Published online Oct 28, 2012. doi: 10.3748/wjg.v18.i40.5729
Revised: July 16, 2012
Accepted: August 14, 2012
Published online: October 28, 2012
AIM: To compare intradermal (ID) and intramuscular (IM) booster doses, which have been used in healthy and high risk subjects, such as healthcare workers, haemodialysis patients, human immunodeficiency virus patients, and renal transplant recipients unresponsive to initial hepatitis B vaccination, in celiac individuals.
METHODS: We conducted our study on 58 celiac patients, vaccinated in the first year of life, whose blood analysis had showed the absence of protective hepatitis B virus (HBV) antibodies. All patients had received the last vaccine injection at least one year before study enrolment and they had been on a gluten free diet for at least 1 year. In all patients we randomly performed an HBV vaccine booster dose by ID or IM route. Thirty celiac patients were revaccinated with recombinant hepatitis B vaccine (Engerix B) 2 μg by the ID route, while 28 celiac patients were revaccinated with Engerix B 10 μg by the IM route. Four weeks after every booster dose, the anti-hepatitis B surface (HBs) antibody titer was measured by an enzyme-linked immune-adsorbent assay. We performed a maximum of three booster doses in patients with no anti-HBs antibodies after the first or the second vaccine dose. The cut off value for a negative anti-HBs antibody titer was 10 IU/L. Patients with values between 10 and 100 IU/L were considered "low responders" while patients with an antibody titer higher than 1000 IU/L were considered "high responders".
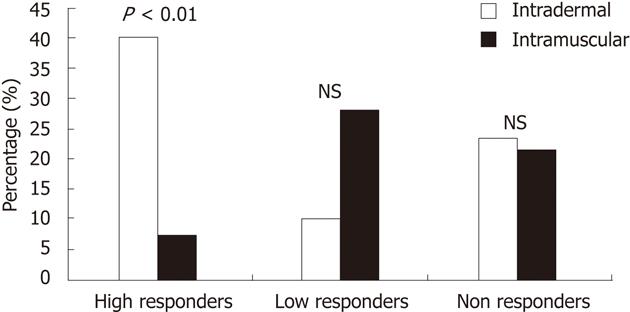
RESULTS: No significant difference in age, gender, duration of illness, and years of gluten intake was found between the two groups. We found a high percentage of "responders" after the first booster dose (ID = 76.7%, IM = 78.6%) and a greater increase after the third dose (ID = 90%, IM = 96.4%) of vaccine in both groups. Moreover we found a significantly higher number of high responders (with an anti-HBs antibody titer > 1000 IU/L) in the ID (40%) than in the IM (7.1%) group, and this difference was evident after the first booster dose of vaccination (P < 0.01). No side effects were recorded in performing delivery of the vaccine by either the ID or IM route.
CONCLUSION: Our study suggests that both ID and IM routes are effective and safe options to administer a booster dose of HBV vaccine in celiac patients. However the ID route seems to achieve a greater number of high responders and to have a better cost/benefit ratio.
-
Citation: Leonardi S, Praticò AD, Lionetti E, Spina M, Vitaliti G, Rosa ML. Intramuscular
vs intradermal route for hepatitis B booster vaccine in celiac children. World J Gastroenterol 2012; 18(40): 5729-5733 - URL: https://www.wjgnet.com/1007-9327/full/v18/i40/5729.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i40.5729
In literature there are several reports describing a non-responsiveness to hepatitis B vaccine in celiac patients[1-4], although the pathogenic mechanism is still unclear. As a matter of fact it seems that this failed response could be linked to the "major histocompatibility complex" (MHC) and human leucocyte antigen (HLA)-II pattern characterizing the disease[5-7], while other studies indicate that gluten intake at the time of vaccination could influence the vaccine-induced immune response[8].
However it is not completely understood whether the unresponsiveness to hepatitis B vaccine in celiac patients is also linked to a weakened immune response in healthy older people or to a physiological loss of humoral immunity with the flow of time[9]. Fisman et al[10] published a meta-analysis on the increased risk of unresponsiveness to hepatitis B vaccination in older subjects, finding a low response even in 30-year-old patients.
An important consideration is that the titer of antihepatitis B surface (anti-HBs) antibodies that should be considered as cut-off for "non-response" to hepatitis B virus (HBV) vaccine is < 10 IU/L, when the measurement is performed a long time after the vaccination. The responsiveness to hepatitis B vaccine should usually be determined by antibody measurement within 2-6 mo after the third vaccine dose. In those patients with anti-HBs < 10 IU/L a booster vaccine schedule should be proposed, but until now there is no consensus on this kind of management.
