Published online Nov 7, 2012. doi: 10.3748/wjg.v18.i41.5986
Revised: August 17, 2012
Accepted: August 25, 2012
Published online: November 7, 2012
This report presents a case of massive mucosal necrosis of the small intestine in a patient with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS), which particularly affects the brain, nervous system and muscles. A 45-year-old Japanese female, with an established diagnosis of MELAS, presented with vomiting. Computed tomography showed portomesenteric venous gas and pneumatosis intestinalis. She underwent a resection of the small intestine. A microscopic study showed necrosis of the mucosa and vacuolar degeneration of smooth muscle cells in the arterial wall. Immunohistochemistry showed anti-mitochondrial antibody to be highly expressed in the crypts adjacent the necrotic mucosa. The microscopic and immunohistochemical findings suggested the presence of a large number of abnormal mitochondria in MELAS to be closely linked to mucosal necrosis of the small intestine.
- Citation: Fukuyama K, Ishikawa Y, Ogino T, Inoue H, Yamaoka R, Hirose T, Nishihira T. Mucosal necrosis of the small intestine in myopathy, encephalopathy, lactic acidosis, and stroke-like episodes syndrome. World J Gastroenterol 2012; 18(41): 5986-5989
- URL: https://www.wjgnet.com/1007-9327/full/v18/i41/5986.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i41.5986
Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) is one of a family of mitochondrial cytopathies[1]. MELAS particularly affects the brain and nervous system and muscles. Gastrointestinal symptoms, such as nausea, vomiting and anorexia, are also common[2]. Although scattered focal necrosis is sporadically seen[3], massive intestinal necrosis in MELAS is rare. Only two cases of massive large intestinal necrosis in MELAS have been reported to date[4,5]. This report presents the case of a MELAS patient with massive mucosal necrosis of the small intestine. This is the first case report of MELAS with massive mucosal necrosis of the small intestine.
A 45-year-old Japanese female presented with vomiting. Abdominal computed tomography (CT) showed portomesenteric venous gas; therefore she was referred to this hospital for further investigation and treatment.
Her medical history revealed that she presented with progressive sensorineural hearing loss at 24 years of age. In addition, she experienced headaches, seizures, homonymous hemianopsia, right hemi paresis, limb weakness and a stroke-like episode at 43 years of age. Her lactic acid and pyruvate level were 112.4 mg/dL (normal < 17 mg/dL) and 2.16 mg/dL (normal < 0.94 mg/dL), respectively. She did not undergo a muscle biopsy. However, the mitochondrial mutation A3243G was detected in her son. A diagnosis of MELAS was therefore established because MELAS is transmitted by maternal inheritance[2,6].
Her height and body weight were 143 cm, and 26.6 kg, respectively, at the time of admission. Physical examinations revealed abdominal distension. Guarding, rebound tenderness or rigidity could not be estimated properly due to the presence of generalized muscle atrophy and difficulties in communicating with the patient. She was in a state of septic shock. Her heart rate and blood pressure were 88 beats/min and 73 mmHg/31 mmHg, respectively. A semi-quantitative measurement showed her procalcitonin level to be ≥ 10 ng/mL.
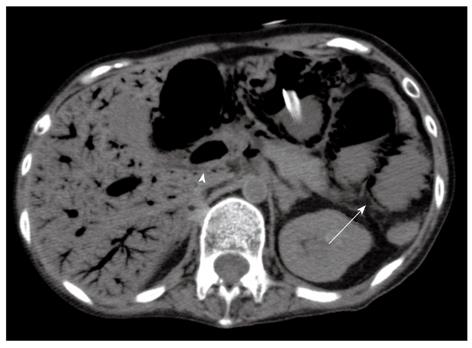
CT showed a massive amount of portomesenteric venous gas and pneumatosis intestinalis involving the duodenum, jejunum and ileum (Figure 1). An emergency operation was indicated. Mucosal necrosis from the duodenum to the ileum was recognized transluminal from the serosal. The pulse of the superior mesenteric artery and the vasa recta was palpable. She underwent a massive resection of the small intestine from 10 cm distal to the Treitz ligament to 30 cm proximal to the ileocecal valve, followed by jejunostomy and ileostomy, as damage control surgery because her vital signs indicated the patient to be in a state of shock.
