Published online Nov 21, 2012. doi: 10.3748/wjg.v18.i43.6308
Revised: July 31, 2012
Accepted: August 26, 2012
Published online: November 21, 2012
AIM: To investigate the effect of different secondary warm ischemia time (SWIT) on bile duct injury in liver-transplanted rats.
METHODS: Forty-eight male inbred Sprague-Dawley rats were randomly assigned into four groups: a sham-operation group and three groups with secondary biliary warm ischemia time of 0 min, 10 min and 20 min. A rat model of autologous liver transplantation under ether anesthesia was established, and six rats were killed in each group and blood samples and the median lobe of the liver were collected for assay at 6 h and 24 h after hepatic arterial reperfusion.
RESULTS: With prolongation of biliary warm ischemia time, the level of vascular endothelial growth factor-A was significantly decreased, and the value at 24 h was higher than that at 6 h after hepatic arterial reperfusion, but with no significant difference. The extended biliary SWIT led to a significant increase in bile duct epithelial cell apoptosis, and a decrease in the number of blood vessels, the bile duct surrounding the blood vessels and bile duct epithelial cell proliferation in the early postoperative portal area. Pathologic examinations showed that inflammation of the rat portal area was aggravated, and biliary epithelial cell injury was significantly worsened.
CONCLUSION: A prolonged biliary warm ischemia time results in aggravated injury of the bile duct and the surrounding vascular plexus in rat autologous orthotopic liver transplantation.
- Citation: Zhu XH, Pan JP, Wu YF, Ding YT. Effects of warm ischemia time on biliary injury in rat liver transplantation. World J Gastroenterol 2012; 18(43): 6308-6314
- URL: https://www.wjgnet.com/1007-9327/full/v18/i43/6308.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i43.6308
Orthotopic liver transplantation (OLT) has proven to be a successful treatment choice for patients with end-stage chronic or acute liver failure. However, biliary complications remain a significant source of morbidity and have been associated with a mortality rate ranging from 8% to 15%[1,2]. With improvement in surgical techniques, the incidence of anastomotic biliary strictures was markedly reduced, whereas non-anastomotic biliary strictures (NAS) have become a predominant biliary complication of liver allografts[3]. Diffuse NAS after OLT have been termed as ischemic-type biliary complications, ischemic cholangitis and ischemic cholangiopathy. NAS remains the most challenging type of biliary complication as it is frequently therapy-resistant and often associated with long-term consequences[4,5].
The cause of NAS is multifactorial, and ischemia/reperfusion injury of the biliary epithelium is considered as one of the major causes[6]. Warm ischemic time in donors after cardiac death (DCD), in addition to subsequent cold ischemia-reperfusion injury, is believed to result in increased damage to biliary epithelial cells[7,8]. The most commonly used procedure for revascularization of the liver graft in clinical practice is initial portal reperfusion and subsequent reconstruction of the hepatic artery. Compared with liver cells, the bile duct epithelial cells experience an extra ischemic process from portal venous recanalization to hepatic arterial recanalization, which is defined as the “secondary warm ischemia time (SWIT) in the biliary tract” or “relative warm ischemia time in the biliary tract”. This is a special phase of biliary warm ischemia in the graft. Because warm ischemia time in the harvesting of donor livers after cardiac death is inevitable, more and more studies have shifted their focus to the effect of SWIT on bile duct injury[9].
The terminal branches of the hepatic artery are represented by either the extra- or intra-hepatic peribiliary arterial plexus (PBP). The function of the intrahepatic biliary tree is linked to its vascular supply sustained by PBP[10]. Alterations of intrahepatic bile duct mass are associated with the architectural changes in the PBP. In this study, we investigated the impact of different SWITs on the bile duct and PBP in a rat autologous liver transplantation model.
Forty-eight male inbred SD rats weighing 220-250 g were purchased from the Animal Center of Yangzhou University (Yangzhou, China). The rats were housed and fed at the Animal Center of Drum Tower Hospital for at least 7 d before transplantation for acclimatization to the environment. All rats were provided with standard laboratory chow and water and housed in accordance with institutional animal care policies. The rats were fasted for 8 h, but allowed free access to water before being used in the study.
The following experimental protocol was approved by the Animal Care and Use Committee of the Drum Tower Hospital and conformed to Guide for the Care and Use of Laboratory Animals from National Institutes of Health.