In the present study we administered a vaccine booster dose against HBV by the intradermal (ID) or intramuscular (IM) route in celiac patients, whose antibodies levels against the HBV were low after the first regimen of hepatitis B vaccine performed in the first year of life.
The aim of our study was to evaluate the possibility to provide a satisfactory immune response against HBV by these procedures, comparing their efficacy.
Our study was a prospective, randomized study, conducted on 58 celiac patients (age, mean ± SD, 9.8 ± 6.2 years) of 116 celiac subjects (age, mean ± SD, 10.2 ± 5.7 years) referred to our Pediatric Department, University of Catania, Italy, whose blood analysis showed the absence of protective HBV antibodies (anti-HBs). In all included patients, the diagnosis of celiac disease was made after one year of age, based on clinical signs and standard serological markers (antigliadin IgA and IgG, tissue trans-glutaminase IgA antibody, anti-endomysial antibody) and on typical histological findings on small bowel biopsies (villous atrophy with crypt hyperplasia and increased intraepithelial lymphocytes).
All patients received the anti-HBV vaccine at 3, 5 and 11 mo of age by IM injection on the front-lateral area of their quadriceps muscle (10 μg of Engerix B, GlaxoSmith and Kline, Belgium), as planned by the Italian standard vaccination schedule.
All patients had received the last vaccine injection at least one year before the study enrolment and they had been on a gluten free diet for at least 1 year. None of the patients had ever been affected by HBV infection.
In all patients we randomly performed an HBV vaccine booster dose by the ID or IM route. Thirty celiac patients were revaccinated by the ID route with a 2 μg dose of recombinant hepatitis B vaccine (Engerix B) administered on the flexor surface of the forearm, using a 1 mL syringe and 26-gauge needle. In all the patients a visible skin weal was noticed as evidence of the ID inoculation.
Twenty-eight celiac patients were revaccinated by the IM route with a 10 μg dose of Engerix B administered in the lateral region of their deltoid muscle, using a 5 mL syringe and 26-gauge needle.
Four weeks after every booster dose, the anti-HBs antibody titer was measured by enzyme-linked immune-adsorbent assay (hepanostica anti-HBs, bioMeriuex, Netherlands). We performed a maximum of three booster doses in patients with no anti-HBs antibodies after the first or the second vaccine dose. The cut off value for a negative anti-HBs antibody titer was 10 mIU/mL. Patients with values between 10 and 100 IU/L were considered "low responders" while patients with an antibody titer higher than 1000 IU/L were considered "high responders"[11].
The Mann-Withney U-test was performed to compare age, duration of illness, years of gluten intake and HBs antibody titer between the two groups of patients. The Fisher exact test was used to compare the gender, the number of non responders, low responders and high responders between the ID and IM groups. P value < 0.05 was considered statistically significant.
The main features of the two groups of patients are reported in Table 1. No significant difference of age, gender, duration of illness, and years of gluten intake was found between the two groups.
The number and the percentage of responders to ID and IM hepatitis B vaccination after every dose injection are reported in Table 2, together with the mean and SD of the anti-HBs titer in the two groups after the first and the third booster.
Both groups of patients showed a similar percentage of responders after the first dose of vaccine (ID = 76.7%, IM = 78.6%) and a major increase after the third dose (ID = 90%, IM = 96.4%). However, we did not find any statistically significant difference between the two groups. We found no statistically significant difference in anti-HBs titer between the two groups, after the first and the third doses.
Finally we found a significantly higher number of high responders (with an anti-HBs antibody titer > 1000 IU/L) in the ID (40%) than in the IM (7.1%) group, and this difference was evident after the first booster dose of vaccination (Figure 1). No side effects were recorded in performing both ID and IM injections.
Literature data describe that 4%-10% of healthy, immune competent individuals fail to elicit protective levels of antibodies to recombinant HBs antigen after completing the standard hepatitis B vaccination schedule[12]. Even though the pathogenic mechanism leading to a failed response to hepatitis B vaccine is still unknown, there are several hypotheses trying to explain this link. Recently Zingone et al[8] reported a possible association with gluten intake at the time of vaccination that may influence the vaccine-induced immune response. Nevertheless the most likely hypothesis is related to a specific pattern of MHC[13] and HLA-II antigens linked to the disease. As a matter of fact, homozygosis for HLA-B8, DR3 and DQ2 alleles was found to have a significantly higher incidence in hepatitis B vaccine non responders[5-7].
