Published online Dec 7, 2012. doi: 10.3748/wjg.v18.i45.6587
Revised: July 30, 2012
Accepted: August 14, 2012
Published online: December 7, 2012
AIM: To assess the rate and risk factors for tumour seeding in a large cohort of patients.
METHODS: Over an 8-year period, 1436 hepatocellular carcinoma (HCC) patients with 2423 tumour nodules underwent 3015 image-guided percutaneous cryoablation sessions [1215 guided by ultrasonography and 221 by spiral computed tomography (CT)]. Follow-up CT or magnetic resonance imaging was performed every 3 mo. The detailed clinical data were recorded to analyse the risk factors for seeding.
RESULTS: The median follow-up time was 18 (range 1-90) mo. Seeding was detected in 11 patients (0.76%) at 1-24 (median 6.0) mo after cryoablation. Seeding occurred along the needle tract in 10 patients and at a distant location in 1 patient. Seeded tumours usually showed similar imaging and histopathological features to the primary HCCs. Univariate analyses identified subcapsular tumour location and direct subcapsular needle insertion as risk factors for seeding. Multivariate analysis showed that only direct subcapsular needle insertion was an independent risk factor for seeding (P = 0.017; odds ratio 2.57; 95%CI: 1.47-3.65). Seeding after cryoablation occurred earlier in patients with poorly differentiated HCC than those with well or moderately differentiated HCC [1.33 ± 0.577 mo vs 11.12 ± 6.896 mo; P = 0.042; 95%CI: (-19.115)-(-0.468)].
CONCLUSION: The risk of seeding after cryoablation for HCC is small. Direct puncture of subcapsular tumours should be avoided to minimise seeding.
- Citation: Wang CP, Wang H, Qu JH, Lu YY, Bai WL, Dong Z, Gao XD, Rong GH, Zeng Z, Yang YP. Tumour seeding after percutaneous cryoablation for hepatocellular carcinoma. World J Gastroenterol 2012; 18(45): 6587-6596
- URL: https://www.wjgnet.com/1007-9327/full/v18/i45/6587.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i45.6587
In patients with hepatocellular carcinoma (HCC) superimposed on cirrhosis, orthotopic liver transplantation, surgical resection and percutaneous ablation are considered radical treatments as they provide better survival rates compared with no treatment[1]. Because of the poor acceptance of surgery and a severe shortage of donor organs, image-guided percutaneous ablation therapies play an important role in the management of HCC. Various local ablation therapies such as percutaneous ethanol injection (PEI), radiofrequency ablation (RFA), microwave (MW) ablation and cryoablation have been developed for the treatment of unresectable HCC. All these procedures require the insertion of long, sharp needles into the liver parenchyma and tumours, which may cause various complications, although the complication rates are low. Tumour seeding is one of the most serious complications, especially in patients who are waiting for liver transplantation[2]. The reported incidence of seeding after other ablation procedures varies widely: 0.2%-1.4% following PEI[3,4], 0.005%-12.5% following RFA[5,6] and 0.75% following MW ablation[7].
Certain factors have been found to increase the likelihood of needle-tract seeding, including a superficial or subcapsular tumour[8], high number of needle insertions[9,10], large needle bore[9,11], end-cutting needle[8,9], absent or thin layer of normal liver parenchyma surrounding the needle tract[9,10], high-grade HCC (moderately or poorly differentiated[3,8,9], high serum alpha-fetoprotein (AFP) level[2], tumour volumes > 2 cm3 and immunosuppression[12].
Argon-helium cryoablation is a new local ablation modality. At one time, this technology caused some authors to question its use in HCC. Most of the bias against this percutaneous setting is based on a theoretical risk of post-procedure haemorrhage. However, the gradual downsizing of cryoprobes has fueled interest in percutaneous use, which offers several potential advantages versus the heat-based ablation modalities[13]. First, multiple cryoprobes can be used simultaneously to generate a large zone of ablation. Second, the size and shape of the developing ice ball can be readily visualized using intra-procedural computed tomography (CT), magnetic resonance imaging (MRI) or ultrasound (US). Third, in contrast to heat-based ablation, percutaneous cryoablation is a relatively painless procedure. Recently, many studies have reported that imaging-guided percutaneous cryoablation is safe and effective for the treatment of HCC[14-16]. In our previous study, the 1-, 2- and 3-year survival rates in patients with HCC < 5 cm in diameter who were treated with cryoablation were 92%, 82% and 64%, respectively, and the rate of serious complications was low[15]. To our knowledge, tumour seeding after percutaneous cryoablation for HCC has not been described to date. The present study was conducted to evaluate the incidence and possible risk factors for seeding after percutaneous cryoablation, by reviewing the prospective database of HCC patients treated by cryoablation in our department.
This study included 1436 consecutive patients with HCC who were treated at the Center of Therapeutic Research for Hepatocellular Carcinoma, 302 Hospital of PLA, between April 2003 and June 2011. HCC was diagnosed based on typical findings on MRI or CT (hyperattenuation in the arterial phase and hypoattenuation in the portal-venous phase) and serum AFP level. The diagnosis was confirmed by histopathological examination of US- or CT-guided biopsy specimens in 736 patients. Until 2007, we biopsied almost all tumours before treatment, and after 2007 we only biopsied cases in which we could not make a definite diagnosis using dynamic CT or MRI. Biopsy specimens for histological examination were obtained with 1-2 passes of a 19.5-gauge end-cutting needle (AutoVac; Angiomed, Karlsruhe, Germany). Histopathological grading of tumour differentiation was performed using the criteria described by Edmondson et al[17]. Tumour stage was defined according to the Barcelona Clinic Liver Cancer (BCLC) classification[18]. Performance status (PS) was defined according to the Eastern Cooperative Oncology Group criteria (ECOG). The 1436 patients had a total of 2423 tumours with a diameter of 1.2-15.0 cm (mean 4.5 ± 2.3 cm). The clinical characteristics of the patients are shown in Table 1. All cryoablation treatments were approved by the Research Ethics Committee at 302 Hospital of PLA. Written informed consent was obtained from all patients who met the inclusion criteria, before blood and tumour specimens were obtained, and before data were collected and analysed.