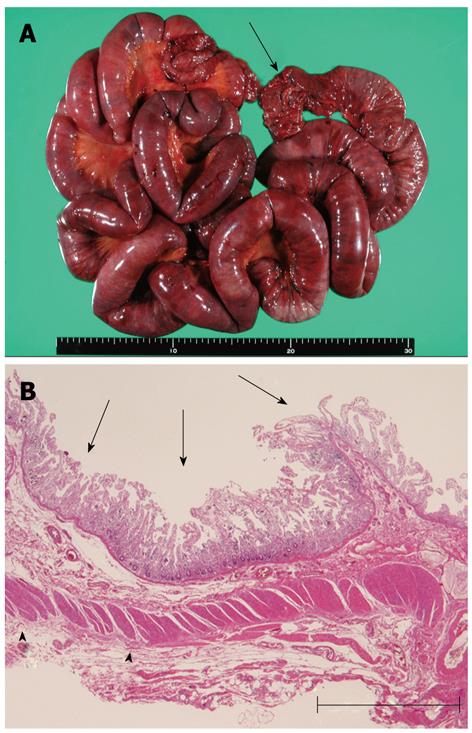
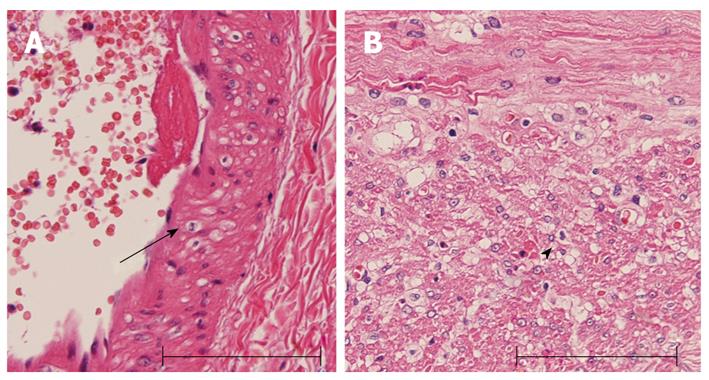
The specimen was 150 cm in length (Figure 2A). Macroscopic observation revealed diffuse mucosal necrosis in two thirds of oral-sided area, and mottled necrosis in the remaining area. The microscopic analysis revealed coagulation necrosis of the mucosa and the lamina propria (Figure 2B). The external longitudinal layer of muscularis propria was also degenerated and partially diminished (Figure 2B). Vacuolar degeneration of smooth muscle cells was observed in the arterial wall and the muscular layer of the intestinal wall (Figure 3).
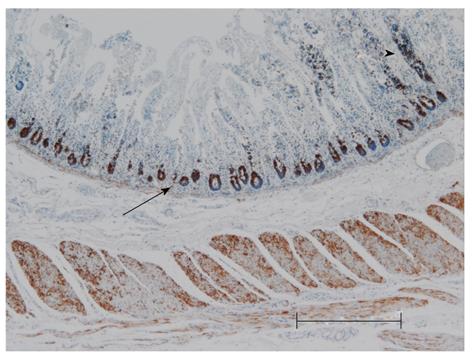
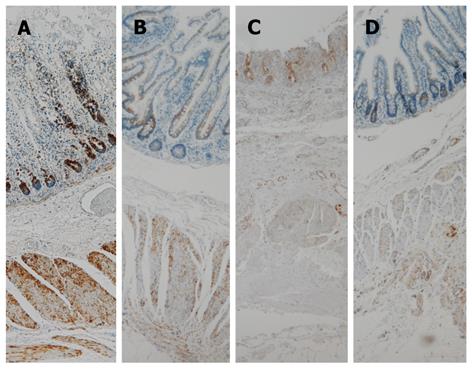
Anti-mitochondrial antibody (AMA), ab92824, was highly expressed in the crypt and slightly in the remaining mucosa that was observed in the necrotic area (Figure 4). On the contrary, AMA expression in the crypts was moderate in the non-necrotic area. The resected strangulated ileum of a non-MELAS patient stored in the pathological department was used as a control. This sample showed moderate AMA expression in the crypt in comparison to that of the current patient (Figure 5; Table 1). Expression patterns of AMA in both specimens are shown in Table 1.
| MELAS patient(in this case) | Non-MELAS patient (in our control) | |||
| Necroticlesion | Non-necrotic lesion | Necrotic lesion | Non-necrotic lesion | |
| Expression of AMA | ||||
| Mucosa | ||||
| Villi | Diminished/+++ | + | Diminished | + |
| Crypt | +++ | +/- | + | + |
| Lamina propria | +/- | +/- | +/- | +/- |
| Muscularis mucosa | + | + | +/- | +/- |
| Submucosa | +/- | +/- | +/- | +/- |
| Muscular layer | ||||
| Inner circular | ++ | ++ | + | + |
| External longitude | ++ | ++ | + | + |
A series of antibiotics were administered based on culture evaluations in order to control infection. She required support for renal insufficiency with continuous hemodiafiltration for a 3-d period after surgery; blood pressure support with catecholamine administration, mechanical ventilation support for a 3-wk period after surgery. The patient’s condition eventually recovered along with the restoration of the necrotic mucosa, based on observations of the oral stoma and thereafter she was discharged. Home parenteral nutrition was indicated because intestinal continuity had not been reestablished in consideration of her general condition. She died of severe metabolic disorder due to MELAS 3 mo after discharge.
MELAS is a rare type of metabolic disorder causing multi-organ disorders, such as brain ischemia and the degeneration of skeletal muscle. MELAS is caused by mutations in mitochondrial DNA encoding transfer RNALEU(UUA/UUG) and it is transmitted by maternal inheritance[2,6]. Though MELAS particularly affects the brain, nervous system and muscles, the occurrence of massive intestinal necrosis due to MELAS is rare.
Many MELAS symptoms caused by mitochondrial A4243G mutation are thought to depend on the mutation load and the tissue distribution of abnormal mitochondria[7]. Therefore, an immunohistochemical study was conducted using AMA, which is a highly sensitive and specific method for identifying the mitochondria[8], to investigate the relationship between mucosal necrosis and the distribution of mitochondria. This method is applied when the distribution of mitochondria cannot be determined by electron microscopy because the tissue was fixed for light microscopy, as in the present case[9]. Though an increase in the number of mitochondria does not always indicate the presence of abnormal mitochondria, an increase in the number of mitochondria is more likely to be a sign of mitochondrial disease and aggregation of large mitochondria a characteristic ultrastructural finding in mitochondrial disease[10,11]. The current patient showed a marked expression of AMA in the crypts, where adjacent villi had almost completely disappeared (Figure 4; Table 1). A marked expression was also observed in the remaining mucosa in the necrotic area. No such marked expression was observed in the crypts of non-necrotic areas in the present case or a necrotic area in the non-MELAS specimen (Figure 5; Table 1). These findings suggest that an increase in the number of mitochondria, which is likely abnormal, therefore, may contribute to mucosal necrosis. The mucosa containing a higher number of abnormal mitochondria may be vulnerable to ischemia.
Microscopic examination revealed vacuolation of the smooth muscle cells in the small arteries in the current case (Figure 3). However, there was no narrowing of the small arteries. An electron microscopic study of cerebral vessels of patients with MELAS showed a striking increase in the number of mitochondria in the smooth muscle and endothelial cells causing the vascular changes[12]. The increase is most prominent in arterioles and small arteries. These MELAS-related vascular changes are likely to attribute to a decrease in blood flow[5]. Therefore, the current patient probably experienced transient non-occlusive ischemia, such as a vasospasm that eventually triggered mucosal necrosis.
In addition to the mucosa, the muscularis mucosa, and inner circular and external longitudinal layer of the muscularis propria were also affected in the present case. Therefore, MELAS may affect any layer of the small intestine.
In conclusion, the presence of abundant abnormal mitochondria may be closely linked to necrosis in the intestinal mucosa, and MELAS-related vascular changes may therefore be a prerequisite for necrosis.
We thank KYODO BYORI. Inc. for the immunohistochemical study using AMA.
Peer reviewers: Dr. José Liberato Ferreira Caboclo, Professor, Rua Antônio de Godoy, São José do Rio Preto 4120, Brazil; Beata Jolanta Jablońska, MD, PhD, Department of Digestive Tract Surgery, University Hospital of Medical University of Silesia, Medyków 14 St., 40-752 Katowice, Poland
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Pavlakis SG, Phillips PC, DiMauro S, De Vivo DC, Rowland LP. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann Neurol. 1984;16:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 763] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 2. | DiMauro S, Hirano M. MELAS. SourceGeneReviews™. Seattle (WA): University of Washington 2010; . [PubMed] |
| 3. | Kuroiwa T, Kuwata T, Nakayama T, Takemura T, Sakuta M, Ichinose S, Goto Y, Okeda R. Mitochondrial encephalomyopathy showing prominent microvacuolation and necrosis of intestinal smooth muscle cells: a case diagnosed by rectal biopsy. Acta Neuropathol. 1998;96:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Hess J, Burkhard P, Morris M, Lalioti M, Myers P, Hadengue A. Ischaemic colitis due to mitochondrial cytopathy. Lancet. 1995;346:189-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Jingu A, Suzuki T, Ishiyama T, Matsumoto H, Yamamoto T, Hasegawa K. A case of mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes with severe mucosal necrosis of the sigmoid colon. J Jpn Surg Assoc. 2011;72:121-125. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Thambisetty M, Newman NJ. Diagnosis and management of MELAS. Expert Rev Mol Diagn. 2004;4:631-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Jeppesen TD, Schwartz M, Frederiksen AL, Wibrand F, Olsen DB, Vissing J. Muscle phenotype and mutation load in 51 persons with the 3243A& gt; G mitochondrial DNA mutation. Arch Neurol. 2006;63:1701-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Shintaku M, Honda T. Identification of oncocytic lesions of salivary glands by anti-mitochondrial immunohistochemistry. Histopathology. 1997;31:408-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Mizutari K, Naganishi H, Tanaka Y. Oncocytic carcinoma in the submandibular gland: report of a case based on anti-mitochondrial immunohistochemical observations. Auris Nasus Larynx. 2005;32:305-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Koenig MK. Presentation and diagnosis of mitochondrial disorders in children. Pediatr Neurol. 2008;38:305-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | DiMauro S, Bonilla E, De Vivo DC. Does the patient have a mitochondrial encephalomyopathy? J Child Neurol. 1999;14 Suppl 1:S23-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Ohama E, Ohara S, Ikuta F, Tanaka K, Nishizawa M, Miyatake T. Mitochondrial angiopathy in cerebral blood vessels of mitochondrial encephalomyopathy. Acta Neuropathol. 1987;74:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 198] [Article Influence: 5.2] [Reference Citation Analysis (0)] |