Cancer is one of the major health problems worldwide, therefore constantly more effective therapeutic strategies are expected. Cancers arise from the uncontrolled proliferation and spreading of malignantly transformed cell clones with the obvious ability to evade protective immunity. In view of immune surveillance selective, specific and effective eradication of various cancer cells by a subsequent active host immune response serving as a widespread therapy option has still been remained unsolved.
Current therapies for cancer mainly are based on chemotherapeutic drugs that kill transformed, dividing cells or block cell division, but unfortunately these treatments may also attack normal proliferating cells, including immunocompetent ones. However, targeted immune responses (immunotherapy) to tumors may be specific, thus making the possibility to avoid normal cell injury. According to therapeutic vaccines killed tumor cells or tumor antigens can efficiently induce anticancer immunity.
So far less attention has been paid on the possible subcellular and molecular impact of chemotherapy-induced cell death regarding induction of host immune responses.
Autophagy
Besides the proteasomal degradation pathway autophagy represents an additional evolutionarily highly conserved multi-step process of cellular self-digestion due to sequestration of excessive, damaged, or aged proteins and intracellular organelles in double-membranous vesicles of autophagosomes, terminally self-digested in lysosomes[2].
Different types of autophagy according to the route of delivery to lysosomes and the main physiological functions have been characterized, like macro- and microautophagy, and chaperon-mediated autophagy[3]. Upon specific targeted degradation of cytosolic proteins, lipids, or organelles (e.g., ribosomes, nucleosomes, mitochondria), selective forms of autophagy can further be classified as lipophagy, or ribophagy, nucleophagy and mitophagy[2].
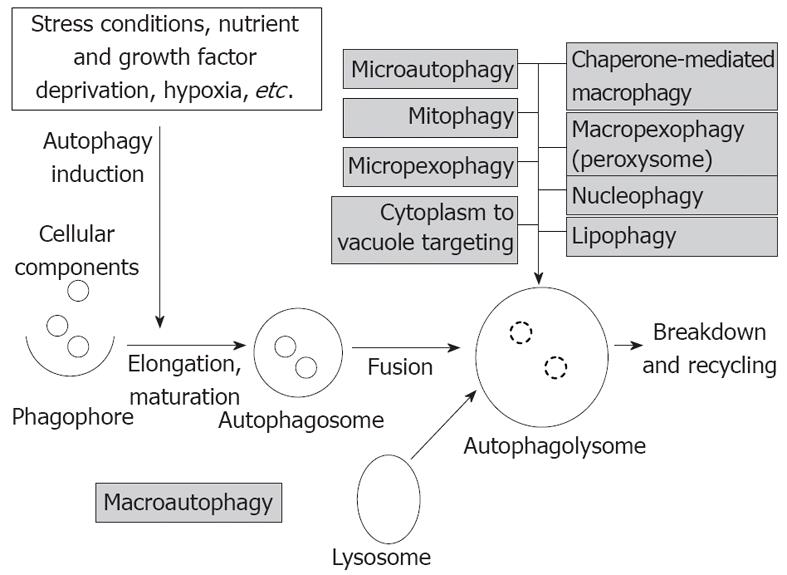
Macroautophagy (hereafter simply termed autophagy) refers to cytoplasmatic bulk, non-selective degradation of subcellular constituents. Within this complex catabolic pathway regulated tightly by a limited number of autophagy genes (atgs) various morphologic stages are distinguishable starting with the formation of phagophore, followed by its elongation and maturation to autophagosome, and finally the fusion with lysosomes[4]. The process of macroautophagy and the types of autophagy are summarized in Figure 1.
Figure 1 The process of macroautophagy and the types of autophagy (gray boxes).
Autophagy is deeply implicated in regulation of numerous physiologic functions including cell development and differentiation, survival and senescence, and it also crucially affects inflammation and innate and adaptive immunity[5]. On a basal level intact autophagy serves constantly and constitutively as a critical adaptive and surveillance mechanism in maintaining cellular homeostasis[3]. However, autophagy is inducible, as well in response to different cellular metabolic stress conditions, including nutrient and growth factor deprivation in order to preserve cell viability. Defects in basal autophagy may yield accumulation of cytotoxic materials, damaged DNA, and thus, genomic instability, while alterations of induced autophagy especially lead to reduced cell survival[4,5].
In general, defective autophagy by compromising cellular fitness has been ultimately related to several disease conditions, such as cancer, certain neurodegenerative, liver, and infectious disorders, aging, and inflammatory conditions, like Crohn’s disease[3,5-7].