| Baseline characteristics | Value |
| Age (yr)1 | 55.9 ± 9.2 |
| Sex | |
| Male | 1176 (81.9) |
| Female | 260 (18.1) |
| Aetiology | |
| HBs-Ag positive only | 1229 (85.6) |
| HCV-Ab positive only | 168 (11.7) |
| Both positive | 19 (1.3) |
| Both negative | 20 (1.4) |
| Child-Pugh score | |
| Class A | 874 (60.9) |
| Class B | 562 (39.1) |
| Tumour size (cm) | |
| ≤ 3 | 411 (28.6) |
| 3-5 | 656 (45.7) |
| ≥ 5 | 369 (25.7) |
| Tumour number | |
| Single | 1213 (84.5) |
| 2-3 | 223 (15.5) |
| Tumour location | |
| Subcapsular | 484 (33.7) |
| No subcapsular | 952 (66.3) |
| Route of needle insertion | |
| Direct subcapsular insertion | 213 (14.8) |
| Deep | 1223 (85.2) |
| BCLC staging | |
| Stage A | 787 (54.8) |
| Stage B | 453 (31.5) |
| Stage C | 196 (13.7) |
| Completed ablation | |
| Yes | 1168 (81.3) |
| No | 268 (18.7) |
| Tumour differentiation2 | |
| Well or moderate | 490 (66.6) |
| Poorly | 246 (33.4) |
| Biopsy performed prior to cryoablation2 | 736 (51.3) |
| Number of sessions | |
| Single | 336 (23.4) |
| 2 | 743 (51.7) |
| > 2 | 357 (24.9) |
| AFP (ng/mL)1 | 575 ± 2039 |
Inclusion criteria for cryoablation were as follows: contraindications to surgical resection or orthotopic liver transplantation, Child-Pugh class A or B liver function, total serum bilirubin level < 51.3 μmol/L, platelet count ≥ 20 × 109/L and performance status ≤ 2. Ascites was controlled before the procedure with diuretics. Patients with early HCC who were reluctant to undergo hepatic resection or transplantation were included. Patients were excluded for the following reasons: BCLC stage D (ECOG PS > 2, Child-Pugh C), tumour thrombosis at the main branch of the portal vein and the size of the thrombosis exceeded 50% of the diameter of the portal vein, extrahepatic metastasis, tumours which were not accessible percutaneously, or a history of other ablation therapies. We generally performed percutaneous cryoablation in patients with up to three lesions, all of which were ≤ 5 cm in diameter, but we performed a combination of repeated cryoablation and transarterial chemoembolisation (TACE) in some patients who did not meet these criteria.
We defined a session as a single treatment consisting of one or more ablations performed on one or more tumours. To assess tumour depth, we categorised tumours as subcapsular or deep. Tumours were defined as subcapsular when they were located adjacent to the surface of the liver, less than 0.5 cm of parenchyma between the tumour and the liver capsule, otherwise, they were defined as deep. Direct subcapsular needle insertion was defined as puncture of subcapsular tumours without traversing a sufficient portion of normal hepatic parenchyma. The number of needle insertions was defined as the total number of needle positions in all sessions.
Argon-helium cryoablation was performed as described in our previous report[16]. Briefly, an argon-helium gas-based CRYOcare system (EndoCare, Irvine, CA, United States) and cryoprobes were used to freeze the tumour with a dual freeze-thaw cycle under US or CT guidance. After determining the most favourable percutaneous approach, we inserted the 3-mm cryoprobe into the tumour through the sheath introducer system under US or CT guidance, and advanced the tip to the distal margin of the targeted lesion. The number of probes used depended on the location and size of the lesions to be ablated. The dual freeze-thaw cycle comprised a 20-min freeze, a 10-min thaw and a further 15-min freeze. The dimensions of the frozen tissue were monitored by US or CT. The cryoprobe temperatures were reduced to -135 °C ± 2 °C within 1 min. After removal of the probes, all tracts were packed with Surgicel (Johnson and Johnson, Inc., Arlington, TX, United States) through the sheath introducer to control bleeding, and the sheath introducer was removed. We aimed to perform curative ablation of all tumours in each session by single or multiple cryoablation, particularly for tumours < 5 cm in diameter. Dynamic CT or MRI was performed 2-3 d after treatment to evaluate treatment efficacy. Complete ablation was defined as non-enhancement of the entire lesion on CT or MRI with a safety margin in the surrounding liver parenchyma. Patients underwent additional ablation sessions until complete ablation was confirmed in all nodules, to a maximum of three sessions. If ablation was incomplete after three sessions, we performed TACE. The cryoablation procedure was performed under conscious sedation. Echocardiography, ventilation and oxygen saturation levels were monitored throughout the procedure. Patients were kept warm during cryoablation with warming mats.
All patients underwent MRI and CT at 1 mo after cryoablation. Patients were then assessed every 2-3 mo, including measurements of liver biochemistry and AFP level, and by CT or MRI. A newly detected tumour attached to the peritoneum or pleura was considered to be seeded, and the diagnosis was confirmed by biopsy and histopathological examination. Seeded tumours were treated with repeat cryoablation, PEI or TACE when feasible. The seeding rate was calculated based on the number of patients.
Potential risk factors for seeding were analysed. The following variables were recorded: age, sex, viral markers, tumour size, number of tumour nodules, tumour location, direct subcapsular needle insertion, tumour differentiation, number of cryoablation sessions, number of needle insertions, percutaneous biopsy prior to cryoablation and serum AFP level. Continuous variables were compared between patients with and without seeding using the Student’s t test. The χ2 test or Fisher’s exact test was used to compare categorical variables between the groups. Variables with P < 0.1 were entered into a multivariate logistic regression model using stepwise selection of variables. Variables with P < 0.05 were considered statistically significant. All analysis were conducted using SPSS software version 13 (SPSS Inc., Chicago, IL, United States).
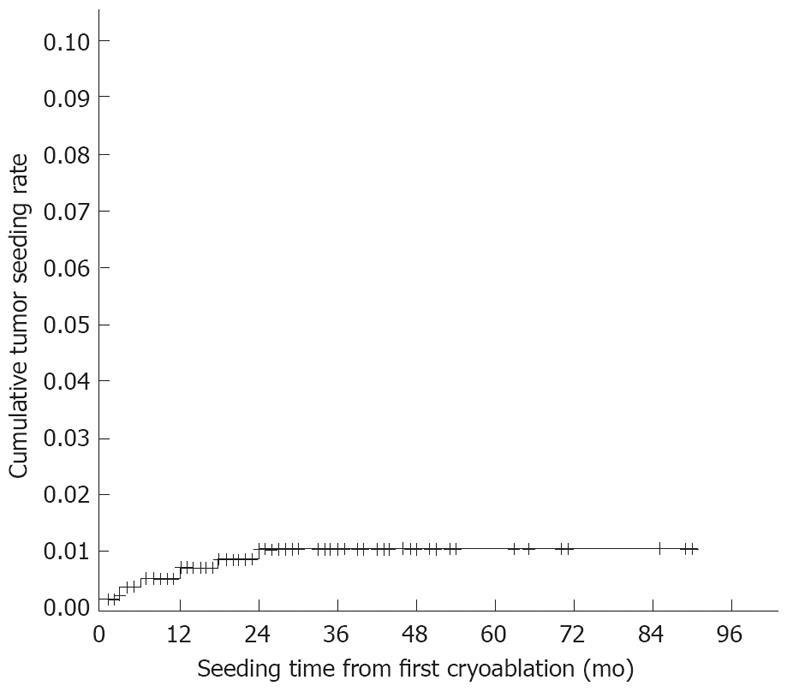
A total of 1436 HCC patients underwent 3015 cryoablation sessions (1215 guided by ultrasonography and 221 guided by CT; average 2.1 sessions per patient) for 2423 nodules. When a patient underwent more than one treatment session, the data from the initial session were used. During the follow-up period (median 18 mo; range 1-90 mo), seeding was diagnosed in 11 patients at an interval of 1-24 (median 6.0) mo after the first cryoablation. The seeding rate was 0.76% per patient (11/1436). The longest interval between the first cryoablation session and detection of seeding was 2 years. The cumulative seeding rates were analysed using Kaplan-Meier estimates, and were 0.49% at 1 year and 1.0% at 2 years (Figure 1). Table 2 shows the baseline characteristics of the 11 patients with seeding. Eight of these patients were male, and the mean patient age was 56.5 ± 9.0 years. Ten patients were hepatitis B surface antigen positive and one patient was hepatitis C virus antibody positive. Eight patients were Child-Pugh class A and three were Child-Pugh class B. The mean tumour size was 4.5 ± 1.9 cm, and the mean number of tumours was 1.3 ± 0.6. The mean number of needle insertions was 3.3 ± 1.7. Direct subcapsular needle insertions were performed in eight patients with subcapsular tumours. Six patients underwent biopsy prior to cryoablation, of which three had poorly differentiated HCC. Four patients were classified as BCLC stage A, six as stage B, and one as stage C. The tumours were completely ablated in eight patients. The mean serum AFP level was 1182.9 ± 2668.6 ng/mL.