This HLA-DQ2 haplotype is present in 90%-95%[14,15] of celiac patients and it seems to explain the relationship between the disease and the non-responsiveness to hepatitis B vaccine. Thus, in celiac non responders a re-vaccination should be recommended because of the worldwide spread of the disease.
Nowadays, there is no consensus on the management of celiac patients with anti-HBs antibody levels < 10 IU/L after the IM vaccine. In healthy people a common practice is to administer a higher dose of HBV recombinant vaccine (HBRV) or a second course of three doses of IM recombinant vaccine (IMRV)[16], but it does not seem to be successful. In fact it has been reported that more than 50% of non responders are not able to acquire an protective anti-HBs titer with at least two additional IMRV booster doses in the primary course[17,18].
In human immunodeficiency virus patients, repeated vaccination is commonly considered as a first satisfactory strategy[19]. Some investigators have even increased the dose of hepatitis B vaccine with varying success[20,21] or have used a double dose of a combined hepatitis A and B vaccine[22].
The United States Center for Disease Control and Prevention recommends the administration of an additional series of three doses of IM vaccine in chronic hemodialysis patients[23]. For those non-responders after two series (six doses of vaccine in total), there is no data to support the use of additional doses to induce an immune response.
Another approach is to administer HBRV vaccine by the ID route. In fact a recent meta-analysis by Fabrizi et al[24] concluded that the ID route is associated with higher anti-HBs antibody levels, although this is not sustained over time. Recently in a pilot study we found an effective response after ID administration of HBRV in celiac patients too[25].
At present this is the first study comparing the ID and IM routes in these patients. In our study we found a high percentage of response after the first dose of vaccination in both groups (ID 76.7% vs IM 78.6%) and a higher response after the third booster dose (ID 90% vs IM 96.4%). Moreover, the percentage of responders in both groups after the three doses of vaccine was similar to those found in vaccinated healthy people[12].
Our data confirms that both routes are effective to perform a booster strategy in celiac patients with low anti-HBs antibodies, as 90% of ID patients and 96.4% of IM subjects showed a protective anti-HBs titer after the third booster dose. However the ID route seems to produce a significantly higher percentage (40%) of high responders (anti-HBs > 1000 IU/L) than the IM route (7.1%).
In our opinion, this result may have an important clinical significance, because a protective anti-HBs titer may persist to 64% after 10 years in normal children if there is a high value of anti-HBs antibody titer at the end of the initial schedule[9].
However, whether the ID route is a better strategy than IM hepatitis B vaccine still remains an open question. In fact several studies in high-risk groups[26-28] showed that low dose ID injections resulted in long term sero-protection in a large number of subjects non responsive to IM vaccination. However, a recent meta-analysis of 757 adults by Sangaré et al[29] demonstrated that ID hepatitis B vaccination was less effective to achieve sero-protection than IM vaccination.
Recently a randomized study on ID vs IM hepatitis B vaccination in human immunodeficiency virus-infected children, without severe immunosuppression, confirmed this issue[30]. In particular, in the study by Medeiros et al[31] on hemodialysis patients, the percentage of responders was very low (13.3%).
Our data seem to suggest that the use of the ID route for the booster dose of hepatitis B vaccine in celiac patients is a better option to obtain an higher titer of antibodies against HBV. Moreover the ID route allows a better cost/efficacy ratio, because of the cost reduction exceeding 50% (2 μg per dose) compared with a standard IM vaccine regimen (10 μg per dose)[32]. In conclusion, it is important to highlight that the ID route could represent an efficacious and cost-saving option for difficult-to-vaccinate and high-risk patients, as reported in other studies[33-35] and also for the observed 4%-10% of healthy people who normally fail to respond to the standard HBV vaccination regimen[36].
The hepatitis B vaccine is a mandatory vaccine for all children. Nevertheless around 4%-10% of healthy subjects and a higher percentage of patients affected by celiac disease do not respond to the standard cycle of vaccination provided by the Ministry of Health. As a matter of fact they show an antibody titer less than 10 IU/L after the standard vaccination doses. This population could be considered at risk for potential exposure to hepatitis B virus (HBV) infection. Thus, it is proposed that the revaccination of "non-responders" at the first cycle of scheduled HBV vaccination, by booster doses, could improve HBV antibody titer and this study compared the efficacy of intramuscular (IM) boosters vs intradermal (ID) vaccination.
