Published online Nov 7, 2012. doi: 10.3748/wjg.v18.i41.5905
Revised: June 13, 2012
Accepted: June 28, 2012
Published online: November 7, 2012
AIM: To evaluate accuracy of in vivo diagnosis of adenomatous vs non-adenomatous polyps using i-SCAN digital chromoendoscopy compared with high-definition white light.
METHODS: This is a single-center comparative effectiveness pilot study. Polyps (n = 103) from 75 average-risk adult outpatients undergoing screening or surveillance colonoscopy between December 1, 2010 and April 1, 2011 were evaluated by two participating endoscopists in an academic outpatient endoscopy center. Polyps were evaluated both with high-definition white light and with i-SCAN to make an in vivo prediction of adenomatous vs non-adenomatous pathology. We determined diagnostic characteristics of i-SCAN and high-definition white light, including sensitivity, specificity, and accuracy, with regards to identifying adenomatous vs non-adenomatous polyps. Histopathologic diagnosis was the gold standard comparison.
RESULTS: One hundred and three small polyps, detected from forty-three patients, were included in the analysis. The average size of the polyps evaluated in the analysis was 3.7 mm (SD 1.3 mm, range 2 mm to 8 mm). Formal histopathology revealed that 54/103 (52.4%) were adenomas, 26/103 (25.2%) were hyperplastic, and 23/103 (22.3%) were other diagnoses include “lymphoid aggregates”, “non-specific colitis,” and “no pathologic diagnosis.” Overall, the combined accuracy of endoscopists for predicting adenomas was identical between i-SCAN (71.8%, 95%CI: 62.1%-80.3%) and high-definition white light (71.8%, 95%CI: 62.1%-80.3%). However, the accuracy of each endoscopist differed substantially, where endoscopist A demonstrated 63.0% overall accuracy (95%CI: 50.9%-74.0%) as compared with endoscopist B demonstrating 93.3% overall accuracy (95%CI: 77.9%-99.2%), irrespective of imaging modality. Neither endoscopist demonstrated a significant learning effect with i-SCAN during the study. Though endoscopist A increased accuracy using i-SCAN from 59% (95%CI: 42.1%-74.4%) in the first half to 67.6% (95%CI: 49.5%-82.6%) in the second half, and endoscopist B decreased accuracy using i-SCAN from 100% (95%CI: 80.5%-100.0%) in the first half to 84.6% (95%CI: 54.6%-98.1%) in the second half, neither of these differences were statistically significant.
CONCLUSION: i-SCAN and high-definition white light had similar efficacy predicting polyp histology. Endoscopist training likely plays a critical role in diagnostic test characteristics and deserves further study.
-
Citation: Chan JL, Lin L, Feiler M, Wolf AI, Cardona DM, Gellad ZF. Comparative effectiveness of
i -SCAN™ and high-definition white light characterizing small colonic polyps. World J Gastroenterol 2012; 18(41): 5905-5911 - URL: https://www.wjgnet.com/1007-9327/full/v18/i41/5905.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i41.5905
Fourteen million colonoscopies are performed annually in the United States, with the majority performed for screening with the goal of detecting and resecting pre-cancerous polyps[1]. Small polyps (≤ 10 mm in size) make up the majority of polyps removed during screening colonoscopy, yet the rate of advanced neoplasia and invasive carcinoma in these polyps is low. The current standard of practice is removal of all detected polyps, regardless of size, for the purpose of histopathologic diagnosis and prevention of colorectal cancer[2]. However, this practice may not be the most cost-effective way to utilize limited endoscopic resources[3]. Accurate methods to predict histology of small polyps in vivo could prevent unnecessary polypectomies and/or promote a “resect and discard” practice[4], thus minimizing risk to patients as well as improving efficiency and cost of endoscopy as a screening tool. However, clinical impression of lesion histology using standard white light colonoscopy has been shown to correlate poorly with neoplasia regardless of endoscopist experience[5]. This climate creates an opportunity to expand the role for advanced colonoscopic techniques to predict histology in vivo and perform polypectomy in a targeted fashion. In fact, chromoendoscopy with optical magnification and pit pattern analysis can be highly accurate in optical diagnosis[6]. Ideally, the benefit of chromoendoscopy in “optical diagnosis” could be harnessed with more time-efficient digital chromoendoscopic techniques that visually enhance mucosal surface aspects or vessel patterns without significantly prolonging endoscopic procedure time[7].
