Published online Dec 7, 2012. doi: 10.3748/wjg.v18.i45.6682
Revised: October 26, 2012
Accepted: October 30, 2012
Published online: December 7, 2012
Neuroendocrine carcinoma (NEC) of the pancreas is rare. We report the case of a 34-year-old man with pancreatic NEC with soft tissue metastasis. The patient presented with right upper abdominal discomfort. Computed tomography revealed a low-density heterogeneous mass in the tail and body of the pancreas that encroached on the greater curvature of the stomach and spleen. We performed exploratory laparotomy and total pancreatectomy with splenectomy and total gastrectomy. Histopathological analysis showed spindle-shaped cells with scanty cytoplasm and hyperchromatic nuclei, confirming a primary pancreatic NEC. One month after the surgery, the patient experienced leg swelling. Positron emission tomography-computed tomography revealed high uptake of fludeoxyglucose in the left leg, and the leg was amputated. Histopathological analysis confirmed metastasis of pancreatic NEC. The patient was followed up and received chemotherapy (etoposide and cisplatin). One month after amputation, the level of tumor marker neuron-specific enolase was 142.70 μg/L and computed tomography scan revealed an aggravated metastatic lesion. The patient suffered from unbearable pain and we treated him with odynolysis. Four months postoperatively, the patient died of respiratory failure.
- Citation: Chen J, Zheng Q, Yang Z, Huang XY, Yuan Z, Tang J. Neuroendocrine carcinoma of the pancreas with soft tissue metastasis. World J Gastroenterol 2012; 18(45): 6682-6685
- URL: https://www.wjgnet.com/1007-9327/full/v18/i45/6682.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i45.6682
Primary neuroendocrine carcinoma (NEC) of the pancreas is very rare, accounting for only 1%-1.4% of all pancreatic cancers[1,2]. Almost all NECs of the pancreas are discovered when the tumor is fairly large (mean: 6.2 cm, range: 2.5-20 cm) and it has metastasized to several distant organs such as the liver, adrenal gland, and brain, which explains the dismal prognosis[3]. We report a rare route of metastasis in this case.
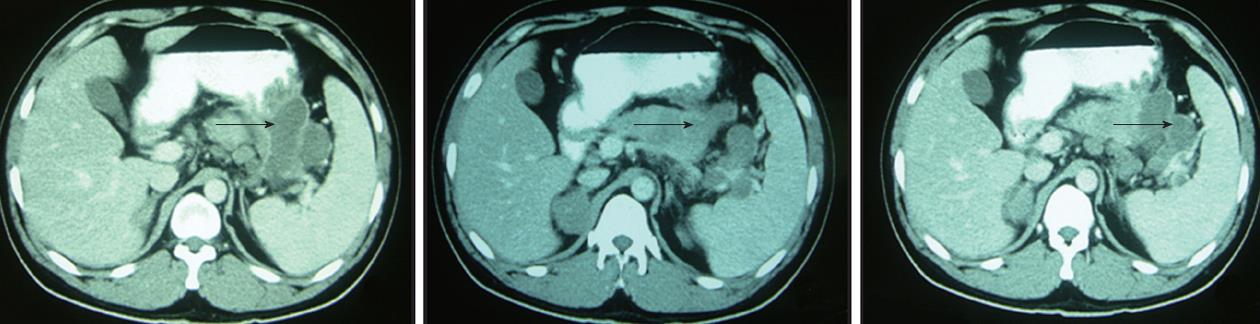
We report a case of pancreatic NEC with soft tissue metastasis. The patient was a 34-year-old man who had no significant past medical history. He visited our hospital on January 1, 2012 with the symptom of right upper abdominal discomfort. A computed tomography (CT) scan revealed a low-density heterogeneous mass of 81 mm × 68 mm in size in the tail and body of the pancreas that invaded the greater curvature of the stomach and the spleen (Figure 1). The laboratory findings were as follows: hemoglobin, 102 g/L; white blood cell count, 6.5 × 109/L; platelets, 374 × 109/L; aspartate aminotransferase, 18 U/L; alanine aminotransferase, 13 U/L; total bilirubin, 9.1 μmol/L; direct bilirubin, 4.0 μmol/L; serum creatinine, 59 μmol/L; carcinoembryonic antigen, 0.86 ng/mL (normal, < 10.0 ng/mL); alpha-fetoprotein, 2.37 ng/mL (normal, < 13.40 ng/mL); and carbohydrate antigen 19-9, 101.7 U/mL (normal, < 27 U/mL). The serum neuron-specific enolase (NSE) level was 59.94 μg/L (normal, < 17 μg/L). Chest X-ray examination revealed no signs of primary lung cancer or metastasis. In addition, there was no evidence of liver metastasis; therefore, exploratory laparotomy was performed. During the abdominal exploration, a 1-cm mass was detected in the head of the pancreas, and an 8-cm mass was detected in the pancreatic tail. The identified mass invaded the greater curvature of the stomach and the spleen. Consequently, we performed total pancreatectomy with splenectomy and total gastrectomy.