Regarding tumorigenesis a dual-faced (Janus) role of autophagy has been proposed, since on one side it may be critical for cancer cell survival and progression, in particular under stressful situations, however it may elicit tumor death signaling pathways. Direction of autophagy toward cytoprotection or tumor cell suppression, thus the pro-survival or pro-death function is context-dependent, and influenced by many intra- and extracellular factors, such as involved tissues, surrounding microenvironment, genetic background, and stages of tumor development, nevertheless its precise relation to cancer networks has not yet been fully elucidated[5,6,8].
The involvement of autophagy in cell death, either in apoptosis (programmed, type I death) or in non-apoptotic or necrotic death, and their possible interactions are rather complicated. Autophagy in tumor cells usually displays a critical, programmed pro-survival function by inhibiting apoptosis or suppressing necrotic death, including programmed (or regulated) cell necrosis of caspase-independent necroptosis, and poly-ADP-ribose polymerase-mediated necrosis[9].
In cases of autophagy deficiency, however, no tumor suppression, but on the contrary, accelerated tumorigenesis can be manifestated. In autophagy-incompetent cells upon induced oxidative stress cell-autonomous mechanisms are exhibited in forms of accumulated DNA damage and chromatin instability[10]. As a non-cell-autonomous mechanism, however, inflammatory events along with defective apoptosis could also contribute independently to cancer progression, partly by favouring cell necrosis[11]. Similar situation has been found in human inflammatory bowel diseases (IBD) with high risk of malignancy, and in experimental cases of atg5-/- or atg7-/- mice displaying inflammatory Paneth cell abnormalities resembling human IBD[7,12].
The atg6/Beclin-1 gene, a Bcl-2/Bcl-xL interacting element has been found to be monoallelically lost in certain human cancers, and confirmed that it functions as a haploinsufficient tumor suppressor[13]. However, this suppressive function of Beclin-1 may be tissue-specific, since even its higher expression has been detected in colorectal and gastric carcinomas[14]. In addition to Beclin-1, alterations of other autophagy-associated genes, e.g., atg4, atg5, UV-irradiation resistance-associated gene (UVRAG), or Bax-binding protein-1 (Bif-1) have also been detected in various cancers, indicating that tumor suppression is attributed to different autophagy elements. Nonsense mutations of UVRAG, and downregulation of Bif-1 have been documented in colon and gastric carcinomas, and in colon adenocarcinomas, respectively[15-17].
Hypothetically, increased autophagic flux via excessively induced autophagy may promote non-apoptotic (programmed, type II) autophagic cell death, acting like a tumor suppressor[18]. Autophagy is also known to stimulate oncogene-induced senescense, thus providing another possible barrier against malignant transformation[19]. Nevertheless, there is no direct evidence regarding the realistic anti-tumor capacity of autophagy.
In human cancers constitutive activation of Ras- and phosphoinositol 3-kinase/Akt-mammalian target of rapamycin (mTOR) pathway is a common phenomenon, and mTOR complex 1 seems to be the main negative regulator of autophagy[20,21]. The tumor suppressor p53 gene exerts a typical dual role in autophagy regulation, depending primarily on its subcellular, nuclear or cytoplasmic distribution[22]. Both stress-responsive cellular degradation pathways of intrinsic and extrinsic apoptosis and of autophagy can fundamentally affect, activate or inhibit each other via an extensive molecular crosstalk, and in fact, cell destiny is determined by their actual functional status and interplay[6,23]. Their crosstalk is regulated primarily by the current status of the Bcl-2/Beclin-1 complex, dissociation of which can be achieved upon activation of mitogen activated phosphokinase-jun kinase or translocation of the damage-associated molecular pattern (DAMP) protein HMGB-1[23]. Nuclear factor (NF)-κB plays also a critical role in malignant transformation, and its constitutive, chronic activation has been observed in the majority of different tumor cells. There is also a complex interaction between autophagy and the NF-κB signaling pathways via positive and negative feedback regulatory loops[24]. The important autophagy selective substrate p62 acts as an adaptor protein to regulate NF-κB, as well[25].