| Case No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| Age (yr) | 66 | 51 | 47 | 51 | 65 | 43 | 49 | 61 | 58 | 58 | 72 |
| Sex | M | F | M | M | F | M | M | M | M | F | M |
| Child-Pugh class | A | A | A | A | B | A | A | B | A | A | B |
| BCLC Stage | A | B | B | B | B | C | A | B | A | A | B |
| AFP (ng/mL) | 7 | 9 | 3550 | 368 | 8589 | 16 | 75 | 33 | 23 | 48 | 294 |
| No. of tumours | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 2 | 1 |
| Tumour size (cm) | 6 | 3 | 5.4 | 6 | 3.2 | 8 | 2.4 | 4.8 | 2.6 | 2 | 5.6 |
| No. of sessions | 2 | 1 | 4 | 3 | 1 | 3 | 1 | 2 | 1 | 4 | 2 |
| No. of needle insertions | 4 | 2 | 6 | 4 | 2 | 6 | 1 | 3 | 1 | 4 | 3 |
| Completed ablation | Yes | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Biopsy | No | Yes | Yes | Yes | Yes | No | No | No | No | Yes | Yes |
| Tumour differentiation | |||||||||||
| Primary HCC | - | Poor | Mod | Mod | Poor | - | - | - | - | Well | Poor |
| Seeding HCC | Mod | Poor | Mod | Mod | - | Mod | Mod | Mod | Mod | Well | - |
| Subcapsular location | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No |
| Direct subcapsular insertion | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No |
| Seeding location | Ip | Ip | Sf | Ip | Pleura | Ip and Im | Ip | Ip | Ip | Ip | Pc |
| Seeding time (mo) | 24 | 2 | 5 | 5 | 1 | 6 | 12 | 7 | 12 | 18 | 1 |
| Overall survival (mo) | 36 | 25 | 9 | 19 | 18 | 12 | 18 | 26 | 36 | 60 | 5 |