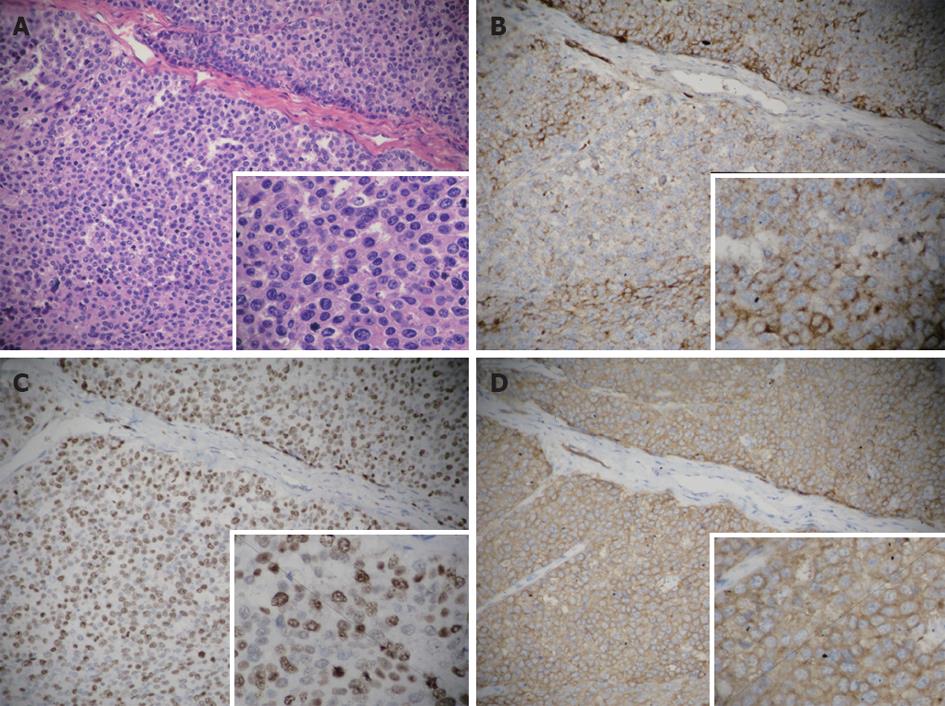
Histological examination revealed spindle-shaped cells with scanty cytoplasm and hyperchromatic nuclei. In addition, 9/12 lymph nodes were positive for metastasis. Hematoxylin and eosin staining (Figure 2) was performed on the paraffin-embedded sections. Immunohistochemical examination revealed chromogranin A and Ki-67 positivity.