A rat model of autologous liver transplantation was established using the technique of Wang et al[11] under ether anesthesia. Rats were randomly assigned into four groups according to the SWIT: a sham-operation group and three groups with the biliary SWIT of 0 min, 10 min and 20 min. In the sham-operation group (group I), the liver was mobilized without cold or warm ischemia-reperfusion injury to exclude the influence of surgery. In group II with no SWIT, simultaneous reperfusion was performed through the portal vein and hepatic artery after cold perfusion. In groups III and IV with SWIT of 10 min and 20 min, hepatic arterial perfusion was performed for 10 min and 20 min, respectively, after portal venous reperfusion.
At 6 h and 24 h after hepatic arterial reperfusion, 6 rats were killed in each group, and blood samples were collected via the infrahepatic vena cava, and the median lobe of liver was obtained for assay. The serum was separated and stored at -70 °C until analysis. After washing with cold saline solution, the liver samples were stored immediately in liquid nitrogen until analysis.
Vascular endothelial growth factor-A (VEGF-A) plasma levels of the samples collected at 6 h and 24 h after hepatic arterial reperfusion were determined with enzyme-linked immunosorbent assay kits (Ruiqi Biotechnology Co. Ltd, Shanghai, China) according to the manufacturer’s instruction.
The liver specimens were fixed with 10% formalin and embedded in paraffin. The liver tissues were cut into sequential slices of 2.5 mm. The bile ducts were immunolocalized by CK19 polyclonal antibody (Boside Biotechnology Co. Ltd, Wuhan, China), and the blood vessels were tagged with rabbit factor VIII-related antigen (Boaoseng Biotechnology Co. Ltd, Beijing, China). In the portal area, the number of bile ducts, blood vessels, and bile ducts with and without blood vessels were counted.
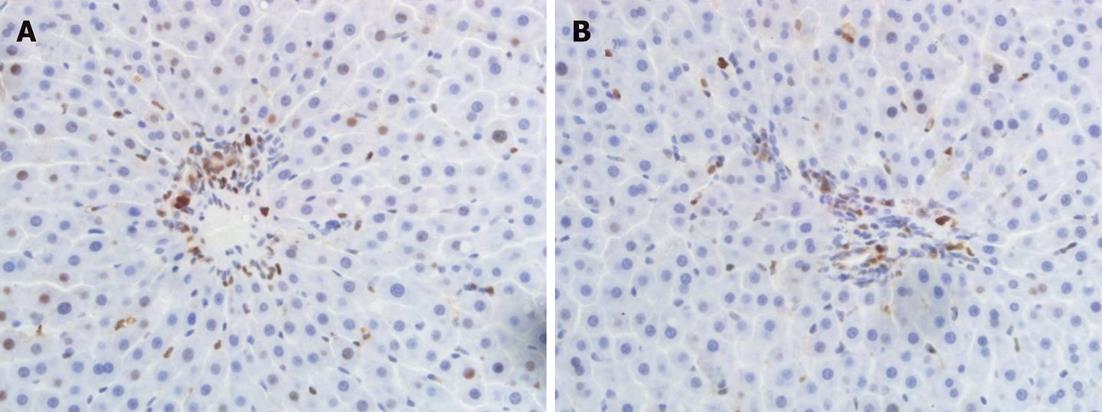
The number of proliferating-cell nuclear antigen (PCNA)-positive cells was used to evaluate cholangiocyte proliferation with immunohistochemistry. After rehydration, the silane-coated slides were treated with 0.3% H2O2 in methyl alcohol for 15 min and then briefly washed in phosphate buffer saline. They were then incubated overnight at 4 °C, with a 1:100 dilution of anti-PCNA monoclonal antibody, and subsequently incubated with Envision Plus Sunpoly-H III HRP rabbit/mouse kit (Boshide Biotech Co. Ltd., Wuhan, China) for 30 min at 37 °C. Finally, the sections were counterstained with hematoxylin and coverslipped. After staining, sections were analyzed in a coded fashion under a light microscope.
PCNA protein expression appeared brown in the cell nucleus. The cholangiocyte proliferation index was measured as the number of PCNA-positive cholangiocytes per 100 cells under high magnification (× 400).