PENTAX i-SCAN™, a PENTAX Medical Company digital chromoendoscopy technology, uses post-processing computer algorithms integrated into the standard high-definition processor to modulate light reflected from mucosa and highlight surface contrast. Several small studies have evaluated the accuracy of i-SCAN to assist physicians in predicting polyp histology in vivo and have shown promising results[8-10]. However, the generalizability of these studies is limited by their reliance on experts with non-validated diagnostic criteria.
The goal of this pilot study is to build on this early work by evaluating the accuracy of i-SCAN in predicting histology of small polyps (less than 10 mm) throughout the colon.
This was a single center, prospective, comparative effectiveness pilot study conducted at a single academic medical center. The study protocol and equipment were approved by the local Institutional Review Board. The sponsor had no role in the conduct, analysis or reporting of study results.
Consecutive outpatient adults at least 45 years of age referred for screening or surveillance endoscopy at Duke University Medical Center between December 1, 2010 and April 1, 2011 were eligible for study enrollment. Based on readily available data from chart review at the time of the referral, we excluded patients with any of the following conditions: history of colorectal cancer or polyposis syndrome, acute gastrointestinal bleed, history of inflammatory bowel disease, use of anti-platelet or anticoagulant agents that prevent biopsy or polypectomy during colonoscopy, ASA Class III or greater, or inability to provide informed consent. All enrolled subjects provided written informed consent. Patient demographics including age, sex, race, and personal history of prior adenomatous polyps were recorded.
Endoscopies were performed using PENTAX high-definition adult and pediatric colonoscopies equipped with i-SCAN. A button-operated control head on the endoscope permits instant switch between high-definition white light (HDWL) and i-SCAN modes.
All colonoscopies were performed by two experienced endoscopists who have completed at least 2000 colonoscopies. Previous work with i-SCAN capitalized on its ability to enhance surface patterns for polyp histology prediction[8,10], and thus Kudo pit pattern analysis was chosen for systematic histology prediction. The endoscopists had not previously used i-SCAN for the purpose of polyp prediction and thus underwent review of Kudo pit pattern characterization prior to enrolling patients. Posters showing pit pattern characteristics were also available for reference during all procedures.
After colonic preparation, patients underwent moderate sedation per endoscopy unit protocol. The endoscope was inserted to the cecum in standard fashion without attempt to detect polyps on insertion. Procedure time and quality of bowel preparation were graded by the endoscopist and recorded.
Upon reaching the cecum, the colonoscope was withdrawn in standard fashion using HDWL to visualize the colonic mucosa. All polyps detected during the procedure were documented for size, location, and morphology. Size was estimated using open biopsy forceps for reference. Small polyps, those defined as less than 10 mm in size, were further evaluated as below.
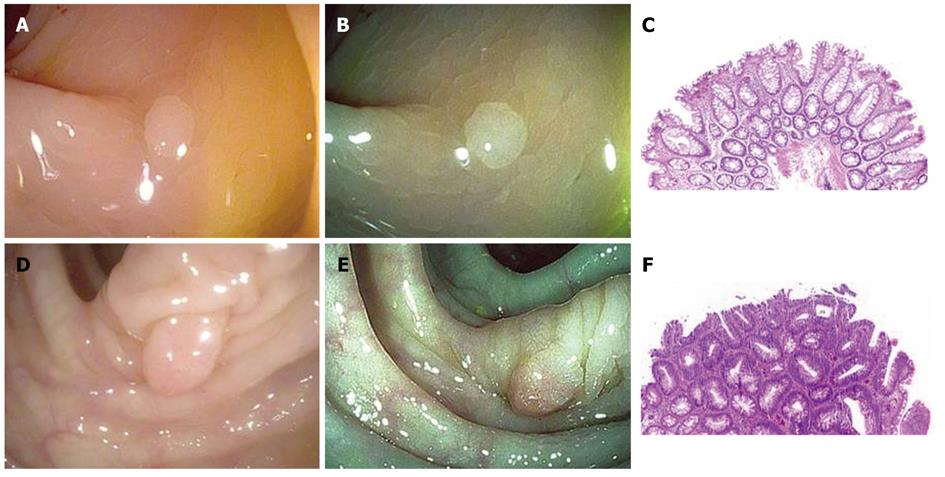
When polyps less than 10 mm in size were identified by white light endoscopy, surface characteristics were first assessed using HDWL followed by i-SCAN. Endoscopists were not limited to any one i-SCAN mode. Endoscopists were asked to predict histology of the polyp in real time using Kudo pit pattern classification for each diagnostic modality. Images of the polyp in HDWL and i-SCAN were captured by the endoscopist (Figure 1). Polyp morphology was described using Paris classification system[11]. Subsequently, the polyp was resected, collected in an individual specimen jar with fresh neutral buffered formalin, and sent to pathology. The samples were processed, and two hematoxylin and eosin sections were created and reviewed by a single surgical pathologist blinded to the colonoscopy findings. A maximum of five consecutive polyps per patient were examined using the i-SCAN modality. This restriction was implemented so as to avoid skewing results by the rare patient who might have multiple hyperplastic polyps in the rectum.
