineteen subjects (56.4 years, 7 male and 12 female) underwent colonoscopy with measurements of the ICV pressures after intubation of the ICV with a colonoscope. Cecal pressures before air insufflation were considered as baseline pressures and all the pressures noted were relative to cecal pressures. If the recorded pressures were less than the baseline cecal pressures, than they were considered negative. The resting pressures in the subjects with normal lactulose breath tests were -1.14 ± 1.44 mmHg and 1.18 ± 0.73 mmHg in TI and ICV respectively. The resting pressures in the subjects with positive lactulose breath tests were -1.31 ± 1.07 mmHg and 1.70± 2.49 mmHg in TI and ICV respectively. Initial readings showed no statistical difference in the resting pressures of the TI or ICV between subjects with positive lactulose breath tests and normal lactulose breath tests.
Ten subjects (5 male and 5 female), (age: 55.8 ± 3.05 years, weight: 86.77 ± 7.29 kg). The average dose of Midazolam and Fentanyl per kg was 0.06 mg and 0.73 mg. The average peak ICV pressure during air insufflation into the cecum in subjects with normal lactulose breath tests was significantly higher than cecal pressures during air insufflation (49.33 ±7.99 mmHg vs 16.40 ± 2.14 mmHg, P = 0.0011). The average percentage difference of the area under the pressure curve of the ICV from the cecum during air insufflations was significant higher (280.72% ± 43.29% vs 100% (P = 0.0006). The average symptom score for all subjects with normal lactulose breath tests was 13.30 ± 4.09.
Nine subjects (2 male and 7 female), (age: 57 ± 4.92 years, weight: 84.35 ± 6.17 kg). The average dose of Midazolam and Fentanyl per kg was 0.08 mg and 0.50 mg. The average peak ICV pressure during air insufflation into the cecum in subjects with positive lactulose breath tests was not significantly different than cecal pressures during air insufflation (21.23 ± 3.52 mmHg vs 16.10 ± 3.39 mmHg). The average percentage difference of the area under the pressure curve of the ICV from the cecum during air insufflation was not significantly different 101.08% ± 7.96% vs 100%. The average total symptom score for all subjects with positive lactulose breath tests was 24.14 ± 6.58.
In some prior studies methane seems to influence smooth muscle function[22]. Analyzing the breath test results, 3 of the subjects were methane producers’ and one of these subjects was also positive for hydrogen. Six subjects were hydrogen producers. There was no significant difference in the peak ICV pressures between methane producers and hydrogen producers (23.7 ± 0.67 mmHg vs 26.8 ± 2.48 mmHg). There was only a weak correlation between the pressure measurements and the hydrogen excretion (r = 0.19) and the pressure measurements and the methane excretion (r = 0.25).
There is no significant difference in age and weight between subjects with normal lactulose breath test and subjects with positive lactulose breath test. There is no significant difference in the drug dose/kg of Midazolam and Fentanyl between the two groups.
The ICV peak pressures during air insufflations were significantly higher in the subjects with normal lactulose breath tests than in the subjects with positive lactulose breath tests (P = 0.005) (Figures 3 and 4). The average percent difference of the area under the pressure curve within the ICV from cecum during air insufflations was significantly higher in subjects with normal lactulose breath tests than in subjects with positive lactulose breath tests (P = 0.0012) (Figure 5). The cecal peak pressures during air insufflations were not significantly different in the subjects with normal lactulose breath tests compared to the subjects with positive lactulose breath tests. The total symptom scores were not significantly different in subjects with normal lactulose breath tests than in subjects with positive lactulose breath tests. However, significantly higher symptom scores were observed in the subjects with positive lactulose breath tests in the individual symptoms of: “not able to finish normal sized meal” (P = 0.036), “feeling excessively full after meals” (P = 0.029), “loss of appetite” (P = 0.018) and “bloating” (P = 0.0536).