| Prognosis | Died | Alive | Died | Died | Died | Alive | Alive | Died | Alive | Alive | Alive |
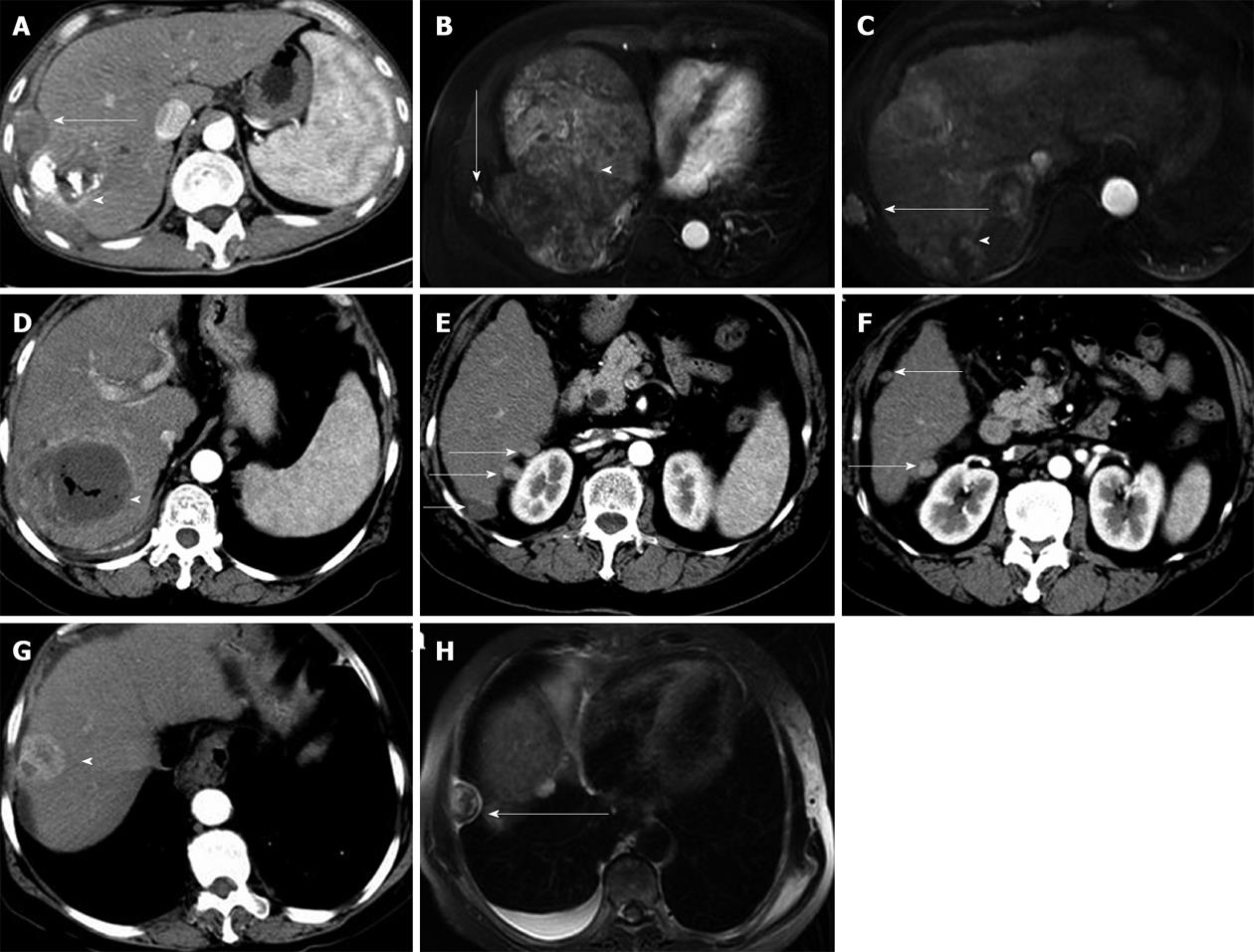
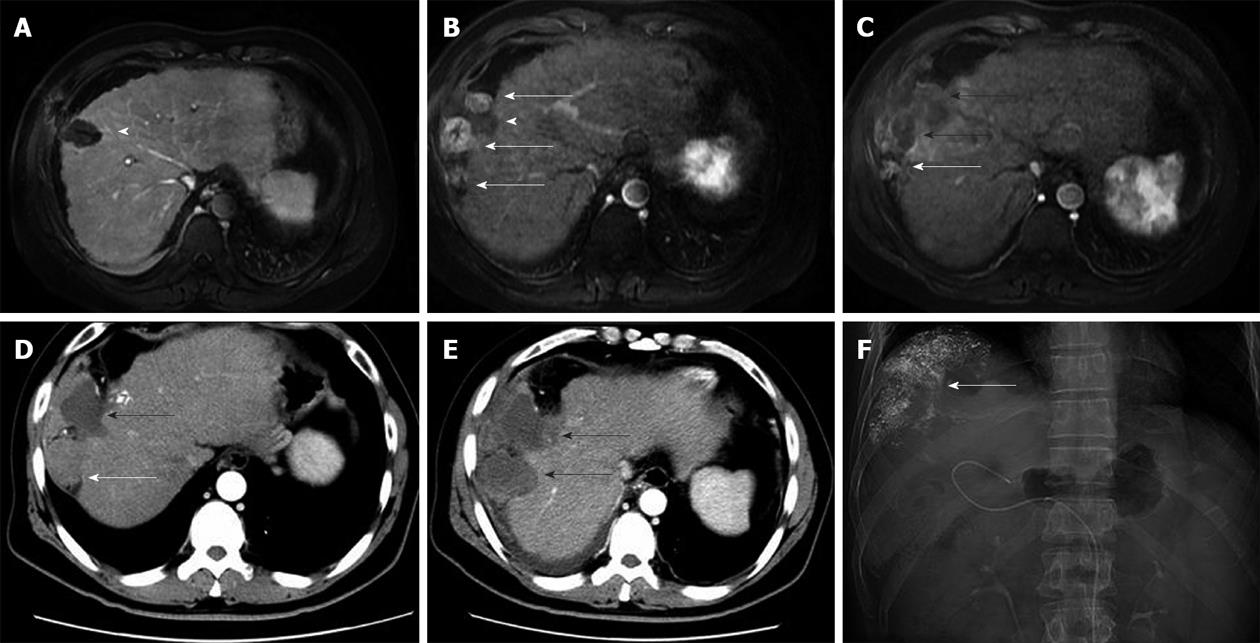
Seeding occurred along the cryoablation needle tract in 10 patients, and at a distant location in 1 patient (Figure 2). The seeding was intraperitoneal in seven patients, intraperitoneal and in the intercostal muscles in one patient, pleural in one patient, and in the abdominal wall (subcutaneous fat) in one patient. One patient had distant seeding in the peritoneal cavity. In ten patients, the seeded tumours were < 3 cm in diameter, and in one patient the tumour was 3 cm in diameter. Nine (81.8%) patients had a single seeded tumour, and the other two (18.2%) patients had two and three seeded tumours, respectively, indicating that multiple seeding was not uncommon. One patient developed treatment-related liver haemorrhage 5 mo before the seeding was detected.
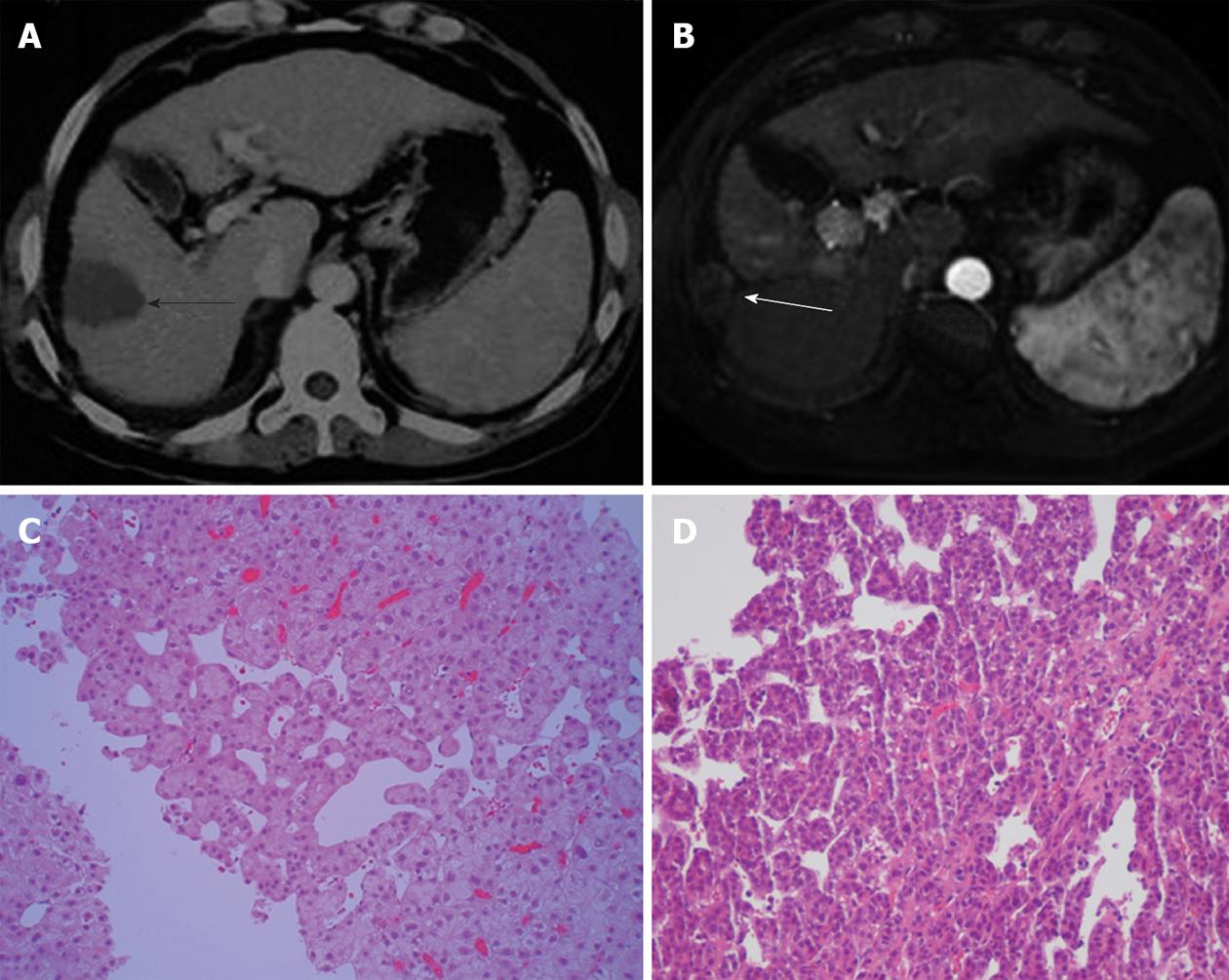
CT and MRI are the preferred imaging modalities for detecting needle-tract seeding. The seeded tumours are usually detected as one or a few round or oval-shaped enhancing nodules along the needle tract, with a few occurring at a distant location. Seeded tumours showed a similar imaging pattern to primary HCCs, with arterial phase hyperattenuation followed by portal-venous phase hypoattenuation (Figure 3).