Overall, there is no doubt that process of autophagy can be considered as an apparently quite difficult regulatory network, being in close connection with other signal transduction pathways and cellular programs. The complex and rather contradictory function of autophagy in tumorigenesis makes itself a promising but challenging therapeutic target both in cancer treatment and prevention. In autophagy-competent tumor cells autophagy increase can often be induced in response to different chemo- and radiotherapies, representing mainly an adaptive survival mechanism, but provoking simultaneously treatment resistance. Therefore it has been hypothesized that concurrent pharmacologic inhibition of autophagy, as an adjuvant may sensitize tumor cells to a spectrum of anticancer drugs[22,26,27]. In cases of autophagy-deficient tumors, however, due to their extreme susceptibility, metabolic stress- and DNA-damage-inducing therapeutic protocols are suggested. However, autophagy induction could also provide an alternative therapeutic option[22,26,27]. Nevertheless, excessive autophagy can potentially act as an active cell death machinery, mainly along with inherent apopotosis defects, so induction of autophagy by antitumor drugs may also be considered as an efficient cytotoxic manipulation.
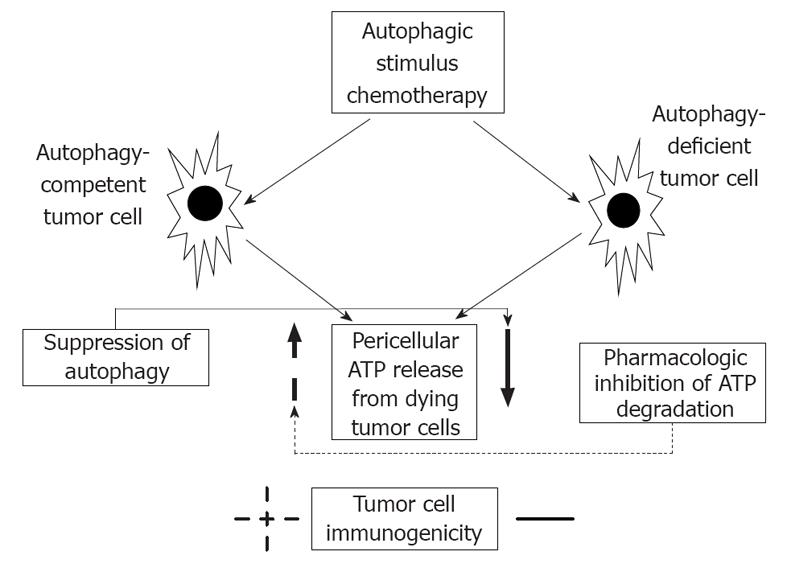
Michaud et al[1] in their experiments, using transplantable murine tumors of CT26 colorectal carcinoma and of MCA205 fibrosarcoma treated either with mitoxantrone or oxalipatin have found that autophagy-competent tumor cells release more ATP comparing with autophagy-deficient ones. Furthermore, pharmacologic inhibition of autophagy reduced chemotherapy-induced ATP release, however induction of autophagy did not trigger it. ATP serves as a danger signal, it is a prominent DAMP molecule. In addition, unlike autophagy-deficient tumor cells chemotherapy in autophagy-competent cancer cells elicited a protective immune response, i.e., attraction of dendritic cells, CD4+ and CD8+ lymphocytes, and priming of T cells. Inhibition of autophagy decreased the immunogenic potential of tumor cells. The authors finally conclude, that upon chemotherapy premortem autophagy is required for tumor immunogenicity by releasing ATP from dying apoptotic cells, and consequently, in case of autophagy defiency the ability of tumor cells to induce an adaptive anticancer immune response is significantly restricted. In that transplantable model dying cancer cells function as a therapeutic vaccine. Nevertheless, in autophagy-deficient tumors of immunocompetent hosts by pharmacologic inhibition of ATP degradation a compensatory increase in pericellular ATP content was achieved, thus successfully restoring the immunogenic capacity, and suggesting a novel adjuvant therapeutic possibility (Figure 2).
Figure 2 The relation of autophagy and anticancer immunity.
Similarly to autophagy-deficient tumor cells inhibition of autophagy results in decreased pericellular ATP secretion, and thus suppressing anticancer immunity. Pharmacologic inhibition of ATP degradation, however, increases ATP level in the microenvironment of tumor cells, and favours tumor cell immunogenicity.
Findings of Michaud et al[1] not only highlight on the complexity and many faces of autophagy in tumorigenesis, but emphasize the rationality of analyzing subcellular, molecular consequences of chemotherapy in respect of influencing host immunity, and thus propose a promising therapeutic strategy to compensate autophagy deficiency-related altered tumor immunogenicity.