At the midpoint of the study as part of a pre-determined intervention, the study team reviewed incorrect predictions with each endoscopist. This intervention included a review of incorrectly predicted polyp images in both HDWL and i-SCAN.
The protocol was an investigator-initiated study funded by PENTAX Medical Company. PENTAX provided funding for the study coordinator and pathology costs. The funding source had no role in the study conduct, data collection, statistical analysis, interpretation, manuscript preparation, or decision to submit the manuscript for publication.
The primary outcome for the analysis is sensitivity, specificity, accuracy, and test characteristics of i-SCAN and HDWL in predicting the histology of small polyps in real time. Diagnostic test characteristics were evaluated by comparing with histopathologic diagnosis as the gold standard.
The test characteristics were calculated as binomial proportions from one-way frequency tables, and 95% confidence intervals were constructed using the exact confidence limits. Patient and polyp characteristics were compared between providers using Wilcoxon rank-sum test for continuous variables and χ2 or Fisher’s exact test for categorical variables. To assess the impact of patient and provider characteristics on the likelihood of accurate prediction, a generalized linear mixed model was used, with random effect of intercept and slope of polyp size to take within-patient dependency into account, and to allow intercept and slope differ across patients. We explored the learning curve with i-SCAN by comparing the endoscopists’ prediction accuracy on polyps from patients from the first half of the study vs those from the second half. All data were analyzed by using the SAS version 9.2 (SAS Institute Inc., Cary, NC).
Eighty-two patients met enrollment criteria; seven patients declined to participate. Thus, 75 patients were enrolled during the study period between December 1, 2010 and April 1, 2011. The cecum was successfully intubated in 100% of cases. Baseline patient demographic data (Table 1) are shown. There were no significant differences between the two endoscopists in terms of patient age, sex, race, family history of colorectal cancer, or personal history of adenomatous polyps.
| All patients | Patients with polyps | |
| Patients (n) | 75 | 43 |
| Age (yr), mean ± SD | 60.6 ± 9.58 | 61.9 ± 8.98 |
| Male sex | 43 (57.3) | 31 (72.1) |
| Race1 | ||
| White | 56 (75.7) | 33 (78.6) |
| Black | 15 (20.3) | 7 (16.67) |
| Other | 3 (4.00) | 2 (4.76) |
| Proportion of patients with +family history of colorectal cancer | 14 (19.2) | 10 (23.8) |
| Proportion of patients with personal history of adenomatous polyps | 24 (32.9) | 32 (74.4) |
| Prep quality | ||
| Excellent | 14 (19.2) | 7 (17.5) |
| Good | 48 (65.8) | 27 (67.5) |
| Fair | 10 (13.7) | 5 (12.5) |
| Poor | 1 (1.4) | 1 (2.5) |
Quality metrics for the two endoscopists involved in the study are shown in Table 2. Due to scheduling variability, the majority of procedures in the study were performed by Endoscopist A. Endoscopist B took longer, on average, to complete procedures. There was no difference in polyp detection (P = 0.47) or polyp size (P = 0.34) between endoscopists.