Tumour biopsies performed before cryoablation in six patients who developed seeding showed that one patient had a well differentiated tumour, two patients had moderately differentiated tumours, and three patients had poorly differentiated tumours. The seeded tumours in the nine patients without distant or pleural seeding were confirmed by biopsy and histopathological examination. The seeded tumour showed similar differentiation features to the primary HCC in four of these patients (Figure 4).
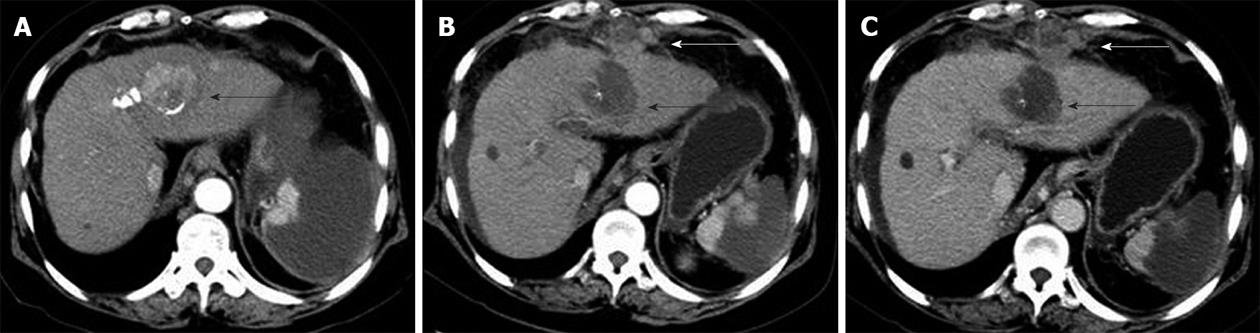
Seeding was treated by PEI in four patients, resection in one patient, cryoablation in two patients, cryoablation plus sorafenib in two patients, and conservative treatment in two patients. Of the nine patients with seeding who were treated, recurrence of seeding after treatment occurred in five (55.6%), including three treated with PEI and two treated with cryoablation plus sorafenib. Three of the patients with recurrent seeding were treated with RFA plus radiation and did not have further recurrence, and the other two patients were treated with sorafenib plus cryoablation and TACE (Figure 5).
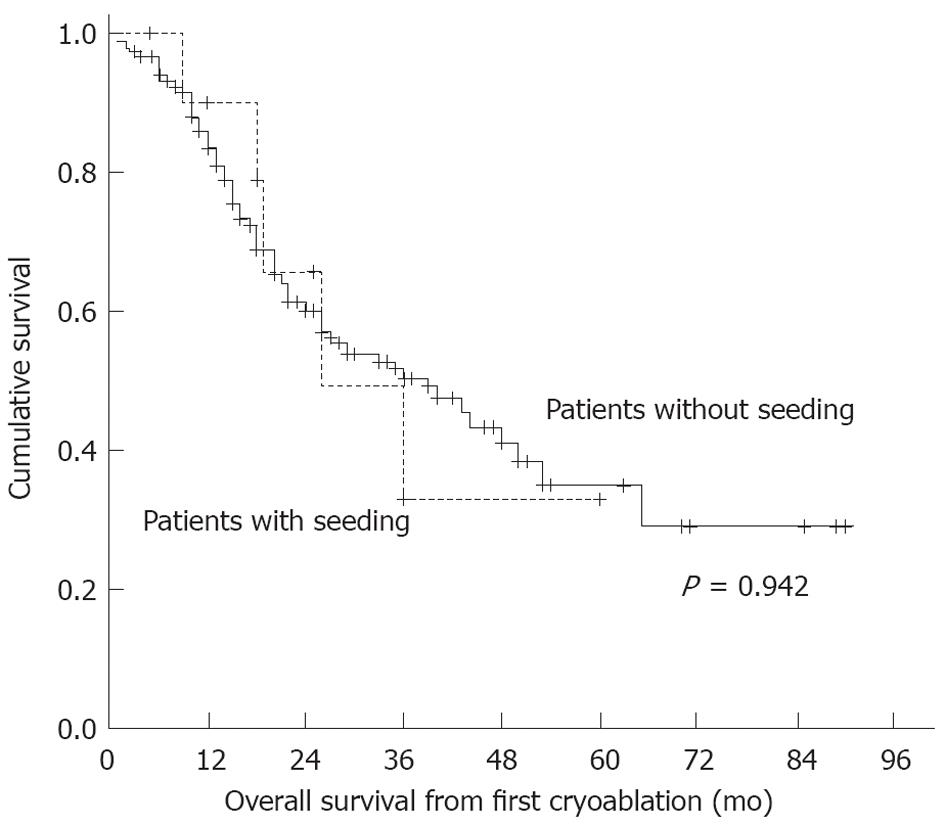
At the end of the follow-up period, five patients with seeding had died. The causes of death were intrahepatic HCC progression and liver failure. No patient died of their seeded tumour nodules. In patients with seeding, the cumulative survival rates were 90% at 1 year, 68% at 2 years, 53% at 3 years, 32% at 4 years and 32% at 5 years. In patients without seeding, the cumulative survival rates were 86% at 1 year, 61% at 2 years, 51% at 3 years, 43% at 4 years and 34% at 5 years. There were no significant differences in survival rates between the two patient groups (P = 0.942) (Figure 6).
Table 3 shows the results of the univariate analysis to identify risk factors for seeding. Direct subcapsular needle insertion (P = 0.0043) and subcapsular tumour location (P = 0.0152) were associated with seeding. There were no significant associations between seeding and age, sex, viral markers, Child-Pugh class, tumour size, number of nodules, number of sessions, number of needle insertions, tumour differentiation, biopsy prior to cryoablation, BCLC stage, incomplete ablation, or serum AFP level. Even though there was no significant association between seeding and tumour differentiation, seeding was detected earlier in patients with poorly differentiated HCC than in those with well or moderately differentiated HCC [1.33 ± 0.577 mo vs 11.12 ± 6.896 mo; 95%CI: (-19.115)-(-0.468); P = 0.042)]. Multivariate analysis showed that the only significant risk factor for seeding was direct subcapsular needle insertion (odds ratio 2.57; 95%CI: 1.47-7.65; P = 0.017).
