Published online Nov 7, 2012. doi: 10.3748/wjg.v18.i41.5932
Revised: March 30, 2012
Accepted: April 13, 2012
Published online: November 7, 2012
AIM: To analyze small intestinal bacterial overgrowth in school-aged children and the relationship between hydrogen and methane production in breath tests.
METHODS: This transversal study included 85 children residing in a slum and 43 children from a private school, all aged between 6 and 10 years, in Osasco, Brazil. For characterization of the groups, data regarding the socioeconomic status and basic housing sanitary conditions were collected. Anthropometric data was obtained in children from both groups. All children completed the hydrogen (H2) and methane (CH4) breath test in order to assess small intestinal bacterial overgrowth (SIBO). SIBO was diagnosed when there was an increase in H2≥ 20 ppm or CH4≥ 10 ppm with regard to the fasting value until 60 min after lactulose ingestion.
RESULTS: Children from the slum group had worse living conditions and lower nutritional indices than children from the private school. SIBO was found in 30.9% (26/84) of the children from the slum group and in 2.4% (1/41) from the private school group (P = 0.0007). Greater hydrogen production in the small intestine was observed in children from the slum group when compared to children from the private school (P = 0.007). A higher concentration of hydrogen in the small intestine (P < 0.001) and in the colon (P < 0.001) was observed among the children from the slum group with SIBO when compared to children from the slum group without SIBO. Methane production was observed in 63.1% (53/84) of the children from the slum group and in 19.5% (8/41) of the children from the private school group (P < 0.0001). Methane production was observed in 38/58 (65.5%) of the children without SIBO and in 15/26 (57.7%) of the children with SIBO from the slum. Colonic production of hydrogen was lower in methane-producing children (P = 0.017).
CONCLUSION: Children who live in inadequate environmental conditions are at risk of bacterial overgrowth and methane production. Hydrogen is a substrate for methane production in the colon.
- Citation: Mello CS, Tahan S, Melli LCF, Rodrigues MSDC, Mello RMP, Scaletsky ICA, Morais MB. Methane production and small intestinal bacterial overgrowth in children living in a slum. World J Gastroenterol 2012; 18(41): 5932-5939
- URL: https://www.wjgnet.com/1007-9327/full/v18/i41/5932.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i41.5932
Recent studies have identified small intestinal bacterial overgrowth (SIBO) as being involved in several clinical conditions, such as chronic constipation[1], irritable bowel syndrome[2,3] and short bowel syndrome[4]. SIBO is also present in tropical/environmental enteropathy[5,6].
SIBO is favored by environmental contamination and inadequate basic sanitation conditions[7], and is often the result of ingesting food and/or water that is not appropriate for consumption[8]. Thus, an excessive quantity of bacteria colonizes the upper intestinal tract, causing diarrhea[9,10] and malnutrition[5,10] due to nutrient malabsorption[9,10]. A reduction in the absorption of carbohydrates, proteins, lipids and some vitamins can be observed, even in asymptomatic patients[11]. Therefore, environmental enteropathy is a condition that may compromise child growth[12].
The breath test is a noninvasive method that has been widely used in the diagnosis of SIBO[10]. The breath test measures the production of hydrogen derived from the fermentation of lactulose by intestinal bacteria[5,7,9,13,14]. In addition to hydrogen, methane may also be detected in the exhaled breath during the test. However, the significance of methane in the diagnosis of bacterial overgrowth in the small intestine is still controversial, and in general, it is not considered in the interpretation of the breath test[5,7,13]. However, some studies[1,2,9,14,15] have suggested that methane is also an indicator of bacterial overgrowth in the small intestine. A universal criterion for breath test interpretation has not been proposed.
The design of the present study was based on the fact that no other community study had used both hydrogen and methane as SIBO diagnostic criteria. In addition, the relationship between methane production, environmental conditions and the presence of SIBO was considered.
Considering the above rationale, the aim of the present study was to analyze SIBO using the lactulose hydrogen and methane breath test in school-aged children from two distinct socioeconomic strata, and to assess the relationship between intestinal production of hydrogen and methane, environmental conditions and SIBO.
A transversal study was performed in the municipality of Osasco, in the state of Sao Paulo, Brazil. The study participants consisted of two groups of children belonging to distinct socioeconomic strata.