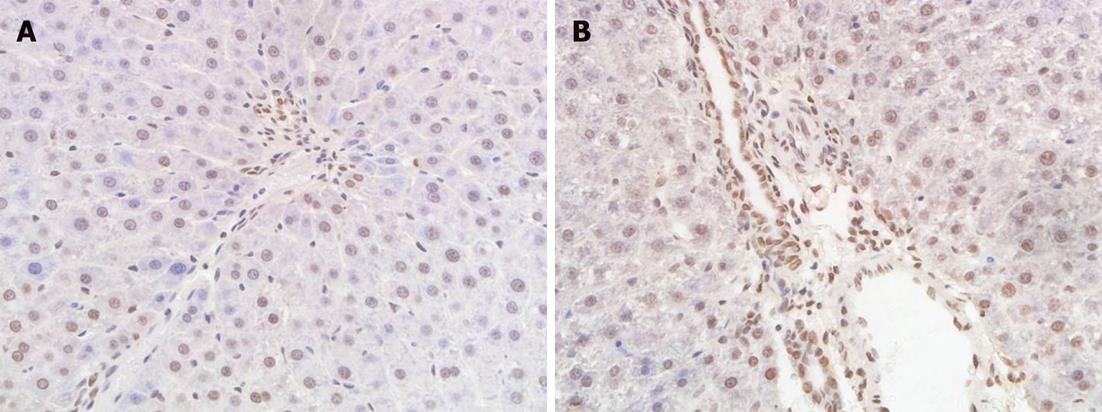
Apoptosis of bile duct epithelial cells was identified by detecting DNA fragmentation in situ in serial sections at 6 h and 24 h after hepatic arterial reperfusion. DNA fragmentation was detected by terminal -deoxynucleotidyl transferase mediated nick end labeling (TUNEL) staining, which was performed on deparaffinized and dehydrated sections using the In Situ Cell Death Detection kit (Zhongshan Biomedical Technology Co., Beijing, China) according to the manufacturer’s instructions. TUNEL-positive cholangiocytes displayed a characteristic morphology of apoptosis, including chromatin condensation, cell fragmentation and apoptotic bodies. Apoptotic cells were examined at original magnification × 400 in 10 randomly selected fields per section. The apoptotic index was calculated as the percentage of apoptotic cells in the total number of cholangiocytes.
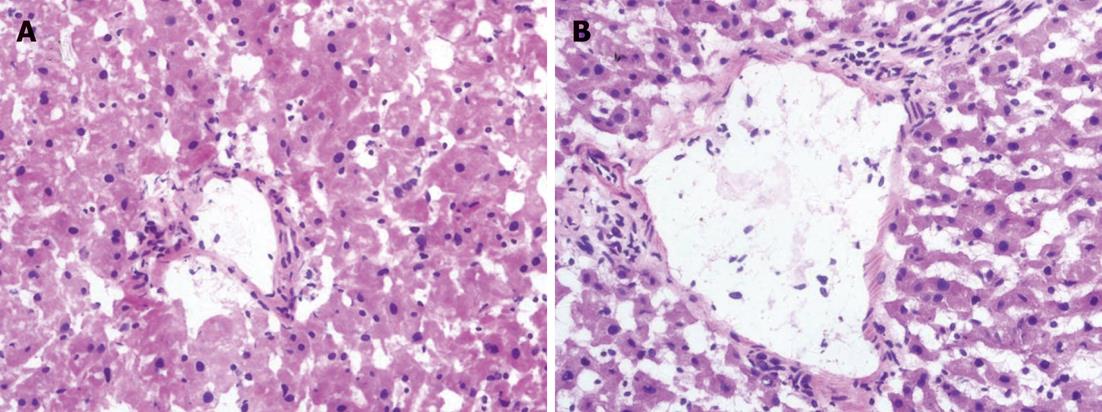
Six liver specimens were collected at 24 h after hepatic arterial reperfusion in each group. The liver specimens for light microscopy were fixed with 10% formalin and embedded in paraffin. The sections were stained with hematoxylin and eosin for histological examination. Bile duct injury in the specimens was semiquantified by calculating a bile duct injury severity score (BDISS)[12] based on the following three components: bile duct damage (graded as 0, absent; 1, mild; 2, moderate; 3, severe; modified from the Banff criteria for acute rejection); ductular proliferation (graded 0-3, using a similar scale as stated earlier); and cholestasis (graded 0-3, using a similar scale as stated earlier). This resulted in a minimal BDISS of zero and a maximum score of 9 points. All examinations were conducted by an experienced pathologist who was unaware of the other study data.