| Variable | Seeding was identified(n = 11) | Seeding was not identified (n = 1425) | P value |
| Age (yr)1 | 56.5 ± 9.0 | 55.5 ± 9.3 | 0.8970 |
| Gender (male/female) | 8/3 | 1168/257 | 0.6895 |
| HBs-Ag positive only | 10 | 1219 | 0.9412 |
| HCV-Ab positive only | 1 | 167 | 1.0000 |
| Both positive | 0 | 19 | 1.0000 |
| Both negative | 0 | 20 | 1.0000 |
| Child-Pugh class (A/B) | 8/3 | 866/559 | 0.6176 |
| Tumour size (cm)1 | 4.5 ± 1.9 | 4.6 ± 3.0 | 0.8800 |
| Number of tumours1 | 1.3 ± 0.6 | 1.3 ± 0.7 | 0.9520 |
| Number of sessions1 | 2.2 ± 1.2 | 2.3 ± 1.1 | 0.8764 |
| Number of needle insertions1 | 3.3 ± 1.7 | 3.8 ± 1.1 | 0.3430 |
| Direct subcapsular insertion | 8 | 205 | 0.0043 |
| Subcapsular location | 8 | 476 | 0.0152 |
| Biopsy performed2 | 6 | 730 | 0.8264 |
| Poorly differentiated tumour | 3 | 243 | 0.6674 |
| BCLC stage (A/B/C) | 4/6/1 | 783/447/195 | 0.2869 |
| Completed ablation (yes/no) | 8/3 | 1161/264 | 0.7235 |
| AFP (ng/mL)1 | 1182.9 ± 2668.6 | 577.0 ± 2038.1 | 0.3270 |
The new modality of imaging-guided percutaneous argon-helium cryoablation has been widely developed in China. Many studies have reported the safety and efficacy of this technique in the treatment of HCC[14-16]. Although many complications have been reported, the majority are minor and can be treated conservatively. In carefully selected patients, the rate of serious complications is low[16]. Because of its minimal invasiveness and resulting large ablation zone, percutaneous cryoablation is a useful treatment modality for HCC[19].
However, occasional tumour seeding after percutaneous cryoablation for HCC remains unavoidable, as in other local ablation treatments such as PEI, RFA and MW ablation. In this study, we systematically searched for evidence of seeding using state-of-the-art imaging over a long follow-up period, and found seeding in 11 of 1436 patients treated with 3015 cryoablation sessions. The seeding rate was 0.76% per patient. The median interval between the first cryoablation session and detection of seeding was 6 (range 1-24) mo, with a median follow-up time of 18 (range 1-90) mo. At the end of the follow-up period, 580 patients had died, and these patients had a median survival time of 15 (range 1 to 65) mo. All patients were under close observation, and no patients were lost to follow-up. Because of the duration and quality of follow-up, the likelihood of having missed a seeded tumour in this study is minimal.
Similar to other percutaneous interventions such as biopsy, PEI and RFA, the sites of seeding after cryoablation were the thoracic wall, abdominal wall, diaphragm and peritoneal cavity[20-22]. Seeding usually occurred along the needle tract, but a few cases were at a distant location, with pleural and peritoneal cavity seeding in one patient each. Regular follow-up with contrast-enhanced CT or MRI from the chest to the pelvis is therefore very important.
The median time to diagnosis of seeding has been reported to be 13 (range 1-58) mo after biopsy[21], 6 (range 2-48) mo after PEI[21] and 28.5 (range 8.6-60.7) mo after RFA[22]. In the present study, the median time to diagnosis was 6.0 (range 1-24) mo after cryoablation. The longest interval from the first cryoablation session to the diagnosis of seeding was 2 years. It can therefore be concluded that it is necessary to carefully monitor patients for at least 2 years after cryoablation for HCC. The reason for the longest interval to the diagnosis of seeding in the present study being shorter than in previous studies on biopsy, PEI and RFA is unknown. There are no reports of growth rates for seeded tumours after cryoablation, but it has been reported that the growth rate of needle-tract seeding after biopsy varies depending on the initial number of implanted tumour cells and the doubling time of the tumour, as well as the microenvironment surrounding the seeded tumour. The doubling time of seeded tumours after biopsy is 112 (range 22-415) d[8], which is comparable to that of primary HCC. Regarding differentiation features, Matsukuma et al[23] reported that peritoneal seeding can infrequently proliferate aggressively with more differentiated features. In the current study, four patients with seeding showed similar tumour differentiation features to the primary HCC, and only three patients had poorly differentiated HCC, so it is difficult to explain the shorter interval in terms of tumour differentiation. The available data report a median HCC diameter of 32.5 mm in patients who underwent PEI and 30 mm in patients who underwent RFA, and the use of 14- to 22-gauge needles for biopsies, 21- to 22-gauge needles for PEI and 14- to 17-gauge needles for RFA[20,21]. The shorter interval to detection of seeding after cryoablation may be related to the use of larger needles (a 3-mm cryoprobe correlates to an 11-gauge needle) and larger nodule size (mean 45 ± 19 mm).
HCC is particularly prone to seeding, with higher seeding rates after biopsy (0%-5.1%)[24] than other solid tumours such as pancreatic tumours (0.003%-0.017%) and other abdominal tumours (0%-0.03%)[9,25].
In a recent review, several factors were suggested to contribute to seeding after percutaneous interventional procedures, which were listed as follows: poorly differentiated tumour, high serum AFP level, subcapsular tumour location, biopsy prior to RFA, high number of sessions and high number of electrode placements[19]. However, only Shirai et al[22] and Imamura et al[26] have reported multivariate analyses of these factors. Imamura et al[26] reported that only poor tumour differentiation was an independent risk factor for seeding. Shirai et al[22] reported that only RFA was an independent risk factor. In the present study, univariate analyses identified subcapsular tumour location and direct subcapsular needle insertion as risk factors. There were no significant associations between seeding and age, sex, viral markers, Child-Pugh class, tumour size, number of nodules, number of sessions, number of needle insertions, tumour differentiation, biopsy prior to cryoablation, BCLC stage, incomplete ablation or serum AFP level. Multivariate analysis showed that only direct subcapsular needle insertion was an independent risk factor for seeding.
Several studies have reported that subcapsular tumour location was a risk factor for seeding[2,27-29]. In a study reporting a 12.5% seeding rate after RFA, all patients with seeding had a subcapsular tumour[2]. This is consistent with the results of our univariate analysis. In our initial experience, percutaneous cryoablation of subcapsular tumours was also associated with liver haemorrhage[16]. In the present study, treatment-related liver haemorrhage occurred in one patient with seeding. We therefore insert our cryoprobe across a portion of normal hepatic parenchyma, and avoid direct subcapsular needle insertion for subcapsular tumours whenever possible. This minimises both liver haemorrhage and needle-tract seeding. This may explain why multivariate analysis only identified direct subcapsular needle insertion as an independent risk factor.