In this study we explored weather patients with positive lactulose breath tests have an incompetent ICV. We tested this hypothesis by measuring ICV pressures during cecal distension in subjects with positive and negative lactulose breath tests.
In designing this study consideration was given to using a sleeve sensor to measure ICV pressures. However, this was rejected in favor of a four port pressure transducer because a sleeve sensor would have measured the peak pressure recorded and would not have been able to distinguish between the terminal ileum, the ICV or the cecum. Consideration was also given to using a barostat to distend the cecum. However, it was decided that a barostat would be very cumbersome to use during routine colonoscopy and would not distend the cecum in the correct position to reproducibly increase ICV pressure. We felt that insufflation of air, into the cecum, was closer to the actual physiology of gas being produced in the colon causing distension of the cecum. We did not use the same volume of gas in each subject. Rather we used a cutoff cecal pressure. We did this for safety purposes since we did not want to go above a certain threshold pressure and risk barotrauma to the subjects. A cutoff pressure of 40 mmHg was used during cecal distension. Finally consideration was given to collection of fluid from the small intestine to assay for SIBO. However, the colonoscopies were performed after a bowel preparation, which flushes bacteria out of the small intestine and this would have resulted in false negative results. In addition the colonoscope needs to pass through the colon before it can enter the small intestine and this could have contaminated the colonoscope with bacteria and could have resulted in false positive results for SIBO.
Yu et al[23] have recently published an important study in which they combined oro-cecal scintigraphy and lactulose hydrogen breath testing demonstrating that breath testing may detect oro-cecal transit, not small intestinal bacterial overgrowth in patients with irritable bowel syndrome (IBS). Thus the lactulose hydrogen breath test may measure small intestinal transit rather than SIBO in IBS-patients. However, the patient population in that study was different than in the current study and the lactulose breath test is still used around the world to diagnose SIBO. Since the results of the lactulose breath test are dependent on motility within the stomach, the small intestine and the colon, we attempted to control for variability in motility between subjects by eliminating patients with known motility disorders, such as gastroparesis and small bowel motility disorders. We eliminated subjects who were taking any medications that would affect motility, such as prokinetics, anticholinergics and narcotics. The dosages of medications given during the colonoscopies were not significantly different between the two groups, so as not to influence motility during the colonoscopy.
We found that the baseline pressure measurements showed no difference in the pressures of the TI, and ICV between the two groups. These findings are similar to the findings of Quigley et al[24], “because ileocecal muscle behaves as a sphincter, we were surprised by the absence of clear tonic pressures across the ICV”. However, when tested for the ICV cecal distension reflex using air insufflation into the cecum, we recorded an increase in the pressure within the ICV in subjects with normal lactulose breath tests which were significantly higher than in subjects with positive lactulose breath test. Subjects, with a positive lactulose breath test commonly had a common cavity effect (equalization of the pressures across all of the compartments) during cecal air insufflation.
When the lactulose breath test is evaluated against the gold standard of jejunal aspiration with bacterial culturing for the diagnosis of SIBO the sensitivity and specificity of the breath test is much lower. We suggest a number of potential explanations for these results. First, perhaps not all SIBO is due to an incompetent ICV. For example, in cases of scleroderma in which there are diverticulum in the small intestine it has been suggested that SIBO is due to bacteria growing within the diverticulum. Second and probably more important is the fact that these cultures were taken from the jejunum which is a great distance from the ICV and TI. Reflux of colonic contents would not be expected to wash backwards beyond the ileum due to the propulsive motion of peristalsis within the small intestine, which is presumably normal in these subjects. Normal peristalsis should keep the jejunal bacterial count relatively low even when refluxing colonic contents into the ileum[14]. Although we did not design this study to determine if subjects undergoing screening colonoscopy with a positive lactulose breath test have gastrointestinal (GI) symptoms, we did find that many of the subjects in our study had GI symptoms. While these symptoms were more common in the group with positive lactulose breath tests, they were not uncommon in the group with negative lactulose breath tests and we found that there was no significant difference in the overall symptom scores between the two groups. On the other hand, significantly higher symptom scores were demonstrated in the subjects with positive lactulose breath tests for the individual symptoms of, “not able to finish a normal sized meal”, “feeling excessively full after meals”, “loss of appetite” and “bloating”.