The results were expressed as mean ± SD. Data were analyzed using the Statistical Analysis System (SAS Institute, Cary, NC, United States). One-way analysis of variance was used for multiple comparisons with Student-Newman-Keulstest. P < 0.05 was considered statistically significant.
There was no significant difference in VEGF-A at 6 h postoperatively between group I and the other three groups, but there was a significant decrease in VEGF-A at 24 h in groups II-IV. The VEGF-A was lower in group IV than in groups II and III, and there were significant differences among these three groups (P < 0.05) (Table 1).
The number of bile ducts in each portal area in group I was 6.10 ± 0.74, and the bile ducts were always accompanied by blood vessels. Compared with group I, there was a significant decrease in the number of bile ducts, blood vessels and bile ducts with blood vessels in the portal area, and a significant increase in bile ducts without blood vessels in the other three groups. No significant difference in the number of bile ducts and blood vessels was found at 6 h postoperatively among groups II, III and IV. The number of bile ducts, blood vessels and bile ducts with blood vessels in portal area at 24 h was significantly lower in group IV than in groups II and III (P < 0.05) (Table 2) .
| Time (h) | Group I | Group II | Group III | Group IV | |
| Bile ducts | 6 | 6.10 ± 0.74 | 3.40 ± 1.17a | 2.80 ± 0.79a | 2.60 ± 0.51a |
| 24 | 3.00 ± 1.15a | 2.40 ± 0.74ac | 1.85 ± 0.63ace | ||
| Blood vessels | 6 | 5.50 ± 0.94 | 2.90 ± 0.74a | 2.40 ± 0.88a | 2.00 ± 0.84a |
| 24 | 2.40 ± 0.93a | 1.67 ± 0.67ac | 1.10 ± 0.83ace | ||
| Bile ducts with blood vessels | 6 | 5.42 ± 1.35 | 2.20 ± 1.23a | 1.60 ± 0.70a | 1.40 ± 0.81a |
| 24 | 1.80 ± 0.53a | 1.10 ± 0.67ac | 0.40 ± 0.65ace | ||
| Bile ducts without blood vessels | 6 | 0.65 ± 0.42 | 1.0 ± 0.67a | 1.20 ± 0.70a | 1.31 ± 0.92a |
| 24 | 1.12 ± 0.53a | 1.40 ± 1.03ac | 1.58 ± 0.61ace |
Compared with group I, the number of PCNA-positive cholangiocytes was significantly reduced at 6 h and 24 h postoperatively in groups II and III (P < 0.05), and a very significant decrease in cholangiocyte proliferation was observed in group IV (P < 0.01). The number of PCNA-positive cholangiocytes in group IV was lower than in groups II and III, and there were significant differences among these three groups at 6 h and 24 h postoperatively (P < 0.05). The number of PCNA-positive cholangiocytes at 24 h after hepatic arterial reperfusion was reduced in all groups compared with the result at 6 h, but no significant differences were noted (Table 3, Figure 1).
| Group | Cholangiocyte proliferation | Apoptosis index | Severity score | |||
| 6 h | 24 h | 6 h | 24 h | 6 h | 24 h | |
| I | 25.81 ± 3.50 | 26.13 ± 2.60 | 0.87 ± 0.50 | 0.53 ± 0.60 | 0 | 0 |
| II | 17.38 ± 4.31a | 16.91 ± 5.67a | 5.83 ± 0.51 | 7.15 ± 0.62 | 2.6 ± 0.3 | 2.8 ± 0.2 |
| III | 14.36 ± 3.69ac | 12.90 ± 2.48ac | 7.57 ± 0.32c | 8.98 ± 0.65c | 3.7 ± 0.3c | 3.8 ± 0.4c |
| IV | 10.19 ± 0.49ce | 9.01 ± 3.65ce | 8.96 ± 0.67ce | 9.92 ± 0.47c | 4.8 ± 0.2ce | 5.0 ± 0.3ce |
There were a few apoptotic bodies in the liver sections of group I. Compared with group II, a significant increase in apoptosis index was found at 6 h and 24 h postoperatively in groups III and IV (P < 0.05). The apoptosis index in group IV was significantly higher than in group III at 6 h postoperatively (P < 0.05). In groups II, III and IV, the apoptosis index at 24 h after hepatic arterial reperfusion was higher than that at 6 h, but no significant differences were noted (Table 3, Figure 2).