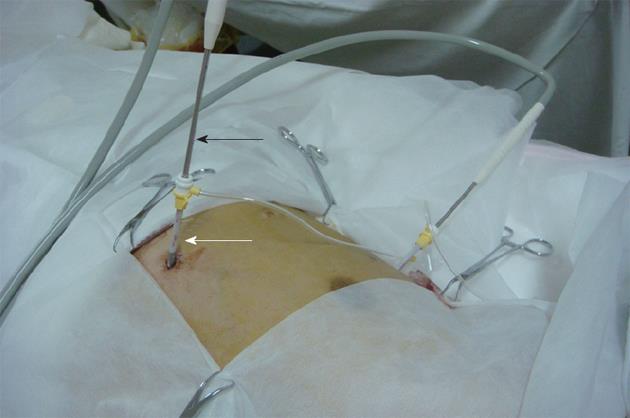
There is still controversy regarding whether tumour biopsy prior to treatment or a poorly differentiated tumour increase the risk of seeding[21-23,30]. In this study, biopsy and a poorly differentiated tumour were not associated with a higher rate of seeding. The current study also did not show a significant association between seeding and tumour size or incomplete cryoablation, which is consistent with the findings of other studies[21,26]. Although the 3-mm cryoprobe was large, the risk of seeding after cryoablation was small. The risk of seeding may be reduced by the use of the sheath introducer system (Figure 7), through which cryoablation needles are inserted and removed. Similarly, Maturen et al[31] reported that no seeding occurred when they used a needle introducer that remained in position during multiple passes of a coaxial cutting needle for biopsies, which may protect the tissue along the needle tract and reduce seeding. Further studies should be conducted to assess the effects of the sheath introducer system on the risk of seeding in percutaneous cryoablation for HCC. In addition, the low risk of cryoablation may be related to the mechanisms of cryoablation. Cryotherapy is believed to kill cells by several mechanisms, including intracellular ice formation, solute-solvent shifts that cause cell dehydration and rupture, small vessel obliteration causing hypoxia and specific anti-tumour immunoreactions that limit tumour growth[32,33]. Several studies found that cryoablation resulted in both local tumour necrosis and necrosis and shrinkage of the tissues adjacent to the tumour, which was thought to indicate ectopic tumour suppression[33]. Preclinical evidence of a cryo-immunologic response as well as some clinical data indicate that cryoablation may generate an anti-tumour response[34,35]. Our previous study indicated that cryoablation not only directly destroys malignant tissues, but also has effects on the adjacent tissues[36]. Cryotherapy resulted in reduced numbers of peripheral Treg cells and a lowering of the CD8-FoxP3+/CD8+FoxP3- ratio in malignant tissues[37]. We therefore speculate that anti-tumour immunoreactions induced by cryoablation may limit seeding. This concept deserves further study.
Although poorly differentiated tumour did not increase the risk of seeding, we found that seeding occurred earlier in patients with poorly differentiated HCC than in those with well or moderately differentiated HCC. It is possible that poorly differentiated HCC lacks cohesiveness[17] and grows more rapidly, allowing the seeding to be identified earlier.
It is not clear whether seeding affects prognosis. Shirai et al[22] and Imamura et al[26] investigated the prognosis of HCC patients with seeding after RFA. They reported that the survival rate was not particularly low in patients with seeding, and that seeding itself did not directly affect survival. The present study also did not find significant differences in the cumulative survival rates of patients with and without seeding. By the end of the follow-up period, five patients with seeding had died of intrahepatic HCC progression and liver failure. No patient died due to the growth of seeded nodules. Nevertheless, the survival rates of patients with seeding tended to be lower from the second year onwards. The reasons for the lack of significant differences in survival rates may be as follows: First, the number of patients with seeding was very small compared with the number without seeding. Second, seeded tumours were treated radically, which may improve outcome[7,38]. It is therefore impossible to claim that seeding does not affect prognosis, and seeded tumours should be treated with the aim of achieving local cure. It is essential to recognise that seeding is difficult to treat successfully. In the present study, the recurrence rate after local radical treatment of seeding was 55.6%.
In conclusion, the relatively low rate of tumour seeding after cryoablation for HCC is considered an acceptable clinical risk. Direct puncture of subcapsular tumours was found to be a risk factor for seeding. Although seeding is sometimes unavoidable, strict attention to detail and knowledge of seeding and its risk factors are helpful for minimising its occurrence.
Imaging-guided percutaneous argon-helium cryoablation is widely used in China, and this technique has been found to be safe and effective for the treatment of hepatocellular carcinoma (HCC). However, details of tumour seeding after this procedure have not been reported to date, even though seeding is one of the most important complications.
This study reports the rate of tumour seeding after percutaneous cryoablation and analyses the risk factors for seeding in a large cohort of HCC patients who were treated with cryoablation sessions over an 8-year period.
Seeding occurred in 11 (0.76%) of 1436 patients treated with percutaneous cryoablation in this study. Only direct puncture of a subcapsular tumour was an independent risk factor for seeding.
This study indicates that the risk of seeding after percutaneous cryoablation for HCC is small and is considered an acceptable clinical risk. This procedure is minimally invasive and results in a large ablation zone, making it a useful treatment modality for HCC. However, direct puncture of a subcapsular tumour should be avoided. The small risk of seeding may be due to the use of an introducer sheath, or to the mechanisms of cryoablation, and further research is warranted.
Percutaneous cryoablation requires the insertion of needle into the liver parenchyma and tumour, which may cause tumour seeding. However, the incidence of HCC seeding after the procedure is low.
The authors analyzed the incidence of HCC tumour seeding after percutaneous cryoablation. It is very interesting study and has a great scientific value for physicians who take care of patients with this pathology. The study is well designed and data is convincing.