The Initial studies evaluating the treatment of IBS patients with poorly absorbed antibiotics, were based on the hypothesis that a significant proportion of these patients actually had occult SIBO[25]. A number of studies have suggested that there are beneficial effects when poorly absorbed antibiotics are used to treat patients with IBS[26,27]. Initial studies reported the presence of SIBO in up to 80% of IBS patients, on the basis of a rapid rise in breath hydrogen during lactulose breath testing. In a recent issue of the New England Journal of Medicine, Pimentel et al[28] reported the results of two large, double-blind, placebo-controlled trials of rifaximin, in patients with IBS without constipation. They found that patients in the group treated with rifaximin had adequate relief of IBS symptoms or bloating. Similarly significant results were obtained in an analysis of relief of symptoms during the 10-wk period after the end of the double-blind treatment phase. Harder et al[29] showed that gas is less well tolerated in the small intestine than in the large bowel. Based on these studies the most likely mode of action of rifaximin is a reduction in overall small bowel bacterial load or a decrease in colonic flora. This may lead to decreased bacterial fermentation and less bloating, possibly in combination with decreased secretion of bacterial products or host responses to bacterial products that contribute to the generation of symptoms. In the future, we plan to perform a study on subjects with documented irritable bowel syndrome, by the Rome III criteria. In order to determine if a subgroup of patients with IBS symptoms is due to a defective ileocecal valve/cecal distension reflex with reflux of fecal flora or gas into the small intestine.
The results of our study suggest that some GI symptoms may be due to a defective ICV cecal distension reflex which either allows gas from the colon to distend the small intestine or lead to reflux of colonic contents into the small intestine, the colonization of the small intestine with fecal flora and the development of SIBO.
We hypothesize that even though a course of non-absorbable antibiotics may clear the small intestine or the colon of colonic flora, the symptomatic effect may not be durable because the underlying defect, the defective ICV cecal distension reflex, is still present and colonic flora or colonic gas may eventually reflux back into the small intestine. The results of the current study suggest that a restoration of the demonstrated dysfunction (pharmacological, endoscopic or surgical) by preventing coloileal reflux may have a more durable effect than antibiotic therapy. Indeed, prevention of coloileal reflux by constructing an artificial ileocolonic valve was suggested by Kellogg in 1913. Bakkevold[30] demonstrated that the nipple valve anastomosis may prevent recurrence of Crohn’s disease, after ileocolic resection.
Pimentel et al[28] suggested that some IBS like symptoms, afflicting many millions of patients, might be due to SIBO and might respond to antibiotic treatment usually used to treat SIBO. In summary, the current study demonstrates that subjects with a positive lactulose breath test have a defective ICV cecal distension reflex. In addition it demonstrates that subjects with a defective ICV cecal distension reflex more commonly have certain GI symptoms. We propose that this pathophysiologic mechanism (defective ICV/cecal distension reflex) which may cause SIBO and IBS like symptoms due to reflux of fecal flora and the production of gas within the small intestine and/or the production of gas within the colon, with reflux of the gas into the small intestine.
We would like to thank Dilek Yarar for her contribution for this project.
Small intestinal bacterial overgrowth (SIBO) refers to a condition in which abnormally large numbers of bacteria are present in the small intestine and the types of bacteria in the small intestine resemble the bacteria of the colon. Irritable bowel syndrome (IBS) is a disorder that leads to abdominal pain and cramping, changes in bowel movements, and other symptoms. SIBO and some forms of IBS may be due to the distension of the small intestine with gas produced by bacteria within the small intestine and/or colon. Recent studies strongly suggest that some IBS like symptoms, afflicting many millions of patients, might be due to small intestinal bacterial overgrowth SIBO.