| Endoscopist A | Endoscopist B | |
| Patients (n) | 48 (64) | 27 (36) |
| Polyps/patient (n/n), mean ± SD | 1.5 ± 1.7 | 1.2 ± 1.9 |
| Polyp size (mm), mean ± SD | 3.67 ± 1.39 | 3.77 ± 1.04 |
| Cases in which polyp identified n (%) | 29 (60.4) | 14 (51.9) |
| Cases in which adenomatous polyp identified n (%) | 22 (45.8) | 10 (37.0) |
| Procedure time (min), mean ± SD | 21 ± 6.8a | 26 ± 9.6a |
One hundred and three small polyps were included in the analysis among the 43 patients with polyps. The average size of the polyps was 3.7 mm (SD 1.3 mm, range: 2-8 mm). Six of the 103 polyps (5.8%) were located in the rectum, 30 (29.1%) in the sigmoid, 7 (6.8%) were located in the descending colon, 35 (34.0%) in the transverse colon, and 25 (24.3%) in the ascending colon/cecum. By morphology, 101 of the 103 (98.1%) were described as by Paris Is, with only 1 polyp described as Paris Ip, and 1 described as Paris IIa.
Pathology revealed 54/103 (52.4%) adenomas, 26/103 (25.2%) hyperplastic, and 23/103 (22.3%) other diagnoses including “lymphoid aggregates”, “non-specific colitis”, and “no pathologic diagnosis”.
The sensitivity, specificity, and accuracy of HDWL for the in vivo prediction of polyp histology are shown in Table 3. Overall sensitivity with all patients combined was 74.1%, specificity was 69.4%, and accuracy was 71.8%. Table 3 shows the sensitivity, specificity, and accuracy of i-SCAN for the in vivo prediction of polyp histology. Test characteristics with all patients combined showed sensitivity of 72.2%, specificity of 71.4%, and accuracy of 71.8%.
| Combined | Endoscopist A | Endoscopist B | |
| White light | |||
| Accuracy | 71.8 (62.1-80.3) | 63.0 (50.9-74.0) | 93.3 (77.9-99.2) |
| Sensitivity | 74.1 (60.4-85.0) | 66.7 (49.0-81.4) | 88.9 (65.3-98.6) |
| Specificity | 69.4 (54.6-81.8) | 59.5 (42.1-75.3) | 100 (73.5-100) |
| Positive predictive value | 72.7 (61.0-84.5) | 61.5 (44.8-77.5) | 100 (79.4-100) |
| Negative predictive value | 70.8 (58.0-83.7) | 64.7(46.5-80.3) | 85.7(57.2-98.2) |
| i-SCAN | |||
| Accuracy | |||
| Total | 71.8 (62.1-80.3) | 63.0 (50.9-74.0) | 93.3 (77.9-99.2) |
| First Half | 71.4 (57.8-82.7) | 59.0 (42.1-74.4) | 100 (80.5-100) |
| Second half | 72.3 (57.4-84.4) | 67.6 (49.5-82.6) | 84.6 (54.6-98.1) |
| Sensitivity | 72.2 (58.4-83.5) | 63.9 (46.2-79.2) | 88.9 (65.3-98.6) |
| Specificity | 71.4 (56.7-83.4) | 62.2 (44.7-77.5) | 100 (73.5-100) |
| Positive predictive value | 73.6 (61.7-85.5) | 62.2 (44.6-76.6) | 100 (79.4-100) |
| Negative predictive value | 70.0 (57.3-82.7) | 63.9 (46.2-79.2) | 85.7 (57.2-98.2) |
In addition to evaluating differences in the accuracy of prediction with i-SCAN between endoscopists, we also assessed for the presence of a learning effect. Namely, we compared the accuracy of i-SCAN in the first and second half of polyps examined by each endoscopist. Endoscopist A increased his accuracy from 59.0% to 67.6% whereas Endoscopist B decreased his accuracy from 100% to 84.6%. These differences were not statistically significant.
In the current study, we did not detect a difference in the diagnostic efficacy of i-SCAN and HDWL in determining small colorectal polyp histology during screening and surveillance colonoscopy. The observed accuracy of HDWL in this study (74.1%) was similar to other studies in the literature[12-14]. This suggests that poor physician performance or effort was less likely to explain suboptimal results. Furthermore, both endoscopists showed a baseline high sensitivity rate using HDWL, thus decreasing room for additional improvement when i-SCAN was then applied. Endoscopist B in particular showed such high baseline sensitivity and specificity for adenoma prediction (88.9% and 100% respectively) using HDWL alone that any additional improvement of i-SCAN as a diagnostic modality was virtually impossible.