The histological findings indicated that the degree of bile duct injury was mild in group I. The main bile duct injuries in group II included cholangiocytes lining in disarray with diversified morphous, edematous, inflammatory cell infiltration, migrated chromatin, and necrotic cell debris in the lumen. The bile duct showed more histological changes in groups III and IV, and more marked injuries in group IV. Microthrombi were found in the microangium around the biliary tract in some sections from groups III and IV.
Compared with group II, a significant increase in BDISS was observed at 6 h and 24 h postoperatively in groups III and IV (P < 0.05). The BDISS in group IV was higher than that in group III, and there was significant difference between the two groups (P < 0.05). BDISS at 24 h after hepatic arterial reperfusion increased in all groups compared with BDISS at 6 h, but no significant differences were noted (Table 3, Figure 3).
Warm ischemia time in DCD is associated with a higher risk of biliary strictures[13], and the incidence of NAS in DCD ranges from 10% to 30% compared with an incidence of 1%-10% in donation after brain death[14,15]. We used a model of rat autologous orthotopic liver transplantation to simulate ischemia-reperfusion injury of the biliary tract, which mimics the whole process of clinical liver transplantation. This model decreases the possibility of blood vessel or vascular anastomosis damage compared with the allogeneic orthotopic liver transplantation, and it minimizes the effects of immunologic rejection. It is a simple model used with a high success rate, which better reflects the pathophysiologic process of bile ducts, and affords a useful tool for the investigation of intrahepatic bile duct damage in liver transplantation caused directly by ischemia-reperfusion injury[16].
Hepatocytes are supplied by both the hepatic artery and the portal vein, but bile ducts entirely rely on arterial blood supply for oxygenation. The terminal branches of the hepatic artery end in the PBP, which is the direct source of blood supply to the intrahepatic bile ducts. Therefore, the changes of PBP often result in alterations of intrahepatic bile duct structure[17]. Post-transplantational hepatic arterial ischemia induces ischemia and occlusion of PBP, thus aggravating ischemia of intrahepatic bile ducts[18,19]. The pathomorphologic changes of bile ducts indicated that there was a time-dependent relationship between secondary ischemia time and pathological injury, and in this study, group IV had the most severe bile duct injury among the four groups. Microthrombi were found in the microangium around the biliary tract in some sections from groups III and IV.
The etiopathogenesis of nonanastomotic stenosis of bile ducts after liver transplantation is complicated, and the factors that cause damage to the bile ducts are mediated by either direct or indirect effects of the PBP. Cholangiocytes can express VEGF and its receptors to regulate the adaptive proliferation of the PBP[20]. In this study, VEGF-A in groups III and IV decreased significantly, and the number of blood vessels and bile ducts with blood vessels in the portal area also decreased significantly.
Cholangiocyte proliferation is regulated by a number of factors including cAMP, gastrointestinal hormones (e.g., gastrin and somatostatin), bile salts, cholinergic, adrenergic and serotoninergic neurotransmitters, and vascular growth factors[21-23]. The number of PCNA-positive cholangiocytes was reduced compared with that in normal rats, most obviously in group IV. And the numbers of bile ducts and bile ducts with blood vessels decreased significantly in group IV.
Bile duct epithelia are highly susceptible to reoxygenation after anoxia[24]. The increased susceptibility to reoxygenation injury by cholangiocytes is associated with increased production of toxic reactive oxygen species by cholangiocytes during reoxygenation, with concomitant low basal levels of the antioxidant glutathione in these epithelial cells[25,26]. There are two mechanisms by which cell death occurs: one is apoptosis and the other is the pathological process of necrosis. Accumulating evidence suggests that apoptosis plays an important role in ischemia-reperfusion injury in organ transplantation[11], and it is widely taken as a reference index to evaluate bile duct epithelial injury. With the prolongation of biliary warm ischemia time, the biliary epithelial cell apoptosis index was significantly elevated.