A percentage of patients with IBS like symptoms have relief of their symptoms when they are treated with non-absorbable antibiotics which suppress the intestinal microbiota. It is thought that suppression of the microflora, within the colon or the small intestine is the mechanism of this therapy.
The initial studies evaluating the treatment of IBS patients with poorly absorbed antibiotics, were based on the hypothesis that a significant proportion of these patients actually had occult SIBO. A number of studies have suggested that there are beneficial effects when poorly absorbed antibiotics are used to treat patients with IBS. Initial studies reported the presence of SIBO in up to 80% of IBS patients, on the basis of a rapid rise in breath hydrogen during lactulose breath testing. The current study is the first study to evaluate the role of ileocecal valve (ICV)/cecal distention reflex in patients suffering with small bowel bacterial overgrowth.
These findings imply that repair of the ICV (pharmacologic, endoscopic or surgical) or restoration of the ICV/cecal distension reflex may be used to treat patients with SIBO and in patients with IBS like symptoms in the future.
The ileocecal valve is a sphincter muscle situated at the junction of the ileum (last portion of your small intestine) and the colon (first portion of your large intestine). Its function is to allow digested food materials to pass from the small intestine into your large intestine. The ileocecal valve also blocks these waste materials from backing back up into your small intestine. It is intended to be a one-way valve, only opening up to allow processed foods to pass through.
The authors examined the ICV/cecal distention reflex in patients with positive lactulose breath test and in subjects with negative lactulose breath test. The authors found that subjects with a positive lactulose breath test have a defective ICV/cecal distension reflex. This defective ICV/cecal distension reflex may cause SIBO and IBS like symptoms due to reflux of fecal flora and the production of gas within the small intestine and/or the production of gas within the colon, with reflux of the gas into the small intestine.
Peer reviewer: Jørgen Valeur, MD, PhD, Department of Medicine, Institute of Medicine, Haukeland University Hospital, University of Bergen, NO-5021 Bergen, Norway
S- Editor Gou SX L- Editor Kerr C E- Editor Lu YJ
| 1. | Dinning PG, Bampton PA, Kennedy ML, Kajimoto T, Lubowski DZ, de Carle DJ, Cook IJ. Basal pressure patterns and reflexive motor responses in the human ileocolonic junction. Am J Physiol. 1999;276:G331-G340. [PubMed] |
| 2. | Kajimoto T, Dinning PG, Gibb DB, de Carle DJ, Cook IJ. Neurogenic pathways mediating ascending and descending reflexes at the porcine ileocolonic junction. Neurogastroenterol Motil. 2000;12:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Rothstein RD, DeRiso J, Ouyang A. Ileocecal sphincter contraction to colonic distension: a tachykinin-mediated spinal reflex. Am J Physiol. 1990;258:G585-G590. [PubMed] |
| 4. | Köhler LW, Heddle R, Miedema BW, Phillips SF, Kelly KA. Response of canine ileocolonic sphincter to intraluminal acetic acid and colonic distension. Dig Dis Sci. 1991;36:1594-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Kumar D, Phillips SF. The contribution of external ligamentous attachments to function of the ileocecal junction. Dis Colon Rectum. 1987;30:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Gazet RJ, Jarrett J. The ileocaeco-colic sphincter. studies in vitro in man, monkey, cat, and dog. Br J Surg. 1964;51:368-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Quigley EM, Phillips SF, Cranley B, Taylor BM, Dent J. Tone of canine ileocolonic junction: topography and response to phasic contractions. Am J Physiol. 1985;249:G350-G357. [PubMed] |
| 8. | Quigley EM, Phillips SF, Dent J. Distinctive patterns of interdigestive motility at the canine ileocolonic junction. Gastroenterology. 1984;87:836-844. [PubMed] |