In general, digital chromoendoscopic techniques including Fujinon intelligent chromoendoscopy (FICE), narrow band imaging, and i-SCAN have been shown to be practical for in vivo differentiation between adenomatous and hyperplastic polyps, but the accuracy has ranged across the literature from 70 to over 90 percent[8-10,15-21]. The accuracy of i-SCAN in our study (71.8%) was lower than we would have expected based on published results and below the accuracy needed for clinical application[22]. Promising studies using i-SCAN thus far have reported up to 90% accuracy[10]. In addition, Hoffman et al[8] showed sensitivity of 82% (9/11 adenomas) and specificity of 96% (52/54 hyperplastic polyps) in the distal 30 cm of the colon.
Our finding may be explained by a number of factors. First, in both of the above studies, endoscopies were performed by a single operator experienced in real-time polyp diagnosis. Our endoscopists, both of whom are experienced faculty members at an academic institution, did not have prior experience with digital chromoendoscopic techniques nor with pit pattern analysis prior to this study and thus underwent training with i-SCAN and pit pattern recognition. A specific, validated method for training practitioners in i-SCAN use and pit pattern recognition has yet to be described. It is promising that training methods have been validated in other chromoendoscopic techniques and have shown to improve diagnostic accuracy and interobserver agreement[23,24]. Our findings highlight the importance of training i-SCAN in a standardized fashion, not only for replication of published results but also for potential future application in a general practice setting.
Another possible explanation for our results rests in the fact that magnification was not used in the study. We felt that the undue increase in procedure time and sedation for our patients, as well as poor quality of stored high magnification images, did not merit using high magnification. However, there may be an important role for high magnification in terms of improving diagnostic efficacy in combination with digital chromoendoscopic technique. For example, Kim et al[17] reported in 2011 that the most significant improvements in diagnostic efficacy were found with FICE in conjunction with high magnification, with a difference in 80.4% accuracy without high magnification to 87.0% with high magnification. In fact, high magnification was particularly helpful when evaluating polyps less than 5 mm, which was the size of the majority of polyps in our analysis.
Finally, it should also be noted that studies have employed a number of endoscopic classification schemes in studying the usefulness of digital chromoendoscopy. These include the Kudo pit pattern classification, the Japanese Society for Cancer of Colon and Rectum criteria, and specific classification schemes developed by the investigators[10,15,25]. It remains unclear how generalizable these classification schemes are, especially when using different virtual chromoendoscopic techniques. Even with other well-studied chromoendoscopic techniques, the importance of standardizing nomenclature for surface pattern characteristics and defining interobserver variability within individual techniques has been recognized[26].
This study does have a number of limitations. First, the training offered to the endoscopists involved in the study was not standardized, and it is unclear to what extent results may have changed with more formal training. We did not detect a significant learning effect during the course of the study though our sample size was small. Secondly, the Kudo polyp classification system used in this study has not been specifically validated for histology prediction using i-SCAN, though several groups have utilized surface characterization patterns to aid polyp histology prediction[8,10]. Further studies validating a specific polyp classification system using i-SCAN may be helpful in this regard. Thirdly, patients were not randomized to the two imaging modalities nor was there a cross-over design. As such, it is unlikely that the accuracy of i-SCAN would be worse than HDWL because the polyp was first evaluated in HDWL.
There has been continued interest in real-time prediction of polyp histology for a number of practical reasons including the avoidance of unnecessary polypectomy, reducing complication risks, and improving cost efficiency from a histopathologic standpoint. While there have been many promising studies using multiple digital chromoendoscopic techniques, including i-SCAN, our study did not identify a benefit to using i-SCAN to predict polyp histology. The markedly different accuracy rate between endoscopists strongly suggests that there are endoscopist factors that predict success with in vivo diagnosis, similar to how endoscopist factors may predict adenoma detection rates[27]. Further understanding these factors will be important to help guide training before the widespread application of virtual chromoendoscopic techniques in clinical practice.