In conclusion, a prolonged biliary warm ischemia time would result in aggravated injury of the bile duct and the surrounding vascular plexus in autologous orthotopic liver transplantation. The secondary biliary warm ischemia time in liver transplantation should be minimized to reduce the injury of the bile duct and its surrounding blood vascular plexus.
We want to thank Dr. Chen Jun for his work in pathological analysis.
With the improvement in surgical techniques, the incidence of anastomotic biliary strictures after liver transplantation decreased markedly, whereas non-anastomotic biliary strictures (NAS) became the major type of biliary complications of liver allografting. Diffuse NAS remain the most challenging type of biliary complication as they are frequently therapy-resistant and are often associated with long-term consequences.
Warm ischemia time in donors after cardiac death, in addition to subsequent cold ischemia-reperfusion injury, is believed to result in increased damage to biliary epithelial cells. Compared with liver cells, the bile duct epithelial cells experience an extra ischemic process from portal venous recanalization to hepatic arterial recanalization, which is defined as the “secondary warm ischemia time (SWIT) in the biliary tract” or “relative warm ischemia time in the biliary tract”. This is a special phase of biliary warm ischemia in the graft; and more and more studies have shifted their focus to the effect of SWIT on bile duct injury in organ transplantation.
The function of the intrahepatic biliary tree is linked with its vascular supply sustained by the intrahepatic peribiliary arterial plexus (PBP). Alterations of intrahepatic bile duct mass are associated with changes in the PBP architecture. In this study, the authors investigated the impact of different SWITs on the bile duct and PBP in a rat autologous liver transplantation model.
The authors concluded that the prolonged biliary warm ischemia time would result in aggravated injury of the bile duct and the surrounding vascular plexus in autologous orthotopic liver transplantation. Therefore, secondary biliary warm ischemia time in liver transplantation should be minimized to reduce injury to the bile duct and its surrounding blood vascular plexus.
This is a concise manuscript, addressing the important problem of non-anastomotic biliary injuries in liver transplantation. The experimental design is clear, methods are valid and the results support the idea that with increasing secondary biliary warm ischemia time, an increase of bile duct injury, mediated by adverse effects of ischemia on the bile duct surrounding blood vascular plexus, could be observed.
Peer reviewers: Alfred Gangl, Professor, Department of Medicine 4, Medical University of Vienna, Allgemeines Krankenhaus, Waehringer Guertel 18-20, Vienna A-1090, Austria; Bijan Eghtesad, Associate Professor, Department of General Surgery, Cleveland Clinic Foundation, 9500 Euclid Avenue, Cleveland, OH 44195, United States
S- Editor Shi ZF L- Editor Cant MR E- Editor Xiong L
| 1. | Wojcicki M, Milkiewicz P, Silva M. Biliary tract complications after liver transplantation: a review. Dig Surg. 2008;25:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Buck DG, Zajko AB. Biliary complications after orthotopic liver transplantation. Tech Vasc Interv Radiol. 2008;11:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Op den Dries S, Sutton ME, Lisman T, Porte RJ. Protection of bile ducts in liver transplantation: looking beyond ischemia. Transplantation. 2011;92:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008;14:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 5. | Howell JA, Gow PJ, Angus PW, Jones RM, Wang BZ, Bailey M, Fink MA. Early-onset versus late-onset nonanastomotic biliary strictures post liver transplantation: risk factors reflect different pathogenesis. Transpl Int. 2012;25:765-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Buis CI, Verdonk RC, Van der Jagt EJ, van der Hilst CS, Slooff MJ, Haagsma EB, Porte RJ. Nonanastomotic biliary strictures after liver transplantation, part 1: Radiological features and risk factors for early vs. late presentation. Liver Transpl. 2007;13:708-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Saidi RF, Bradley J, Greer D, Luskin R, O'Connor K, Delmonico F, Kennealey P, Pathan F, Schuetz C, Elias N. Changing pattern of organ donation at a single center: are potential brain dead donors being lost to donation after cardiac death? Am J Transplant. 2010;10:2536-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Abt P, Crawford M, Desai N, Markmann J, Olthoff K, Shaked A. Liver transplantation from controlled non-heart-beating donors: an increased incidence of biliary complications. Transplantation. 