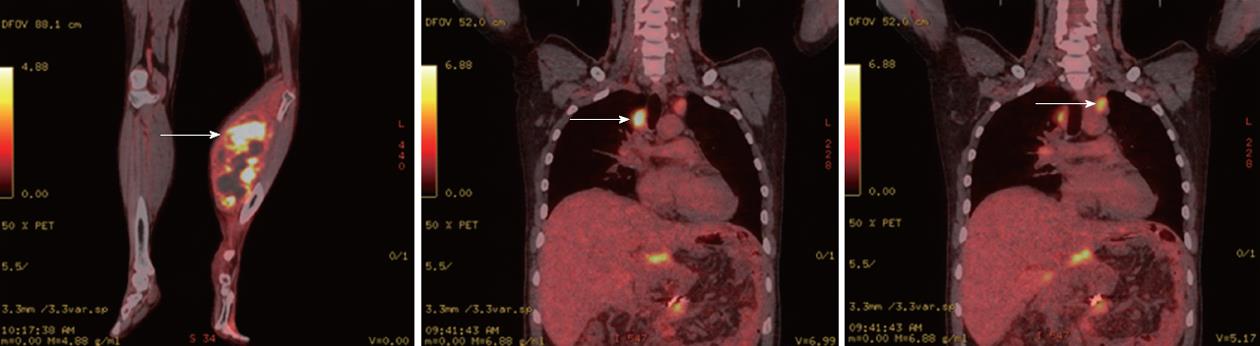
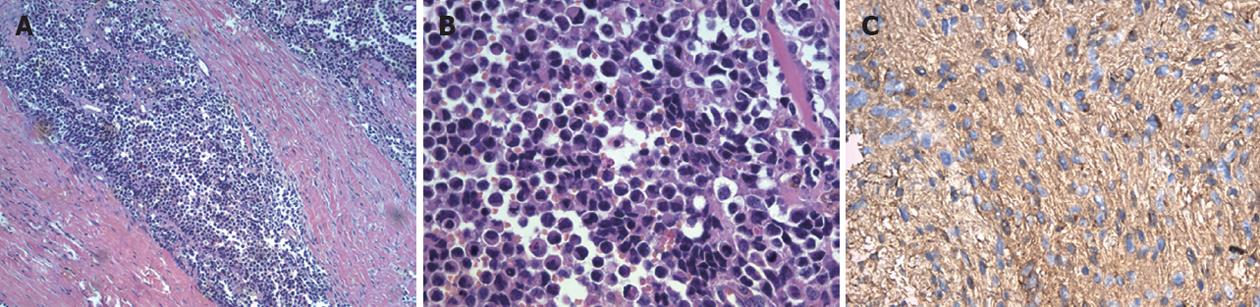
One month after surgery, the patient exhibited leg swelling. Positron emission tomography-CT revealed high fludeoxyglucose uptake in the left leg and the relapse of carcinoma in both hila of the lungs (Figure 3). An orthopedist obtained a biopsy of the left leg, and the frozen section results indicated NEC. Therefore, the left leg of the patient was amputated below the knee. The postoperative pathology of the left leg was metastatic NEC of the pancreas (Figure 4). The patient was followed up, and he received chemotherapy consisting of etoposide and cisplatin.
One month after amputation, the level of the tumor marker NSE was 142.70 μg/L, and a CT scan revealed an aggravated metastatic lesion. The patient reported unbearable pain, and he was treated by odynolysis. Four months postoperatively, he died of respiratory failure.
In a review of all published cases of pancreatic NEC, 91% of patients had metastases at the time of the initial diagnosis. According to the report by Vos et al[4], the most common sites of metastasis are the peripancreatic lymph nodes (62%), liver (38%), lungs (14%), bone marrow (14%), bone (10%), colon (10%), and adrenal gland (10%); rarer sites of metastasis include the spleen, gallbladder, kidneys, skin, and brain.
NSE can be considered a tumor marker that can be used in the diagnosis or assessment of treatment efficacy in patients with pancreatic NEC[5,6]. In our case, NSE was continuously aggravated.