The majority of polyps detected and removed during screening colonoscopy are small polyps (less than 10 mm in size) that are unlikely to represent advanced neoplasm or invasive carcinoma. Accurate methods to predict histopathology of small polyps in vivo could potentially prevent unnecessary polypectomies or encourage a cost-effective “resect and discard” strategy during screening colonoscopy. PENTAX i-SCAN, a digital chromoendoscopy technology, might aid in vivo prediction of polyp histology. In this study, they assessed the accuracy of in vivo histology prediction of small colonic polyps using i-SCAN as compared to high-definition white light, using formal histopathology as the gold standard comparison.
Several small studies using i-SCAN have shown promising results in improving accuracy of in vivo polyp histology prediction. However, the reliance on experienced experts with non-validated diagnostic criteria limits generalizability of published results.
The authors found no significant difference between the accuracy of i-SCAN and high-definition white light, with high baseline accuracy using high-definition white light. Interestingly, they did find a significant difference in accuracy between endoscopists, regardless of imaging modality. This suggests an important role for individual endoscopist factors and experience.
Understanding endoscopist factors and standardizing training using i-SCAN may improve not only the ability to reproduce published results, but also the future possibility to apply these technologies in a general practice setting.
PENTAX i-SCAN is a digital chromoendoscopy technology that uses post-processing computer algorithms integrated into the standard high-definition processor to modulate light reflected from mucosa. This highlights surface contrast by “virtual chromoendoscopy” technique, analogous to the way conventional chromoendoscopy highlights surface contrast using indigo carmine dye.
This study provides potentially useful information for improving the clinical applications of i-SCAN. It explains that histologic assessment of polyps is high using white light, that interobserver variability is high, and that the new refinements probably facilitate the general use of tissue recognition but may not be essential for experienced endoscopists. Endoscopist training likely plays a critical role and deserves further study and standardization.
Peer reviewers: Dr. Josep M Bordas, Department of Gastroenterology, Hospital Clinic, Llusanes 11-13, 08022 Barcelona, Spain; Lin Zhang, Associate Professor, Department of Pharmacology and Chemical Biology, University of Pittsburgh, UPCI Research Pavilion, Room 2.42, Pittsburgh, PA 15214, United States
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Seeff LC, Manninen DL, Dong FB, Chattopadhyay SK, Nadel MR, Tangka FK, Molinari NA. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? Gastroenterology. 2004;127:1661-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [PubMed] |
| 3. | Hassan C, Pickhardt PJ, Rex DK. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol. 2010;8:865-89, 865-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 4. | Ignjatovic A, East JE, Suzuki N, Vance M, Guenther T, Saunders BP. Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpect ChAracterise Resect and Discard; DISCARD trial): a prospective cohort study. Lancet Oncol. 2009;10:1171-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 287] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 5. | Lawrance IC, Sherrington C, Murray K. Poor correlation between clinical impression, the small colonic polyp and their neoplastic risk. J Gastroenterol Hepatol. 2006;21:563-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Fu KI, Sano Y, Kato S, Fujii T, Nagashima F, Yoshino T, Okuno T, Yoshida S, Fujimori T. Chromoendoscopy using indigo carmine dye spraying with magnifying observation is the most reliable method for differential diagnosis between non-neoplastic and neoplastic colorectal lesions: a prospective study. Endoscopy. 2004;36:1089-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 190] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Wallace MB, Kiesslich R. Advances in endoscopic imaging of colorectal neoplasia. Gastroenterology. 2010;138:2140-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Hoffman A, Kagel C, Goetz M, Tresch A, Mudter J, Biesterfeld S, Galle PR, Neurath MF, Kiesslich R. Recognition and characterization of small colonic neoplasia with high-definition colonoscopy using i-Scan is as precise as chromoendoscopy. Dig Liver Dis. 2010;42:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Hoffman A, Sar F, Goetz M, Tresch A, Mudter J, Biesterfeld S, Galle PR, Neurath MF, Kiesslich R. High definition colonoscopy combined with i-Scan is superior in the detection of colorectal neoplasias compared with standard video colonoscopy: a prospective randomized controlled trial. Endoscopy. 2010;42:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Lee CK, Lee SH, Hwangbo Y. Narrow-band imaging versus I-Scan for the real-time histological prediction of diminutive colonic polyps: a prospective comparative study by using the simple unified endoscopic classification. Gastrointest Endosc. 2011;74:603-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1328] [Article Influence: 60.4] [Reference Citation Analysis (4)] |