The sample size was calculated taking into account a power of 80% and an alpha of 5%. Two aspects of the study were considered: (1) The prevalence of SIBO in the slum community was the first parameter determined. The population in this community included 1220 children aged between 6 and 10 years. The expected prevalence of SIBO was 35%[5], with a maximum variation of 10%. Epi Info 3.4.3 software was used which indicated that 82 children in the slum group were needed to define the prevalence of SIBO; and (2) For comparison with the slum group, the necessary sample size of the private school group was estimated taking into account an expected prevalence of 35% for SIBO in the slum group and of 6% in the private school group[5]. According to Epi Info 3.4.3, each group should have at least 36 children.
The first group was composed of children whose families lived in poor socioeconomic conditions and who resided in a slum in the vicinity of a landfill without adequate housing or basic sanitation conditions. To obtain a representative sample of the population, the children were selected at random by performing a survey of households in the slum area. Of the 100 children randomly selected, 85 agreed to participate in the study.
The second group was composed of children from a private school who belonged to socioeconomically advantaged families and had satisfactory housing and basic sanitation conditions. Of the 60 children whose parents or guardians showed an interest in participating in the study, 43 (71.6%) completed the study.
To be included in the study, the children had to be between 6 and 10 years of age and not have suffered from diarrhea for at least 30 d. The presence of clinical evidence that could mark a serious illness, such as heart disease, nephropathy, or neuropathy, was a criterion for exclusion from participation in the study. In addition, the use of antibiotics up to 15 d before the breath test was considered a criterion for exclusion from the study.
This project was evaluated and approved by the Research Ethics Committee of the Federal University of Sao Paulo. A signed informed consent form was obtained from the parents or guardians of each participant at the time of admission into the study.
A questionnaire was given to the parents and/or guardians of the children who participated in the study. Information regarding the presence of a water supply, a sewage system (destination of waste) and garbage collection was obtained.
An analysis of the water used for household consumption by the children in the slum group was performed. To determine the levels of total and fecal coliforms, the water was stored in 100 mL sterile plastic bags containing a sodium thiosulfate tablet, which neutralizes the action of chlorine used in water treatment. The samples were transported and then incubated at 37 °C for 18 and 24 h, respectively, on the same day as the material was collected. Subsequently, the samples were analyzed using the Colilert Substrate method (IDEXX Laboratories, Maine, United States), which enumerates the levels of total and fecal coliforms (Escherichia coli) simultaneously in the same water sample, according to the manufacturer’s instructions.
The weight and height of the children participating in the study were measured according to the recommendations of Jelliffe[16]. To measure weight, a mechanical balance with a capacity of 150 kg and a sensitivity of 100 g was used. For height measurements, a portable vertical anthropometer with the capacity to measure up to 190 cm and a sensitivity of 0.1 cm was used.
Z-scores were calculated for weight-for-age, height-for-age, and body mass index (BMI), with adjustments for age and sex[17]. The anthropometric indicators were calculated using the Epi Info version 3.4.3 program and the reference values from the National Center for Health Statistics[18].
The lactulose breath test was performed in the morning following oral hygiene using 0.05% chlorhexidine. The children fasted for a period of 12 h prior to the test.
Breath samples were collected using a non-rebreathing valve setup (QuinTron Instrument Co. Inc., Menomonee Falls, Wisconsin, United States). After collection of the fasting breath, 10 g of lactulose (Daiichi Sankyo, Sao Paulo, Brazil) was administered orally as a 10% aqueous solution. Subsequently, new breath samples were collected 15, 30, 45, 60, 90, 120, and 180 min after ingestion of lactulose.
The levels of hydrogen (H2) and methane (CH4) in the samples were simultaneously measured by gas chromatography using a 12i model QuinTron MicroLyzer unit (QuinTron Instrument Company, Milwaukee, Wisconsin, United States). Study participants were considered to exhibit SIBO when an increase in H2≥ 20 ppm or CH4≥ 10 ppm with respect to the fasting value was observed up to 60 min after the ingestion of lactulose[1]. The study participants were treated as methane producers when the concentration of methane in the breath was higher than (or equal to) 3 ppm with respect to the concentration of methane in the environment[19].
Information was also collected on the intestinal habits of the children from both groups, taking into account the existence of an association between the production of methane and evacuation disorders.
For data analysis, Sigma Stat 3.5 and Epi Info 3.4.3 software were used, setting 5% as the level to reject the null hypothesis.
Of the children studied, 85 were living in a slum and 43 were enrolled in a private school. Table 1 describes the demographic data, nutritional status indicators, and basic sanitation conditions of the studied population. There were no statistical differences in age or gender between the children in the two groups. The children in the slum group exhibited lower Z-score values for weight-for-age, height-for-age, and BMI when compared with children in the private school group. It was found that the majority of families in the slum did not have access to public service sewage or household garbage collection. Clandestine water supplies existed in 41.2% (35/85) of the households, and water analysis revealed the presence of total coliforms in 65 (77.4%) and fecal coliforms in 43 (51.2%) of the 84 samples analyzed.
| Slum(n = 85) | Private school(n = 43) | P value | |
| Age (yr) | 8.2 ± 1.4 | 8.4 ± 1.3 | 0.5321 |
| Gender (%) | |||
| Male | 48 (56.5) | 37 (43.5) | 0.0612 |
| Female | 16 (37.2) | 27 (62.8) | |
| Z-score | |||
| Age-weight | -0.56 ± 1.03 | 0.57 ± 1.18 | < 0.0011 |
| Age-height | -0.31 ± 1.01 | 0.29 ± 1.14 | 0.0031 |
| BMI | -0.58 ± 1.06 | 0.56 ± 1.25 | < 0.0011 |
| Access to public water network (%) | 50 (58.8) | 43 (100.0) | < 0.00012 |
| Access to public sewage (%) | 8 (9.4) | 43 (100.0) | < 0.00012 |
| Public collection of household garbage (%) | 2 (2.4) | 43 (100.0) | < 0.00012 |
During the study, three children did not perform the breath test; one child was from the slum group, and two children were from the private school group. SIBO was found in 30.9% (26/84) of the children in the slum group and in 2.4% (1/41) of the children in the private school group (χ2 test, P = 0.0007). In the slum group, 65.4% (17/26) of the children with SIBO had increased hydrogen production with respect to the fasting value (H2≥ 20 ppm), 23.1% (6/26) had increased methane concentrations with respect to the fasting value (CH4≥ 10 ppm) and 11.5% (3/26) fulfilled both criteria for SIBO. In the private school group, the one child with SIBO fulfilled only the hydrogen criterion.
The demographic data, nutritional status indicators, and basic sanitation conditions of the children from the slum group with or without SIBO are shown in Table 2. No statistically significant differences for any of the variables analyzed (P > 0.05) were observed. It is important to emphasize that all the cases of SIBO were asymptomatic.
| With SIBO (n = 26) | Without SIBO (n = 58) | P value | |
| Age (yr) | 8.3 ± 1.2 | 8.2 ± 1.5 | 0.8171 |
| Z-score | |||
| Age-weight | -0.76 ± 1.05 | -0.46 ± 1.02 | 0.2131 |
| Age-height | -0.38 ± 0.93 | -0.28 ± 1.07 | 0.6691 |
| BMI | -0.80 ± 1.06 | -0.46 ± 1.05 | 0.1761 |
| Water contamination (%) | |||
| Fecal coliforms | 21/26 (80.8) | 44/574 (77.2) | 0.5942 |
| Total coliforms | 13/26 (50.0) | 30/574 (52.6) | 0.9412 |
| Access to public water network (%) | 13 (50.0) | 37 (63.8) | 0.2362 |
| Access to public sewage (%) | 3 (11.5) | 5 (8.6) | 0.4743 |
| Public collection of household garbage (%) | 0 (0.0) | 2 (3.4) | 0.4743 |
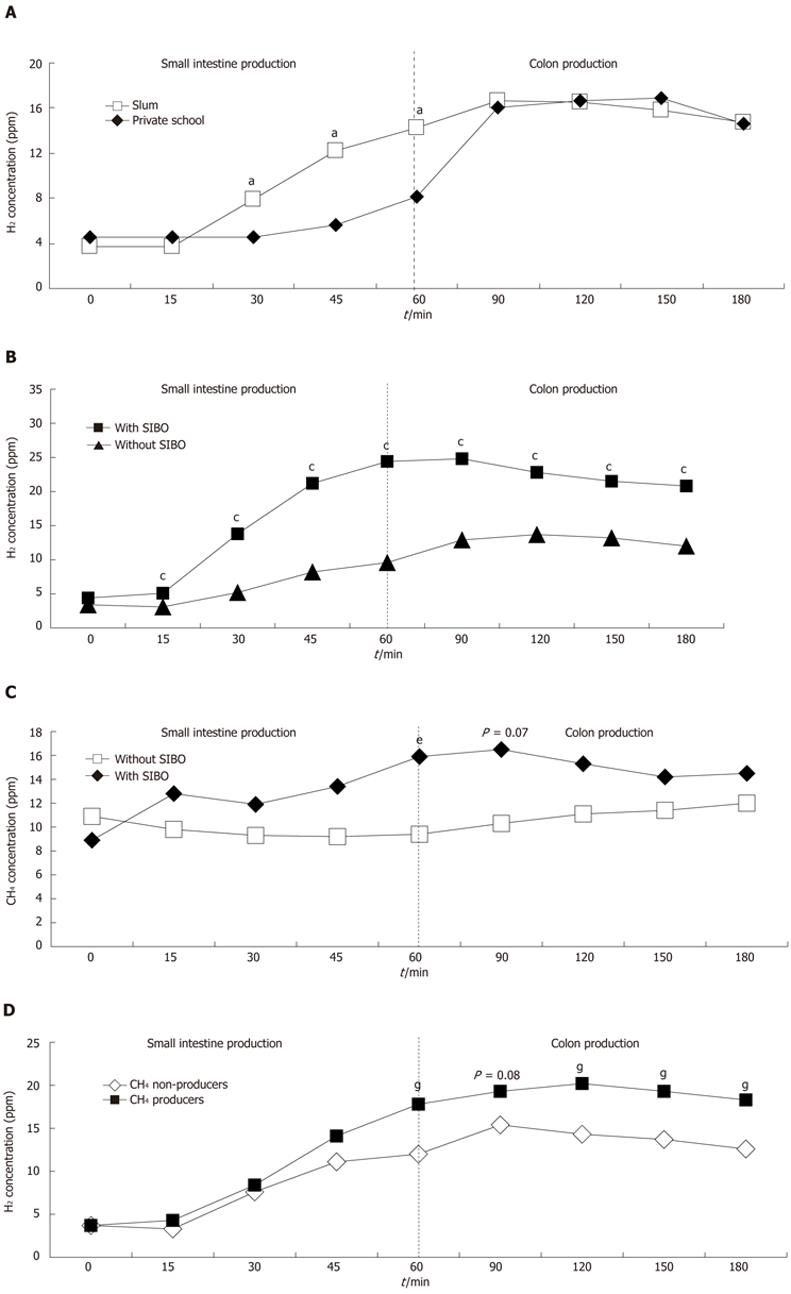
The hydrogen concentrations (ppm) obtained by the lactulose breath test were analyzed for the areas under the individual curves. It was found that the children in the slum group exhibited greater (Student’s t-test, P = 0.007) hydrogen production during the first hour of the test, which presumably originated from the small intestine, when compared with the children in the private school group (491.16 ± 369.05 ppm vs 314.45 ± 251.49 ppm per min, respectively). Between 60 min and 180 min of the test, the period during which hydrogen production occurs predominantly in the large intestine, the concentration of hydrogen in the breath of the children in the slum and private school groups were similar (4363.93 ± 1045.63 ppm vs 4275.0 ± 1390.55 ppm per min, respectively, P = 0.690) (Figure 1A).
Figure 1B shows the mean hydrogen concentrations (ppm) obtained from the breath tests of children with and without bacterial overgrowth in the slum group. A greater area under the curve for the small intestine was observed among the 26 children with SIBO compared with the 58 children without SIBO up to 60 min after the ingestion of lactulose (344.22 ± 185.23 ppm vs 818.94 ± 460.55 ppm per min; Student’s t-test, P < 0.001). A similar response was observed for the colon during the 60 to 180 min of the test (4021.03 ± 711.73 ppm vs 5128.85 ± 1262.40 ppm per min; Student’s t-test, P < 0.001).
Methane production was observed in 63.1% (53/84) of the children in the slum group and in 19.5% (8/41) of the children in the private school group (χ2 test, P < 0.0001). The mean methane concentration remained relatively constant in both groups during the breath test and did not vary after lactulose ingestion. Among the 8 children in the private school group who were methane producers, 3 (37.5%) had intestinal constipation without fecal incontinence. There were no cases of constipation among the 53 children in the slum group who were methane producers (χ2 test, P < 0.0001).
Figure 1C shows the mean methane concentrations (ppm) obtained from the breath tests of children with and without bacterial overgrowth in the slum group. In the small intestine, differences in the area under the curve were not observed among the 26 children with SIBO in relation to the 58 children without SIBO up to 60 min after the ingestion of lactulose (730.96 ± 829.56 ppm vs 576.72 ± 573.72 ppm per min; Student’s t-test, P = 0.327). In addition, no significant difference was observed in the area under the curve for the colon during the 60 min to 180 min of the test (3835.34 ± 1159.71 vs 4324.04 ± 2053.62 ppm per min; Student’s t-test, P = 0.168).
Methane production was observed in 38 (65.5%) of the 58 children who did not exhibit bacterial overgrowth and in 15 (57.7%) of the 26 children with bacterial overgrowth (χ2 test, P = 0.658).
Figure 1D shows the concentration of hydrogen (ppm) in the breath for individual methane producers (n = 53) and non-producers (n = 31) in the slum group. An analysis of the area under the curve revealed that between 0 min and 60 min after lactulose ingestion, no difference existed in the levels of hydrogen production between the methane-producing and nonproducing children (448.16 ± 316.03 ppm vs 564.68 ± 441.40 ppm per min; Student’s t-test, P = 0.164). In the period between 60 and 180 min of the test, less hydrogen production was observed among the methane-producing children (4157.55 ± 952.64 ppm per min) than among the methane non-producers (4716.77 ± 1117.13 ppm per min; Student’s t-test, P = 0.017).
SIBO was found in 30.9% of the children in the slum group and in 2.4% of the children in the private school group. These values are similar to those previously reported in Brazil[5] for children living in a slum and those attending a private health clinic (37.5% and 2.1% SIBO in each group, respectively). In Australia, SIBO was found in 27.2% of aboriginal children under 5 years old[13]. These studies[5,13] did not evaluate the methane concentration in the breath test. In the present study, 6 (23.1%) of the 26 children were diagnosed as having SIBO only by an increase in the breath methane level compared with the fasting values. Therefore, using the criteria that takes into account not only breath hydrogen but also breath methane, the diagnosis of SIBO may be more comprehensive. The higher frequency of bacterial overgrowth in the groups living in slums may be hypothetically explained by the different environmental and socioeconomic conditions to which they are exposed (Table 1).
Table 1 shows that the weight and height of children in the slum group were lower than those of the children in the private school group. This situation may be caused, at least in part, by the existence of digestive-absorptive abnormalities linked to bacterial overgrowth and tropical enteropathy[5]. In addition, two factors may support the occurrence of the anthropometric deficit in the children in the slum group. The first factor corresponds to a diet probably deficient in quality and quantity. Data from the food surveys showed that children in the slum group had less caloric intake, less protein consumption, less lipid consumption and less iron, calcium and vitamin A ingestion compared with children in the private school group (data not shown). The second factor corresponds to the inadequate basic sanitation conditions to which the children are exposed, exemplified by the high rates of water contamination, which may be directly related to recurrent infectious outbreaks and, consequently, to recurrent harm during physical development[20].
In the lactulose breath test, the elevated concentrations of hydrogen observed in the breath of children in the slum group after 60 min of the test (Figure 1A) are consistent with the greater prevalence of bacterial overgrowth in these children.
One result of the present study that has yet to be explored in the literature involves the greater colonic production of hydrogen after the first hour of the breath test in the children in the slum group with SIBO (Figure 1B). It was found that these children, in addition to exhibiting greater hydrogen production in the small intestine up to 60 min after the start of the breath test, also showed elevated production of hydrogen from 60 to 180 min after the start of the test when compared with those without SIBO. This difference was found to be statistically significant with respect to the values corresponding to the area under the curve. It is likely that children with bacterial overgrowth possess greater quantities of fermenting bacteria, both in the small intestine and in the colon, when compared with children without SIBO who live in the same environmental conditions. Hydrogen excretion in the colon depends on fermentable substrates supplied by the colonic bacteria[21].
The presence of Lactobacilli and Bifidobacteria in the feces was also evaluated in this same group of children; however, no difference in the counts in the children with or without SIBO from the slum group was found (data not shown). Nevertheless, the number of Lactobacilli and Bifidobacteria colonies in the feces of children in the slum group was lower than that observed in the children in the private school group[22,23]. There is currently no published information which relates SIBO with differences in the colonic microbiota.
Another interesting result of our study was the elevated prevalence of methane producers (63.1%) in the slum group. Considering the proximity of the slum to the municipal landfill, methane is produced by the intense anaerobic degradation of organic waste[24], and methanogenic bacteria which may be present in the soil could potentially colonize the human intestine. These effects constitute an indication of the effects of the environment on the biological condition of individuals exposed to methane gas. Early age, close contact and poor personal hygiene may be factors explaining the high percentage of methane producers[21] in the slum group.
Furthermore, there is concern about the association between the production of methane and the occurrence of diseases related to intestinal motility[25,26]. It should be stressed that, in children, methane production has been related to intestinal constipation with fecaloma and fecal escape[1,27,28], which are associated with a greater slow small intestine[29] and colonic transit time[28]. In our study, an association was not found between methane production and constipation in the children from the slum. However, 3 (37.5%) of the 8 children in the private school group who were methane producers had intestinal constipation without fecal incontinence.
No differences were observed in the methane production between the children from the slum group with or without SIBO (Figure 1C), both in the colon and in the small intestine. Ingestion of non-absorbable disaccharide did not influence breath CH4 excretion, different to that which occurred in H2 excretion[21].
The children in the slum group who were characterized as methane producers exhibited lower hydrogen concentrations in the colon when compared with the methane non-producers (Figure 1D); this result is similar to previously reported results in adults[6]. Assuming that methane production is a good indicator of intracolonic metabolism[19,21,30], this profile may be a consequence of the transformation of hydrogen into methane by methanogenic bacteria present in the colon[14,30]. Methane is synthesized by bacteria in the intestine, where four mmols of hydrogen and one mmol of carbon dioxide create one mmol of methane and water[10]. Methanobrevibacter smithii is the main methanogenic bacterium found in humans, and preferentially colonizes the left colon[31]. Approximately 15% of individuals in the general population are producers of methane instead of hydrogen[15], which is lower than the frequency observed in the slum group.
A previous study performed with adults[14] raised the hypothesis that methane production may be responsible for the false-negative results obtained from studies on SIBO. In previous studies of SIBO in children, the concentration of methane in the breath was not determined. Despite the consistent absence in the literature of methane, in addition to hydrogen, as an indicator of SIBO, the data from our current study indicate that this possibility should be analyzed in future research.
In conclusion, in the present study, we observed a high prevalence of methane producers in children with or without SIBO who were exposed to poor living conditions. However, there was no direct relationship between the presence of SIBO and increased methane production in these children. Thus, the presence of breath methane seems to be a common condition in individuals exposed to inadequate environmental factors, as previously reported[21]. Methane production appeared to be relatively constant during the course of the 3 h breath test. The value of the inclusion of a methane increment as an additional criterion for the diagnosis of SIBO should be evaluated in further studies. With respect to the use of hydrogen as a substrate for methane production in the colon, our hypothesis was confirmed, based on our observation regarding increased hydrogen production in the colon of methane non-producers in the slum.
Small intestine bacterial overgrowth (SIBO) is a clinical disorder characterized by an excessive quantity of bacteria in the upper intestinal tract. SIBO occurrence is common when associated with environmental enteropathy. Poverty associated with ingestion of contaminated water and foods are involved in the etiology of this disease. Some of the consequences of SIBO are diarrhea and malnutrition, however, asymptomatic cases are observed. Breath tests are non-invasive tests used in the diagnosis of SIBO.
In breath tests, hydrogen and methane can be detected. Both gases originate from bacterial fermentation. However, the relationship between methane production and SIBO diagnosis is still unclear.
This is the first study in which methane production was observed in individuals residing in a slum area. In the present study, high methane production was observed in children from a slum area with or without a diagnosis of SIBO. Thus, in addition to literature data relating methane production with severe constipation, methane can also be characterized as an indicator of environmental contamination. These results confirm the hypothesis that hydrogen is used as a substrate for methane production in the colon.
The results of this study suggest that the respiratory tests, characterized by the production of hydrogen and methane, can be performed in the research of SIBO in individuals exposed to unsanitary/unhealthy environments.
SIBO: Clinical disorder characterized by the presence of contaminating bacteria in the small intestine; Environmental enteropathy: Syndrome characterized by a set of nonspecific changes, functional and/or morphological, in the small intestine associated with or without gastrointestinal symptoms. This is a clinical condition associated with environmental contamination; Breath test: The test consists of administering a carbohydrate which is degraded and metabolized by bacteria in the intestine, producing an increase in hydrogen levels in expired air. Others gases can be detected in the expired air, for example, methane.
This is an original study looking for methane and hydrogen expiration in two children communities, as a token of SIBO.
Peer reviewers: Dan Lucian Dumitrascu, Professor, 2nd Medical Department, UMF Iuliu Hatieganu Cluj, Str Clinicilor 4, RO 400006 Cluj, Romania; Orhan Sezgin, Professor, Gastroenteroloji Bilim Dali, Mersin Üniversitesi Tip Fakültesi, Mersin 33190, Turkey
S- Editor Wu X L- Editor Webster JR E- Editor Zhang DN
| 1. | Leiby A, Mehta D, Gopalareddy V, Jackson-Walker S, Horvath K. Bacterial overgrowth and methane production in children with encopresis. J Pediatr. 2010;156:766-70, 770.e1. [PubMed] |
| 2. | Reddymasu SC, Sostarich S, McCallum RW. Small intestinal bacterial overgrowth in irritable bowel syndrome: are there any predictors? BMC Gastroenterol. 2010;10:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Pimentel M. An evidence-based treatment algorithm for IBS based on a bacterial/SIBO hypothesis: Part 2. Am J Gastroenterol. 2010;105:1227-1230. [PubMed] |
| 4. | Cole CR, Frem JC, Schmotzer B, Gewirtz AT, Meddings JB, Gold BD, Ziegler TR. The rate of bloodstream infection is high in infants with short bowel syndrome: relationship with small bowel bacterial overgrowth, enteral feeding, and inflammatory and immune responses. J Pediatr. 2010;156:941-97, 947.e1. [PubMed] |
| 5. | dos Reis JC, de Morais MB, Oliva CA, Fagundes-Neto U. Breath hydrogen test in the diagnosis of environmental enteropathy in children living in an urban slum. Dig Dis Sci. 2007;52:1253-1258. [PubMed] |
| 6. | Campbell DI, Murch SH, Elia M, Sullivan PB, Sanyang MS, Jobarteh B, Lunn PG. Chronic T cell-mediated enteropathy in rural west African children: relationship with nutritional status and small bowel function. Pediatr Res. 2003;54:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 137] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Espinoza JM, Brunser OT, Araya MQ. Colonización bacteriana anormal del intestino delgado alto en escolares de nivel socioeconómico alto y bajo. Rev Chil Pediatr. 1993;64:364-370. |
| 8. | Galpin L, Manary MJ, Fleming K, Ou CN, Ashorn P, Shulman RJ. Effect of Lactobacillus GG on intestinal integrity in Malawian children at risk of tropical enteropathy. Am J Clin Nutr. 2005;82:1040-1045. [PubMed] |
| 9. | Kerckhoffs AP, Visser MR, Samsom M, van der Rest ME, de Vogel J, Harmsen W, Akkermans LM. Critical evaluation of diagnosing bacterial overgrowth in the proximal small intestine. J Clin Gastroenterol. 2008;42:1095-1102. [PubMed] |
| 10. | Bures J, Cyrany J, Kohoutova D, Förstl M, Rejchrt S, Kvetina J, Vorisek V, Kopacova M. Small intestinal bacterial overgrowth syndrome. World J Gastroenterol. 2010;16:2978-2990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 383] [Cited by in RCA: 364] [Article Influence: 24.3] [Reference Citation Analysis (4)] |
| 11. | Gasbarrini A, Lauritano EC, Gabrielli M, Scarpellini E, Lupascu A, Ojetti V, Gasbarrini G. Small intestinal bacterial overgrowth: diagnosis and treatment. Dig Dis. 2007;25:237-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Lunn PG, Northrop-Clewes CA, Downes RM. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet. 1991;338:907-910. [PubMed] |
| 13. | Pereira SP, Khin-Maung-U TD, Duncombe VM, Nyunt-Nyunt-Wai JM. A pattern of breath hydrogen excretion suggesting small bowel bacterial overgrowth in Burmese village children. J Pediatr Gastroenterol Nutr. 1991;13:32-38. [PubMed] |
| 14. | Pimentel M, Mayer AG, Park S, Chow EJ, Hasan A, Kong Y. Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Dig Dis Sci. 2003;48:86-92. [PubMed] |
| 15. | Romagnuolo J, Schiller D, Bailey RJ. Using breath tests wisely in a gastroenterology practice: an evidence-based review of indications and pitfalls in interpretation. Am J Gastroenterol. 2002;97:1113-1126. [PubMed] |
| 16. | Jelliffe DB. Evaluación del estado de nutrición de la comunidad. 1st ed. Ginebra: Organizacion Mundial de La Salud 1968; 291. |
| 17. | World Health Organization. Physical status: the use and interpretation of anthropometry. Geneva: World Health Organization 1995; 114-179. |
| 18. | Centers for Disease Control and Prevention, National Center for Health Statistics. United States: 2000 CDC growth charts [on line]. Hyaltsville: CDC, 2002. Cited 2002-05-11. Available from: http//www.cdc.gov/growthcharts. |
| 19. | Levitt MD, Bond JH. Volume, composition, and source of intestinal gas. Gastroenterology. 1970;59:921-929. [PubMed] |
| 20. | Müller O, Krawinkel M. Malnutrition and health in developing countries. CMAJ. 2005;173:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 518] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 21. | Bond JH, Engel RR, Levitt MD. Factors influencing pulmonary methane excretion in man. An indirect method of studying the in situ metabolism of the methane-producing colonic bacteria. J Exp Med. 1971;133:572-588. [PubMed] |
| 22. | Mello RM, Morais MB, Tahan S, Melli LC, Rodrigues MS, Mello CS, Scaletsky IC. Lactobacilli and bifidobacteria in the feces of schoolchildren of two different socioeconomic groups: children from a favela and children from a private school. J Pediatr (. Rio J). 2009;85:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Vandenplas Y. Gastrointestinal flora composition and health. J Pediatr (. Rio J). 2009;85:285-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Scheutz C, Kjeldsen P, Bogner JE, De Visscher A, Gebert J, Hilger HA, Huber-Humer M, Spokas K. Microbial methane oxidation processes and technologies for mitigation of landfill gas emissions. Waste Manag Res. 2009;27:409-455. [PubMed] |
| 25. | Scanlan PD, Shanahan F, Marchesi JR. Human methanogen diversity and incidence in healthy and diseased colonic groups using mcrA gene analysis. BMC Microbiol. 2008;8:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Attaluri A, Jackson M, Valestin J, Rao SS. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am J Gastroenterol. 2010;105:1407-1411. [PubMed] |
| 27. | Fiedorek SC, Pumphrey CL, Casteel HB. Breath methane production in children with constipation and encopresis. J Pediatr Gastroenterol Nutr. 1990;10:473-477. [PubMed] |
| 28. | Soares AC, Lederman HM, Fagundes-Neto U, de Morais MB. Breath methane associated with slow colonic transit time in children with chronic constipation. J Clin Gastroenterol. 2005;39:512-515. [PubMed] |
| 29. | de Morais MB, Soares AC, Tahan S. Constipation, breath methane, and orocecal transit time for a bean meal test. J Pediatr. 2011;159:171; author reply 171-172. [PubMed] |
| 30. | Bjorneklett AB, Jenssen E. Relationship between hydrogen and methane production in man. Scand J Gastroenterol. 1982;17:985-992. |
| 31. | Miller TL, Weaver GA, Wolin MJ. Methanogens and anaerobes in a colon segment isolated from the normal fecal stream. Appl Environ Microbiol. 1984;48:449-450. [PubMed] |