Pancreatic NEC is a rare type of pancreatic cancer that has a poor prognosis[7]. The clinical course is typically aggressive, often characterized by disseminated disease at presentation and poor survival. In patients with extensive disease, a median survival as short as 2 mo has been reported, whereas in patients with limited disease, a median survival of up to 34 mo has been described[8]. Min Sung Chung reported a case of primary pancreatic NEC with unusually long-term survival after multimodal therapy. This patient remains in good health 36 mo after surgery[9]. In our case, the tumor extended beyond the pancreas with regional lymph node involvement. Because there was extension beyond the locoregional boundaries (extensive disease), we could perform palliative surgery. The patient in our case survived 4 mo on combined chemotherapy.
Some trials have revealed improved oncologic outcomes when patients are treated with regimens similar to those used for small cell cancers of the lungs[10]. The regimen of cisplatin, etoposide, and radiation is generally favored for pancreatic NEC[11]. This is the first reported case of pancreatic NEC with soft tissue metastasis. As mentioned previously, pancreatic NEC is a rare type of pancreatic cancer that has a poor prognosis. These adjuvant approaches should be considered in addition to surgery.
Peer reviewer: Keiji Hanada, MD, PhD, Chief, Center for Gastroendoscopy, Onomichi General Hospital, Clinical Professor, Hiroshima University, School of Medicine, 7-19, Kohama, Onomichi 722-8508, Japan
S- Editor Gou SX L- Editor Logan S E- Editor Li JY
| 1. | Reyes CV, Wang T. Undifferentiated small cell carcinoma of the pancreas: a report of five cases. Cancer. 1981;47:2500-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Cubilla AL, Fitzgerald PJ. Classification of pancreatic cancer (nonendocrine). Mayo Clin Proc. 1979;54:449-458. [PubMed] |
| 3. | Matsubayashi H, Fujiwara S, Kobayashi Y, Iiri T, Mitra A, Goggins M, Hruban RH, Moriyasu F. A small cell carcinoma of the pancreas with a high level of serum ProGRP. J Clin Gastroenterol. 2004;38:834-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Vos B, Awada A, Hendlisz A. Primary small-cell carcinoma of the pancreas: An extensive review of the literature with emphasis on therapy and prognosis. Cancer Therapy. 2008;6:857-864. |
| 5. | Nakamura Y, Tajiri T, Uchida E, Arima Y, Aimoto T, Katsuno A, Naito Z. Changes to levels of serum neuron-specific enolase in a patient with small cell carcinoma of the pancreas. J Hepatobiliary Pancreat Surg. 2005;12:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | O'Connor TP, Wade TP, Sunwoo YC, Reimers HJ, Palmer DC, Silverberg AB, Johnson FE. Small cell undifferentiated carcinoma of the pancreas. Report of a patient with tumor marker studies. Cancer. 1992;70:1514-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Wang D, Rong Y, Wu W, Jin D. Primary small cell carcinoma of the pancreas: rare type of pancreatic cancer and review of the literatures. World J Surg Oncol. 2012;10:32. [PubMed] |
| 8. | Haider K, Shahid RK, Finch D, Sami A, Ahmad I, Yadav S, Alvi R, Popkin D, Ahmed S. Extrapulmonary small cell cancer: a Canadian province's experience. Cancer. 2006;107:2262-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Chung MS, Ha TK, Lee KG, Paik SS. A case of long survival in poorly differentiated small cell carcinoma of the pancreas. World J Gastroenterol. 2008;14:4964-4967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Hoskins PJ, Swenerton KD, Pike JA, Lim P, Aquino-Parsons C, Wong F, Lee N. Small-cell carcinoma of the cervix: fourteen years of experience at a single institution using a combined-modality regimen of involved-field irradiation and platinum-based combination chemotherapy. J Clin Oncol. 2003;21:3495-3501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Winter JM, Narang AK, Mansfield AS, Herman JM, Cameron JL, Laheru D, Eckhauser FE, Olson MT, Hruban RH, Miller RC. Resectable pancreatic small cell carcinoma. Rare Tumors. 2011;3:e5. [PubMed] |