| 12. | Rastogi A, Early DS, Gupta N, Bansal A, Singh V, Ansstas M, Jonnalagadda SS, Hovis CE, Gaddam S, Wani SB. Randomized, controlled trial of standard-definition white-light, high-definition white-light, and narrow-band imaging colonoscopy for the detection of colon polyps and prediction of polyp histology. Gastrointest Endosc. 2011;74:593-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 13. | Rastogi A, Keighley J, Singh V, Callahan P, Bansal A, Wani S, Sharma P. High accuracy of narrow band imaging without magnification for the real-time characterization of polyp histology and its comparison with high-definition white light colonoscopy: a prospective study. Am J Gastroenterol. 2009;104:2422-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | Rogart JN, Jain D, Siddiqui UD, Oren T, Lim J, Jamidar P, Aslanian H. Narrow-band imaging without high magnification to differentiate polyps during real-time colonoscopy: improvement with experience. Gastrointest Endosc. 2008;68:1136-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | van den Broek FJ, Reitsma JB, Curvers WL, Fockens P, Dekker E. Systematic review of narrow-band imaging for the detection and differentiation of neoplastic and nonneoplastic lesions in the colon (with videos). Gastrointest Endosc. 2009;69:124-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | dos Santos CE, Lima JC, Lopes CV, Malaman D, Salomão AD, Garcia AC, Teixeira CR. Computerized virtual chromoendoscopy versus indigo carmine chromoendoscopy combined with magnification for diagnosis of small colorectal lesions: a randomized and prospective study. Eur J Gastroenterol Hepatol. 2010;22:1364-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Kim YS, Kim D, Chung SJ, Park MJ, Shin CS, Cho SH, Kim JS, Song IS. Differentiating small polyp histologies using real-time screening colonoscopy with Fuji Intelligent Color Enhancement. Clin Gastroenterol Hepatol. 2011;9:744-749.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Kuiper T, van den Broek FJ, Naber AH, van Soest EJ, Scholten P, Mallant-Hent RCh, van den Brande J, Jansen JM, van Oijen AH, Marsman WA. Endoscopic trimodal imaging detects colonic neoplasia as well as standard video endoscopy. Gastroenterology. 2011;140:1887-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Sato R, Fujiya M, Watari J, Ueno N, Moriichi K, Kashima S, Maeda S, Ando K, Kawabata H, Sugiyama R. The diagnostic accuracy of high-resolution endoscopy, autofluorescence imaging and narrow-band imaging for differentially diagnosing colon adenoma. Endoscopy. 2011;43:862-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Pohl J, Nguyen-Tat M, Pech O, May A, Rabenstein T, Ell C. Computed virtual chromoendoscopy for classification of small colorectal lesions: a prospective comparative study. Am J Gastroenterol. 2008;103:562-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Pohl J, Lotterer E, Balzer C, Sackmann M, Schmidt KD, Gossner L, Schaab C, Frieling T, Medve M, Mayer G. Computed virtual chromoendoscopy versus standard colonoscopy with targeted indigocarmine chromoscopy: a randomised multicentre trial. Gut. 2009;58:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Rex DK, Kahi C, O'Brien M, Levin TR, Pohl H, Rastogi A, Burgart L, Imperiale T, Ladabaum U, Cohen J. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2011;73:419-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 464] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 23. | Raghavendra M, Hewett DG, Rex DK. Differentiating adenomas from hyperplastic colorectal polyps: narrow-band imaging can be learned in 20 minutes. Gastrointest Endosc. 2010;72:572-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Ignjatovic A, Thomas-Gibson S, East JE, Haycock A, Bassett P, Bhandari P, Man R, Suzuki N, Saunders BP. Development and validation of a training module on the use of narrow-band imaging in differentiation of small adenomas from hyperplastic colorectal polyps. Gastrointest Endosc. 2011;73:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Rex DK. Narrow-band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology. 2009;136:1174-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 26. | Ng SC, Lau JY. Narrow-band imaging in the colon: limitations and potentials. J Gastroenterol Hepatol. 2011;26:1589-1596. [PubMed] [DOI] [Full Text] |
| 27. | Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol. 2007;102:856-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 306] [Article Influence: 17.0] [Reference Citation Analysis (0)] |