| 9. | Kumar D, Phillips SF, Brown ML. Reflux from ileum to colon in the dog. Role of external ligamentous attachments. Dig Dis Sci. 1988;33:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | King CE, Toskes PP. Small intestine bacterial overgrowth. Gastroenterology. 1979;76:1035-1055. [PubMed] |
| 11. | Burt AV. Pneumatic rupture of the intestinal canal: With experimental data showing the mechanism of perforation and the pressure required. Archives of Surgery. 1931;22:875-902. [RCA] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 127] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Harned RK, Consigny PM, Cooper NB, Williams SM, Woltjen AJ. Barium enema examination following biopsy of the rectum or colon. Radiology. 1982;145:11-16. [PubMed] |
| 13. | Kozarek RA, Earnest DL, Silverstein ME, Smith RG. Air-pressure-induced colon injury during diagnostic colonoscopy. Gastroenterology. 1980;78:7-14. [PubMed] |
| 14. | Luchette FA, Doerr RJ, Kelly K, Kulaylat M, Stephan RM, Hassett JM. Colonoscopic impaction in left colon strictures resulting in right colon pneumatic perforation. Surg Endosc. 1992;6:273-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Noveroske RJ. Intracolonic pressures during barium enema examination. Am J Roentgenol Radium Ther Nucl Med. 1964;91:852-863. [PubMed] |
| 16. | Sandhu KS, Cohen H. Cecal perforation following fiberoptic flexible sigmoidoscopy. Am J Med. 1987;82:546-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Tzelepis GE, Nasiff L, McCool FD, Hammond J. Transmission of pressure within the abdomen. J Appl Physiol. 1996;81:1111-1114. [PubMed] |
| 18. | Parodi A, Capurso G, Perri F, Cuoco L, Lauritano EC. H2-breath testing for small-intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2009;29:18-22. |
| 19. | Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, Vorisek V, Kopacova M. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16:2978-2990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 383] [Cited by in RCA: 364] [Article Influence: 24.3] [Reference Citation Analysis (4)] |
| 20. | Kerlin P, Wong L. Breath hydrogen testing in bacterial overgrowth of the small intestine. Gastroenterology. 1988;95:982-988. [PubMed] |
| 21. | Riordan SM, McIver CJ, Walker BM, Duncombe VM, Bolin TD, Thomas MC. The lactulose breath hydrogen test and small intestinal bacterial overgrowth. Am J Gastroenterol. 1996;91:1795-1803. [PubMed] |
| 22. | Pimentel M, Lin HC, Enayati P, van den Burg B, Lee HR, Chen JH, Park S, Kong Y, Conklin J. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1089-G1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 23. | Yu D, Cheeseman F, Vanner S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut. 2011;60:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 24. | Quigley EM, Borody TJ, Phillips SF, Wienbeck M, Tucker RL, Haddad A. Motility of the terminal ileum and ileocecal sphincter in healthy humans. Gastroenterology. 1984;87:857-866. [PubMed] |
| 25. | Kerckhoffs AP, Visser MR, Samsom M, van der Rest ME, de Vogel J, Harmsen W, Akkermans LM. Critical evaluation of diagnosing bacterial overgrowth in the proximal small intestine. J Clin Gastroenterol. 2008;42:1095-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Koo HL, DuPont HL. Rifaximin: a unique gastrointestinal-selective antibiotic for enteric diseases. Curr Opin Gastroenterol. 2010;26:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Singh VV, Toskes PP. Small bowel bacterial overgrowth: presentation, diagnosis, and treatment. Curr Gastroenterol Rep. 2003;5:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 691] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 29. | Harder H, Serra J, Azpiroz F, Passos MC, Aguadé S, Malagelada JR. Intestinal gas distribution determines abdominal symptoms. Gut. 2003;52:1708-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Bakkevold KE. Nipple valve anastomosis for preventing recurrence of Crohn disease in the neoterminal ileum after ileocolic resection. A prospective pilot study. Scand J Gastroenterol. 2000;35:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |