Published online Nov 21, 2012. doi: 10.3748/wjg.v18.i43.6250
Revised: August 19, 2012
Accepted: August 25, 2012
Published online: November 21, 2012
AIM: To evaluate the role of sequential therapy and Lactobacillus reuteri (L. reuteri) supplementation, in the eradication treatment of Helicobacter pylori (H. pylori).
METHODS: H. pylori infection was diagnosed in 90 adult dyspeptic patients. Patients were excluded if previously treated for H. pylori infection or if they were taking a proton pump inhibitor (PPI), H2-receptor antagonist or antibiotics. Patients were assigned to receive one of the following therapies: (1) 7-d triple therapy (PPI plus clarithromycin and amoxicillin or metronidazole) plus L. reuteri supplementation during antibiotic treatment; (2) 7-d triple therapy plus L. reuteri supplementation after antibiotic treatment; (3) sequential regimen (5-d PPI plus amoxicillin therapy followed by a 5-d PPI, clarithromycin and tinidazole) plus L. reuteri supplementation during antibiotic treatment; and (4) sequential regimen plus L. reuteri supplementation after antibiotic treatment. Successful eradication therapy was defined as a negative urea breath test at least 4 wk following treatment.
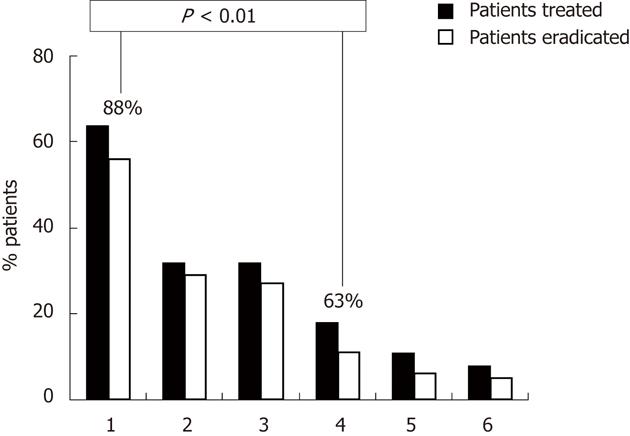
RESULTS: Ninety adult dyspeptic patients were enrolled, and 83 (30 male, 53 female; mean age 57 ± 13 years) completed the study. Nineteen patients were administered a 7-d triple treatment: 11 with L. reuteri supplementation during and 8 after therapy. Sixty-four patients were administered a sequential regimen: 32 with L. reuteri supplementation during and 32 after therapy. The eradication rate was significantly higher in the sequential group compared with the 7-d triple regimen (88% vs 63%, P = 0.01). No difference was found between two types of PPI. No difference in eradication rates was observed between patients submitted to L. reuteri supplementation during or after antibiotic treatment. Compliance with therapy was excellent in all patients. No difference in adverse effects was observed between the different antibiotic treatments and between patients submitted to L. reuteri supplementation during and after antibiotic treatment. There was a low incidence of adverse effects in all groups of patients with sequential therapy, probably due to the presence of the L. reuteri supplementation.
CONCLUSION: The sequential treatment regimen achieved a significantly higher eradication rate of H. pylori compared with standard 7-d regimen. L. reuteri supplementation could reduce the frequency and the intensity of antibiotic-associated side-effects.
-
Citation: Efrati C, Nicolini G, Cannaviello C, O’Sed NP, Valabrega S.
Helicobacter pylori eradication: Sequential therapy andLactobacillus reuteri supplementation. World J Gastroenterol 2012; 18(43): 6250-6254 - URL: https://www.wjgnet.com/1007-9327/full/v18/i43/6250.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i43.6250
Helicobacter pylori (H. pylori) infection is a worldwide disease causing significant morbidity. At present, the role of this infection is well known, particularly in peptic ulcer disease, gastric neoplasia (mucosa-associated lymphoid tissue-lymphoma and carcinoma), non-ulcer dyspepsia (chronic gastritis) and the possible interaction with non-steroidal anti-inflammatory drugs to damage the gastric mucosa[1-3].
There are numerous treatment options for curing H. pylori infection and many are still under investigation. The eradication rate of H. pylori following 7-d triple treatment [proton pump inhibitor (PPI) plus clarithromycin and amoxicillin or metronidazole] is decreasing due to an increasing prevalence of bacterial resistance, poor patient compliance and the occurrence of antibiotic adverse effects[4]. Therefore, further approaches aimed to improve standard triple therapy efficacy should be attempted. In some large studies[5-7], a sequential regimen, i.e., simple 5-d dual (PPI plus amoxicillin) therapy followed by a 5-d triple therapy (PPI, clarithromycin and tinidazole) was more effective than 7-d triple treatment (PPI plus clarithromycin and amoxicillin or metronidazole), with few adverse effects in children, adults and elderly patients. Moreover, the Italian Working Group of the Cervia II meeting advised the use of sequential therapy as an alternative to 7-14 d triple therapy as first-line treatment[8].
As reported by the Maastricht III Consensus Report, probiotics could also play a relevant role in the management of H. pylori infection by improving treatment tolerability and increasing eradication rates[4]. Indeed, some Lactobacilli have been shown to possess antagonistic activity against H. pylori, both in vitro and in vivo[9,10]. L. reuteri ATCC 55730, a probiotic of human origin, has been demonstrated to reduce adverse effects during antibiotic therapy and to increase eradication of the H. pylori infection[11-13]. Further studies support that L. reuteri colonizes the human gastric mucosa, inhibits the binding of H. pylori to gastric epithelial cell lines and suppresses H. pylori urease activity[14-16].
The primary end-point of our prospective study was to compare the eradication rate of 7-d triple treatment with a sequential regimen. The secondary end-point was to evaluate the role of L. reuteri supplementation in H. pylori infection.
Between January 2008 to December 2009, 90 adult dyspeptic outpatients aged > 18 years were consecutively referred to our Division of Gastroenterology. One hundred and five outpatients were screened for enrolment and only 15 of these were excluded. The exclusion criteria were the following: (1) the presence, at endoscopy evaluation, of an active gastro-duodenal ulcer; (2) previous treatment for H. pylori infection; (3) PPI, H2-receptor antagonist or antibiotic treatment in the 4 wk before the study; and (4) a known allergy to the antibiotics used in the present study. No subjects had gastric malignancy at endoscopy.
Patients were enrolled if H. pylori infection was detected. H. pylori infection was diagnosed based on an upper endoscopy with gastric biopsy (2 samples from the antrum and 2 samples from the corpus) or by means of the H. pylori stool antigen-test (SAT) or the 13C urea breath test (UBT). The gold standard for H. pylori diagnosis was an upper endoscopy with multiple gastric biopsies. All but 5 patients underwent endoscopy and, in 3 of these, H. pylori status was assessed by UBT and by SAT in the remaining 2 patients.
Urea breath test: Citric acid (1.5 g) as test meal and 75 mg of 13C-urea as water solution were given to the patients after collection of a baseline sample, obtained by blowing through a disposable plastic straw into a 20 mL container, and a further breath sample was collected 30 min later. The breath samples were considered positive if there was a greater than 5 per 1000 of 13CO2 difference over baseline, according to the manufacturer’s recommendations.
H. pylori stool antigen-test:H. pylori in stool specimens was investigated by a commercial enzymatic immunoassay test (Bioscience). The enzyme immunoassay utilized for the detection of H. pylori antigens in human stool was the Premier Platinum HpSA Plus. The test utilizes monoclonal anti-H. pylori capture antibody adsorbed to microwells. Diluted patient samples and a peroxidase-conjugated polyclonal antibody were added to the wells and incubated for 1 h at room temperature. A wash was performed to remove unbound material. Substrate was added and incubated for 10 min at room temperature. Color developed in the presence of bound enzyme. Stop solution was added and the results were interpreted visually or spectrophotometrically.
The 7-d triple therapy included a PPI 20 mg bid plus clarithromycin 500 mg bid and amoxicillin 1 g bid for 7 d, while the sequential regimen consisted of 5-d dual (PPI 20 mg bid plus amoxicillin 1 g bid) therapy followed by a 5-d triple therapy (PPI 20 mg bid, clarithromicin 500 mg bid and tinidazole 500 mg bid). The PPI (lansoprazole or pantoprazole) was continued for 30 d at a dose of 20 mg daily. A Reuflor tablet (kindly provided by Italchimici, Rome) containing L. reuteri (ATCC 55730; 108 CFU) was taken once a day. Some patients received L. reuteri supplementation after the 7th or 10th day of antibiotic therapy, 12 h after administration of the last tablet of antibiotic. The other patients received the L. reuteri on the first day of antibiotic treatment.
For each therapy regimen, the PPI was prescribed 30 min before breakfast and dinner, whereas all antibiotics were given immediately after these meals.
Consecutive patients were assigned to receive one of the following therapies: (1) 7-d triple therapy plus L. reuteri supplementation during the antibiotic treatment; (2) 7-d triple therapy plus L. reuteri supplementation after the antibiotic treatment; (3) 10-d sequential regimen plus L. reuteri supplementation during the antibiotic treatment; and (4) 10-d sequential regimen plus L. reuteri supplementation after the antibiotic treatment.
In detail, the patients enrolled between 1 January 2008 and 30 November 2008 were treated with 7-d triple therapy, while those enrolled between 1 December 2008 and 31 December 2009 were treated with sequential therapy.
Informed consent was obtained from all patients enrolled in the study. The local Ethical Committee approved the study protocol.
Patients were asked to return at the end of treatment to assess the compliance with therapy and to determine possible adverse effects. Compliance was defined as consumption of > 90% of the prescribed drugs. Adverse effects were evaluated using a structured questionnaire by personal interview. Bacterial eradication was checked in all patients at least 4 wk following the eradication treatment by using UBT.
The data are expressed as mean ± SE. The statistical analysis was conducted by χ2 test. P < 0.05 was considered statistically significant.
Overall, ninety adult dyspeptic patients were enrolled in the study. All but 7 patients completed the study, with one lost to follow-up and 6 noncompliant. Therefore, 83 patients (30 male, 53 female; mean age 57 ± 13 years) completed the study. Nineteen patients were administrated a 7-d triple treatment: 11 with L. reuteri supplementation during and 8 after therapy. Sixty-four patients were administered the sequential regimen: 32 with L. reuteri supplementation during and 32 after therapy (Table 1).
| Therapy | Patients | Mean age (yr) | Male/female |
| 7-d standard triple therapy plus L. reuteri during therapy | 11 | 54 ± 8 | 6/5 |
| 7-d standard triple therapy plus L. reuteri post therapy | 8 | 59 ± 5 | 2/6 |
| 10-d sequential therapy plus L. reuteri during therapy | 32 | 57 ± 2 | 7/25 |
| 10-d sequential therapy plus L. reuteri post therapy | 32 | 60 ± 2 | 11/21 |
The eradication rate was significantly higher in the sequential group compared with the 7-d triple therapy; 88% vs 63%, P = 0.01 (Figure 1). No difference in eradication rates was observed between patients submitted to L. reuteri supplementation during or after antibiotic treatment. No difference was found between the two different types of PPI (lansoprazole or pantoprazole).
The reported compliance with therapy was excellent in all patients. All adverse effects, mainly mild diarrhea and abdominal pain, were self-limiting after the end of therapy. No difference in the number and type of adverse effects was observed between the different antibiotic regimens and between patients submitted to L. reuteri supplementation during or after antibiotic treatment.
In the last few years, with an increasing prevalence of antimicrobial resistance, the H. pylori cure rate with standard triple therapy has declined to unacceptable levels (i.e., 80% or less) in most countries. Two very large meta-analyses showed that standard 7-14 d triple therapies fail to eradicate H. pylori infection in up to 25% of patients[17,18]. More recent data have demonstrated that triple therapy with amoxicillin, clarithromycin and a PPI has an eradication rate of only 74%-76%[19]. Therefore, several tretment regimens have emerged for cure of H. pylori infection. In Italy, since the prevalence of primary clarithromycin resistance is higher than 15%[20,21], the Cervia II Working Group advised the use of 7-14 d triple therapies or a sequential therapy, as first-line treatment. To date, the efficacy of sequential therapy has been investigated in 22 trials including more than 2000 patients. The success rate of the sequential regimen was distinctly higher than that achieved by standard triple therapies[22,23]. Zullo et al[18] found that the sequential regimen was significantly superior to either 7-d and 10-d standard triple therapies with an overall eradication rate of 93.7%, 75.9% and 79.6%, respectively. These results were confirmed in 2 other recent meta-analyses[24,25]. It was known that a dual therapy (PPI plus amoxicillin) administered for less than 7 d was able to achieve a cure rate of up to 50%, and that the efficacy of a triple therapy (PPI, clarithromycin and tinidazole) was inversely related to the bacterial load, with higher eradication rates being achieved in those with a low bacterial density in the stomach. Because amoxicillin acts on the bacterial cell wall and damages it, the initial phase of treatment may prevent the development of efflux channels by weakening the cell wall of the bacterium. An important limitation is that data regarding the efficacy of the sequential regimen mainly came from Italian studies, and the use of metronidazole instead of tinidazole in different studies performed in other geographic areas could reduce the eradication rate[26,27].
The primary aim of this study was to evaluate the efficacy of sequential therapy compared with standard triple therapy. We found that the eradication rate was significantly higher in the sequential group as compared with the 7-d triple therapy group (88% vs 63%; P = 0.01). These results confirm that 10-d sequential therapy is superior to standard regimens (range: 91%-96% vs 71%-83%), as reported by systematic reviews and meta-analyses of randomized, controlled trials comparing these 2 treatments and published until October 2008[24,25,28]. Some prospective randomized Italian studies also more recently[6,29,30], confirmed these results, constantly achieving very high (range: 92%-95%) eradication rates in children, adults, and elderly patients treated with sequential therapy compared with 7-d or 10-d triple treatment (range: 74%-77%).
The secondary end-point was the assessment the role of L. reuteri on the outcome of H. pylori infection. Among probiotics, it was demonstrated that L. reuteri colonizes the human gastric mucosa, inhibits the binding of H. pylori to gastric epithelial cell lines and suppresses H. pylori urease activity[14-16]. Interestingly, in some studies[12,31], monotherapy with L. reuteri showed a reduction in the H. pylori bacterial load.
In our population, all patients were assigned to receive L.reuteri supplementation. In the sequential therapy group, the eradication rate of H. pylori with this probiotic was 88%, similar to that reported in literature. In a double-blind placebo-controlled study[16], H. pylori-positive adult subjects were given L. reuteri for 4 wk before antibiotic therapy or placebo. As with our results after the 10-d sequential regimen, the rate of H. pylori eradication in those who had received the probiotic was 88%.
In our study, in the 7-d therapy group, the eradication rate of H. pylori was 63%, greater than 53% reported by a prospective, pilot study[32] in a subgroup of patients assigned to receive the same therapy with L. reuteri supplementation.
In our population, it a low incidence of adverse effects was observed in all groups of patients with sequential therapy, probably due to the presence of L. reuteri.
In conclusion, sequential therapy appears to be more effective than standard 7-d triple therapy, and L. reuteri supplementation could play a role in the eradication of H. pylori, but a large, double-blind, controlled study is needed to confirm these results and to explain the exact function of L. reuteri. Indeed, it may improve the tolerability to antibiotic therapy by decreasing adverse effects or play a primary role in the eradication of H. pylori infection.
Reuflor was kindly provided by Italchimici (Rome).
The Helicobacter pylori (H. pylori) cure rate following standard triple therapies is decreasing worldwide. The need for alternative treatment strategies for H. pylori infections has created an interest in control of this pathogen with further antibiotic protocols and probiotics.
In some large studies, the sequential regimen, i.e., simple 5-d dual [proton pump inhibitor (PPI) plus amoxicillin] therapy followed by a 5-d triple therapy (PPI, clarithromycin and tinidazole), is more effective than 7-d triple treatment (PPI plus clarithromycin and amoxicillin or metronidazole). Lactobacillus reuteri (L. reuteri) ATCC 55730, a probiotic of human origin, has been demonstrated to reduce adverse effects during antibiotic therapy and to increase eradication of the H. pylori infection. Further studies support that L. reuteri colonizes the human gastric mucosa, inhibits the binding of H. pylori to gastric epithelial cell lines and suppresses H. pylori urease activity.
The H. pylori eradication rate was significantly higher in the sequential group compared with 7-d triple therapy. These results confirmed that 10-d sequential therapy is superior to standard regimens, as reported by systematic reviews and meta-analyses of randomized trials. Moreover, L. reuteri supplementation could play a role in the eradication of H. pylori and improve the tolerability to antibiotic therapy, despite conflicting results in previous studies.
Sequential therapy appears to be more effective than standard 7-d therapy and is a well-tolerated, promising therapy and should be recommended as first-line treatment. L. reuteri supplementation could play a role in the eradication of H. pylori, but a large, double-blind, controlled study is needed to confirm these results and to explain its exact function. It may improve the tolerability to the antibiotic therapy or play a primary role in reducing H. pylori infection.
The authors investigated standard triple drug therapy treatment of H. pylori infection of human patients versus sequential therapy, with all therapies also including L. reuteri probiotic. In 83 patients, they found that eradication rates were higher in the sequential therapy group over that of the standard triple therapy group.
Peer reviewer: David J McGee, PhD, Associate Professor, Department of Microbiology and Immunology, Louisiana State University Health Sciences Center-Shreveport, 1501 Kings Highway, Shreveport, LA 71130, United States
S- Editor Gou SX L- Editor Cant MR E- Editor Xiong L
| 1. | Nardone G. Risk factors for cancer development in Helicobacter pylori gastritis. Dig Liver Dis. 2000;32 Suppl 3:S190-S192. [PubMed] |
| 2. | Stolte M, Bayerdörffer E, Morgner A, Alpen B, Wündisch T, Thiede C, Neubauer A. Helicobacter and gastric MALT lymphoma. Gut. 2002;50 Suppl 3:III19-III24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 125] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Fiorucci S, Romano M. Helicobacter pylori and non-steroidal anti-inflammatory drugs. Dig Liver Dis. 2000;32 Suppl 3:S211-S213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1352] [Article Influence: 75.1] [Reference Citation Analysis (1)] |
| 5. | Zullo A, Vaira D, Vakil N, Hassan C, Gatta L, Ricci C, De Francesco V, Menegatti M, Tampieri A, Perna F. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther. 2003;17:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 166] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | De Francesco V, Zullo A, Hassan C, Della Valle N, Pietrini L, Minenna MF, Winn S, Monno R, Stoppino V, Morini S. The prolongation of triple therapy for Helicobacter pylori does not allow reaching therapeutic outcome of sequential scheme: a prospective, randomised study. Dig Liver Dis. 2004;36:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Scaccianoce G, Hassan C, Panarese A, Piglionica D, Morini S, Zullo A. Helicobacter pylori eradication with either 7-day or 10-day triple therapies, and with a 10-day sequential regimen. Can J Gastroenterol. 2006;20:113-117. [PubMed] |
| 8. | Caselli M, Zullo A, Maconi G, Parente F, Alvisi V, Casetti T, Sorrentino D, Gasbarrini G. “Cervia II Working Group Report 2006”: guidelines on diagnosis and treatment of Helicobacter pylori infection in Italy. Dig Liver Dis. 2007;39:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Gotteland M, Brunser O, Cruchet S. Systematic review: are probiotics useful in controlling gastric colonization by Helicobacter pylori? Aliment Pharmacol Ther. 2006;23:1077-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Hamilton-Miller JM. The role of probiotics in the treatment and prevention of Helicobacter pylori infection. Int J Antimicrob Agents. 2003;22:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Imase K, Tanaka A, Tokunaga K, Sugano H, Ishida H, Takahashi S. Lactobacillus reuteri tablets suppress Helicobacter pylori infection--a double-blind randomised placebo-controlled cross-over clinical study. Kansenshogaku Zasshi. 2007;81:387-393. [PubMed] |
| 12. | Saggioro A. Caroli M, Pasini M, Bortoluzzi F, Girardi L, Pilone G. Helicobacter pylori eradication with Lactobacillus reuteri. A double-blind placebo-controlled study. Dig Liver Dis. 2005;37 Supple 1:A 88. |
| 13. | Lionetti E, Miniello VL, Castellaneta SP, Magistá AM, de Canio A, Maurogiovanni G, Ierardi E, Cavallo L, Francavilla R. Lactobacillus reuteri therapy to reduce side-effects during anti-Helicobacter pylori treatment in children: a randomized placebo controlled trial. Aliment Pharmacol Ther. 2006;24:1461-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Mukai T, Asasaka T, Sato E, Mori K, Matsumoto M, Ohori H. Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri. FEMS Immunol Med Microbiol. 2002;32:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 164] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Valeur N, Engel P, Carbajal N, Connolly E, Ladefoged K. Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Appl Environ Microbiol. 2004;70:1176-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 245] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 16. | Francavilla R, Lionetti E, Castellaneta SP, Magistà AM, Maurogiovanni G, Bucci N, De Canio A, Indrio F, Cavallo L, Ierardi E. Inhibition of Helicobacter pylori infection in humans by Lactobacillus reuteri ATCC 55730 and effect on eradication therapy: a pilot study. Helicobacter. 2008;13:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Fuccio L, Minardi ME, Zagari RM, Grilli D, Magrini N, Bazzoli F. Meta-analysis: duration of first-line proton-pump inhibitor based triple therapy for Helicobacter pylori eradication. Ann Intern Med. 2007;147:553-562. [PubMed] |
| 18. | Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007;56:1353-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 211] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 19. | Buzás GM. First-line eradication of Helicobacter pylori: are the standard triple therapies obsolete? A different perspective. World J Gastroenterol. 2010;16:3865-3870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (2)] |
| 20. | de Francesco V, Margiotta M, Zullo A, Hassan C, Valle ND, Burattini O, Cea U, Stoppino G, Amoruso A, Stella F. Primary clarithromycin resistance in Italy assessed on Helicobacter pylori DNA sequences by TaqMan real-time polymerase chain reaction. Aliment Pharmacol Ther. 2006;23:429-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Toracchio S, Marzio L. Primary and secondary antibiotic resistance of Helicobacter pylori strains isolated in central Italy during the years 1998-2002. Dig Liver Dis. 2003;35:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Marshall B. Sequential therapy for Helicobacter pylori: a worthwhile effort for your patients. Ann Intern Med. 2008;148:962-963. [PubMed] |
| 23. | Moayyedi P. Sequential regimens for Helicobacter pylori eradication. Lancet. 2007;370:1010-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923-931. [PubMed] |
| 25. | Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009;104:3069-379; quiz 1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 219] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 26. | Murakami K, Fujioka T, Okimoto T, Sato R, Kodama M, Nasu M. Drug combinations with amoxycillin reduce selection of clarithromycin resistance during Helicobacter pylori eradication therapy. Int J Antimicrob Agents. 2002;19:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | De Francesco V, Margiotta M, Zullo A, Hassan C, Troiani L, Burattini O, Stella F, Di Leo A, Russo F, Marangi S. Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. Ann Intern Med. 2006;144:94-100. [PubMed] |
| 28. | Tong JL, Ran ZH, Shen J, Xiao SD. Sequential therapy vs. standard triple therapies for Helicobacter pylori infection: a meta-analysis. J Clin Pharm Ther. 2009;34:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Zullo A, Gatta L, De Francesco V, Hassan C, Ricci C, Bernabucci V, Cavina M, Ierardi E, Morini S, Vaira D. High rate of Helicobacter pylori eradication with sequential therapy in elderly patients with peptic ulcer: a prospective controlled study. Aliment Pharmacol Ther. 2005;21:1419-1424. [PubMed] |
| 30. | Urgesi R, Pelecca G, Cianci R, Masini A, Zampaletta C, Riccioni ME, Faggiani R. Helicobacter pylori infection: is sequential therapy superior to standard triple therapy? A single-centre Italian study in treatment-naive and non-treatment-naive patients. Can J Gastroenterol. 2011;25:315-318. [PubMed] |
| 31. | Tummala S, Sheth SG, Goldsmith JD, Goldar-Najafi A, Murphy CK, Osburne MS, Mullin S, Buxton D, Wagner DA, Kelly CP. Quantifying gastric Helicobacter pylori infection: a comparison of quantitative culture, urease breath testing, and histology. Dig Dis Sci. 2007;52:396-401. [PubMed] |
| 32. | Scaccianoce G, Zullo A, Hassan C, Gentili F, Cristofari F, Cardinale V, Gigliotti F, Piglionica D, Morini S. Triple therapies plus different probiotics for Helicobacter pylori eradication. Eur Rev Med Pharmacol Sci. 2008;12:251-256. [PubMed] |