Peer reviewers: Salvatore Gruttadauria, MD, Assistant Professor, Abdominal Transplant Surgery, ISMETT, Via E. Tricomi, 190127 Palermo, Italy; Zenichi Morise, MD, PhD, Professor and Chairman, Department of Surgery, Banbuntane Houtokukai Hospital, Fujita Health University School of Medicine, 3-6-10 Otobashi Nakagawa-ku, Nagoya, Aichi 454-8509, Japan
S- Editor Shi ZF L- Editor Webster JR E- Editor Li JY
| 1. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3282] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 2. | Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 529] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 3. | Ishii H, Okada S, Okusaka T, Yoshimori M, Nakasuka H, Shimada K, Yamasaki S, Nakanishi Y, Sakamoto M. Needle tract implantation of hepatocellular carcinoma after percutaneous ethanol injection. Cancer. 1998;82:1638-1642. [PubMed] |
| 4. | Tarantino L, Francica G, Esposito F, Pisaniello D, Parmeggiani D, Marzullo G, Sordelli IM, Sperlongano P. Seeding from hepatocellular carcinoma after percutaneous ablation: color Doppler ultrasound findings. Abdom Imaging. 2006;31:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Smith EH. The hazards of fine-needle aspiration biopsy. Ultrasound Med Biol. 1984;10:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 108] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Bonatti H, Bodner G, Obrist P, Bechter O, Wetscher G, Oefner D. Skin implant metastasis after percutaneous radio-frequency therapy of liver metastasis of a colorectal carcinoma. Am Surg. 2003;69:763-765. [PubMed] |
| 7. | Yu J, Liang P, Yu XL, Cheng ZG, Han ZY, Dong BW. Needle track seeding after percutaneous microwave ablation of malignant liver tumors under ultrasound guidance: analysis of 14-year experience with 1462 patients at a single center. Eur J Radiol. 2012;81:2495-2499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Chang S, Kim SH, Lim HK, Lee WJ, Choi D, Lim JH. Needle tract implantation after sonographically guided percutaneous biopsy of hepatocellular carcinoma: evaluation of doubling time, frequency, and features on CT. AJR Am J Roentgenol. 2005;185:400-405. [PubMed] |
| 9. | Smith EH. Complications of percutaneous abdominal fine-needle biopsy. Review. Radiology. 1991;178:253-258. [PubMed] |
| 10. | Kim SH, Lim HK, Lee WJ, Cho JM, Jang HJ. Needle-tract implantation in hepatocellular carcinoma: frequency and CT findings after biopsy with a 19.5-gauge automated biopsy gun. Abdom Imaging. 2000;25:246-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 92] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Huang GT, Sheu JC, Yang PM, Lee HS, Wang TH, Chen DS. Ultrasound-guided cutting biopsy for the diagnosis of hepatocellular carcinoma--a study based on 420 patients. J Hepatol. 1996;25:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 96] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Arrivé L, Vurgait A, Monnier-Cholley L, Lewin M, Balladur P, Poupon R, Tubiana JM. Long-term follow-up after neoplastic seeding complicating percutaneous ethanol injection for treatment of hepatocellular carcinoma. Eur Radiol. 2002;12:74-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | McWilliams JP, Yamamoto S, Raman SS, Loh CT, Lee EW, Liu DM, Kee ST. Percutaneous ablation of hepatocellular carcinoma: current status. J Vasc Interv Radiol. 2010;21:S204-S213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Xu KC, Niu LZ, He WB, Guo ZQ, Hu YZ, Zuo JS. Percutaneous cryoablation in combination with ethanol injection for unresectable hepatocellular carcinoma. World J Gastroenterol. 2003;9:2686-2689. [PubMed] |
| 15. | Wang C, Lu Y, Chen Y, Feng Y, An L, Wang X, Su S, Bai W, Zhou L, Yang Y. Prognostic factors and recurrence of hepatitis B-related hepatocellular carcinoma after argon-helium cryoablation: a prospective study. Clin Exp Metastasis. 2009;26:839-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Yang Y, Wang C, Lu Y, Bai W, An L, Qu J, Gao X, Chen Y, Zhou L, Wu Y. Outcomes of ultrasound-guided percutaneous argon-helium cryoablation of hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2011;Dec 21; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462-503. [PubMed] |
| 18. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2877] [Article Influence: 110.7] [Reference Citation Analysis (1)] |
| 19. | Hinshaw JL, Lee FT. Cryoablation for liver cancer. Tech Vasc Interv Radiol. 2007;10:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Chang S, Kim SH, Lim HK, Kim SH, Lee WJ, Choi D, Kim YS, Rhim H. Needle tract implantation after percutaneous interventional procedures in hepatocellular carcinomas: lessons learned from a 10-year experience. Korean J Radiol. 2008;9:268-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Stigliano R, Marelli L, Yu D, Davies N, Patch D, Burroughs AK. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. 2007;33:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 214] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 22. | Shirai K, Tamai H, Shingaki N, Mori Y, Moribata K, Enomoto S, Deguchi H, Ueda K, Maekita T, Inoue I. Clinical features and risk factors of extrahepatic seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2011;41:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Matsukuma S, Sato K. Peritoneal seeding of hepatocellular carcinoma: clinicopathological characteristics of 17 autopsy cases. Pathol Int. 2011;61:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Stigliano R, Burroughs AK. Should we biopsy each liver mass suspicious for HCC before liver transplantation?--no, please don't. J Hepatol. 2005;43:563-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Fornari F, Civardi G, Cavanna L, Di Stasi M, Rossi S, Sbolli G, Buscarini L. Complications of ultrasonically guided fine-needle abdominal biopsy. Results of a multicenter Italian study and review of the literature. The Cooperative Italian Study Group. Scand J Gastroenterol. 1989;24:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 98] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Imamura J, Tateishi R, Shiina S, Goto E, Sato T, Ohki T, Masuzaki R, Goto T, Yoshida H, Kanai F. Neoplastic seeding after radiofrequency ablation for hepatocellular carcinoma. Am J Gastroenterol. 2008;103:3057-3062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 933] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 28. | Jaskolka JD, Asch MR, Kachura JR, Ho CS, Ossip M, Wong F, Sherman M, Grant DR, Greig PD, Gallinger S. Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol. 2005;16:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Shiozawa K, Watanabe M, Wakui N, Ikehara T, Iida K, Sumino Y. Analysis of patients with tumor seeding after percutaneous radiofrequency ablation of hepatocellular carcinoma. Mol Med Report. 2008;1:851-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Silva MA, Hegab B, Hyde C, Guo B, Buckels JA, Mirza DF. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57:1592-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 376] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 31. | Maturen KE, Nghiem HV, Marrero JA, Hussain HK, Higgins EG, Fox GA, Francis IR. Lack of tumor seeding of hepatocellular carcinoma after percutaneous needle biopsy using coaxial cutting needle technique. AJR Am J Roentgenol. 2006;187:1184-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Sabel MS. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology. 2009;58:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 215] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 33. | Osada S, Imai H, Tomita H, Tokuyama Y, Okumura N, Matsuhashi N, Sakashita F, Nonaka K. Serum cytokine levels in response to hepatic cryoablation. J Surg Oncol. 2007;95:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Frielink C, Toonen LW, Boerman OC, Figdor CG, Ruers TJ, Adema GJ. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer. 2006;95:896-905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 35. | Sabel MS, Su G, Griffith KA, Chang AE. Rate of freeze alters the immunologic response after cryoablation of breast cancer. Ann Surg Oncol. 2010;17:1187-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Zhou L, Wang CP, Lu YY, Bai WL, Qu JH, Lou M, Zeng Z, Wu Y, Chen Y, Wang H. Decrease in size of non-treated lesions after cryoablation for hepatocellular carcinoma. Hepatogastroenterology. 2012;59:252-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 37. | Zhou L, Fu JL, Lu YY, Fu BY, Wang CP, An LJ, Wang XZ, Zeng Z, Zhou CB, Yang YP. Regulatory T cells are associated with post-cryoablation prognosis in patients with hepatitis B virus-related hepatocellular carcinoma. J Gastroenterol. 2010;45:968-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Hashimoto K, Sasaki Y, Yokoyama S, Hiraki M, Matsumoto S, Tokuoka M, Matsuyama J, Morita S, Morimoto T, Fukushima Y. A long-term survival case after two resections of the peritoneal metastasis from hepatocellular carcinoma. Gan To Kagaku Ryoho. 2011;38:2466-2468. [PubMed] |