2003;75:1659-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 239] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Abou Abbass A, Abouljoud M, Yoshida A, Kim DY, Slater R, Hundley J, Kazimi M, Moonka D. Biliary complications after orthotopic liver transplantation from donors after cardiac death: broad spectrum of disease. Transplant Proc. 2010;42:3392-3398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Ren J, Lu MD, Zheng RQ, Lu MQ, Liao M, Mao YJ, Zheng ZJ, Lu Y. Evaluation of the microcirculatory disturbance of biliary ischemia after liver transplantation with contrast-enhanced ultrasound: preliminary experience. Liver Transpl. 2009;15:1703-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Wang Z, Zhou J, Lin J, Wang Y, Lin Y, Li X. RhGH attenuates ischemia injury of intrahepatic bile ducts relating to liver transplantation. J Surg Res. 2011;171:300-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Geuken E, Visser D, Kuipers F, Blokzijl H, Leuvenink HG, de Jong KP, Peeters PM, Jansen PL, Slooff MJ, Gouw AS. Rapid increase of bile salt secretion is associated with bile duct injury after human liver transplantation. J Hepatol. 2004;41:1017-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Grewal HP, Willingham DL, Nguyen J, Hewitt WR, Taner BC, Cornell D, Rosser BG, Keaveny AP, Aranda-Michel J, Satyanarayana R. Liver transplantation using controlled donation after cardiac death donors: an analysis of a large single-center experience. Liver Transpl. 2009;15:1028-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 14. | Foley DP, Fernandez LA, Leverson G, Anderson M, Mezrich J, Sollinger HW, D'Alessandro A. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann Surg. 2011;253:817-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 337] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 15. | Jay CL, Lyuksemburg V, Ladner DP, Wang E, Caicedo JC, Holl JL, Abecassis MM, Skaro AI. Ischemic cholangiopathy after controlled donation after cardiac death liver transplantation: a meta-analysis. Ann Surg. 2011;253:259-264. [PubMed] |
| 16. | Zhou B, Zhang PJ, Tian T, Jin C, Li Y, Feng M, Liu XY, Jie L, Tao LD. Role of vascular endothelial growth factor in protection of intrahepatic cholangiocytes mediated by hypoxic preconditioning after liver transplantation in rats. Transplant Proc. 2010;42:2457-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Dacha S, Barad A, Martin J, Levitsky J. Association of hepatic artery stenosis and biliary strictures in liver transplant recipients. Liver Transpl. 2011;17:849-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Buis CI, Hoekstra H, Verdonk RC, Porte RJ. Causes and consequences of ischemic-type biliary lesions after liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 166] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Nishida S, Nakamura N, Kadono J, Komokata T, Sakata R, Madariaga JR, Tzakis AG. Intrahepatic biliary strictures after liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:511-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Ueno Y, Meininger CJ, Franchitto A, Onori P, Marzioni M. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130:1270-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Mancinelli R, Onori P, Gaudio E, DeMorrow S, Franchitto A, Francis H, Glaser S, Carpino G, Venter J, Alvaro D. Follicle-stimulating hormone increases cholangiocyte proliferation by an autocrine mechanism via cAMP-dependent phosphorylation of ERK1/2 and Elk-1. Am J Physiol Gastrointest Liver Physiol. 2009;297:G11-G26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Glaser S, Lam IP, Franchitto A, Gaudio E, Onori P, Chow BK, Wise C, Kopriva S, Venter J, White M. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology. 2010;52:204-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Chen G, Wang S, Bie P, Li X, Dong J. Endogenous bile salts are associated with bile duct injury in the rat liver transplantation model. Transplantation. 2009;87:330-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Noack K, Bronk SF, Kato A, Gores GJ. The greater vulnerability of bile duct cells to reoxygenation injury than to anoxia. Implications for the pathogenesis of biliary strictures after liver transplantation. Transplantation. 1993;56:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 134] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Accatino L, Pizarro M, Solís N, Arrese M, Koenig CS. Bile secretory function after warm hepatic ischemia-reperfusion injury in the rat. Liver Transpl. 2003;9:1199-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Rüdiger HA, Graf R, Clavien PA. Liver ischemia: apoptosis as a central mechanism of injury. J Invest Surg. 2003;16:149-159. [PubMed] |