The ID route of vaccination is an effective way to vaccinate people, it is safe and it seems to be easier to practice than the IM route. This way should be considered in "non-responder" celiac patients, not only for the high response to vaccination, but also because it allows a better cost/efficacy ratio, with a cost reduction exceeding 50% compared with an IM vaccine regimen. This cost decrease is linked to the lower dosage of vaccine used in the ID route (2 μg per dose) than in the IM route (10 μg per dose).
The problems of responsiveness to vaccinations such as anti HBV vaccine have been widely studied, and researchers have demonstrated how this lack of response could be linked to genetic and environmental factors. Actually there are few studies that respond to the question on how to solve this non-responsiveness to HBV vaccine. Some studies found a good strategy was revaccination by the IM route, others demonstrated a better outcome with the ID route. Moreover, other researchers find the practice of applying booster doses to "non-responders" not useful, as they conserve their intra-cellular immunity. Nevertheless, as the problem has widely interested the scientific community in these last years, it could be useful to establish a common behavior to solve this question. For this reason, studies on new vaccination schedules could be useful and this study is the first one that compares two vaccination routes in order to better understand the way the research should address its efforts.
The study results suggest that ID route of HBV vaccination is a potential therapeutic strategy that could be used in improving responsiveness to HBV vaccine and in preventing a potential infection in a "non-responders" population.
This is a good prospective, randomized study in which the authors analyzed the efficacy of ID and IM routes in HBV vaccinated children to improve their responsiveness to standard vaccination. The results are interesting and suggest that the ID route of booster vaccination is not only as effective as the IM one, but it is also less expensive, so it could easily be considered as a successful strategy for booster doses of HBV vaccine in "non-responder" patients.
Peer reviewers: Alessandro Grasso, MD, Internal Medicine and Gastroenterology Unit, San Paolo Hospital, 17100 Savona, Italy; Ourania M Andrisani, PhD, Professor, Center for Cancer Research, Purdue University, B038 Hansen Bldg, West Lafayette, IN 47907, United States
S- Editor Gou SX L- Editor O’Neill M E- Editor Xiong L
| 1. | Park SD, Markowitz J, Pettei M, Weinstein T, Sison CP, Swiss SR, Levine J. Failure to respond to hepatitis B vaccine in children with celiac disease. J Pediatr Gastroenterol Nutr. 2007;44:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Noh KW, Poland GA, Murray JA. Hepatitis B vaccine nonresponse and celiac disease. Am J Gastroenterol. 2003;98:2289-2292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Ahishali E, Boztas G, Akyuz F, Ibrisim D, Poturoglu S, Pinarbasi B, Ozdil S, Mungan Z. Response to hepatitis B vaccination in patients with celiac disease. Dig Dis Sci. 2008;53:2156-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Leonardi S, Spina M, Spicuzza L, Rotolo N, La Rosa M. Hepatitis B vaccination failure in celiac disease: is there a need to reassess current immunization strategies? Vaccine. 2009;27:6030-6033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Wang C, Tang J, Song W, Lobashevsky E, Wilson CM, Kaslow RA. HLA and cytokine gene polymorphisms are independently associated with responses to hepatitis B vaccination. Hepatology. 2004;39:978-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Martinetti M, De Silvestri A, Belloni C, Pasi A, Tinelli C, Pistorio A, Salvaneschi L, Rondini G, Avanzini MA, Cuccia M. Humoral response to recombinant hepatitis B virus vaccine at birth: role of HLA and beyond. Clin Immunol. 2000;97:234-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Godkin A, Davenport M, Hill AV. Molecular analysis of HLA class II associations with hepatitis B virus clearance and vaccine nonresponsiveness. Hepatology. 2005;41:1383-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Zingone F, Morisco F, Zanetti A, Romanò L, Portella G, Capone P, Andreozzi P, Tortora R, Ciacci C. Long-term antibody persistence and immune memory to hepatitis B virus in adult celiac patients vaccinated as adolescents. Vaccine. 2011;29:1005-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Zanetti AR, Mariano A, Romanò L, D'Amelio R, Chironna M, Coppola RC, Cuccia M, Mangione R, Marrone F, Negrone FS. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet. 2005;366:1379-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 268] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 10. | Fisman DN, Agrawal D, Leder K. The effect of age on immunologic response to recombinant hepatitis B vaccine: a meta-analysis. Clin Infect Dis. 2002;35:1368-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 144] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | European Consensus group on Hepatitis B Immunity, Immunity. Are booster immunisations needed for lifelong hepatitis B immunity? European Consensus Group on Hepatitis B Immunity. Lancet. 2000;355:561-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 370] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 12. | Coates T, Wilson R, Patrick G, André F, Watson V. Hepatitis B vaccines: assessment of the seroprotective efficacy of two recombinant DNA vaccines. Clin Ther. 2001;23:392-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Alper CA, Kruskall MS, Marcus-Bagley D, Craven DE, Katz AJ, Brink SJ, Dienstag JL, Awdeh Z, Yunis EJ. Genetic prediction of nonresponse to hepatitis B vaccine. N Engl J Med. 1989;321:708-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 291] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | van Heel DA, West J. Recent advances in coeliac disease. Gut. 2006;55:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 176] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Kapitány A, Tóth L, Tumpek J, Csípo I, Sipos E, Woolley N, Partanen J, Szegedi G, Oláh E, Sipka S. Diagnostic significance of HLA-DQ typing in patients with previous coeliac disease diagnosis based on histology alone. Aliment Pharmacol Ther. 2006;24:1395-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Sjogren MH. Prevention of hepatitis B in nonresponders to initial hepatitis B virus vaccination. Am J Med. 2005;118 Suppl 10A:34S-39S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Zuckerman JN, Sabin C, Craig FM, Williams A, Zuckerman AJ. Immune response to a new hepatitis B vaccine in healthcare workers who had not responded to standard vaccine: randomised double blind dose-response study. BMJ. 1997;314:329-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Averhoff F, Mahoney F, Coleman P, Schatz G, Hurwitz E, Margolis H. Immunogenicity of hepatitis B Vaccines. Implications for persons at occupational risk of hepatitis B virus infection. Am J Prev Med. 1998;15:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 195] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | Rey D, Krantz V, Partisani M, Schmitt MP, Meyer P, Libbrecht E, Wendling MJ, Vetter D, Nicolle M, Kempf-Durepaire G. Increasing the number of hepatitis B vaccine injections augments anti-HBs response rate in HIV-infected patients. Effects on HIV-1 viral load. Vaccine. 2000;18:1161-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 154] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Fonseca MO, Pang LW, de Paula Cavalheiro N, Barone AA, Heloisa Lopes M. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine. 2005;23:2902-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Cornejo-Juárez P, Volkow-Fernández P, Escobedo-López K, Vilar-Compte D, Ruiz-Palacios G, Soto-Ramírez LE. Randomized controlled trial of Hepatitis B virus vaccine in HIV-1-infected patients comparing two different doses. AIDS Res Ther. 2006;3:9. [PubMed] |
| 22. | Department of Health. Immunisation Against Infectious Disease 2006 (The Green Book). Edinburgh: Stationery Office 2006; Chapter 18, Hepatitis B. |
| 23. | Cornejo-Juárez P; CDC. Recommendations for preventing transmission of infections among chronic haemodialysis patients. MMWR. 2001;50:1–43. |
| 24. | Fabrizi F, Dixit V, Martin P, Messa P. Meta-analysis: the impact of diabetes mellitus on the immunological response to hepatitis B virus vaccine in dialysis patients. Aliment Pharmacol Ther. 2011;33:815-821. [PubMed] [DOI] [Full Text] |
| 25. | Leonardi S, Del Giudice MM, Spicuzza L, Spina M, La Rosa M. Hepatitis B vaccine administered by intradermal route in patients with celiac disease unresponsive to the intramuscular vaccination schedule: a pilot study. Am J Gastroenterol. 2010;105:2117-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Ghebrehewet S, Baxter D, Falconer M, Paver K. Intradermal recombinant hepatitis B vaccination (IDRV) for non-responsive healthcare workers (HCWs). Hum Vaccin. 2008;4:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Micozkadioglu H, Zumrutdal A, Torun D, Sezer S, Ozdemir FN, Haberal M. Low dose intradermal vaccination is superior to high dose intramuscular vaccination for hepatitis B in unresponsive hemodialysis patients. Ren Fail. 2007;29:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Morais EO, Resende MR, Oliveira AM, Sinkoc VM, Garcia MT, Angerami RN, da Silva LJ. Intradermal hepatitis B vaccination in patients with advanced chronic renal failure: immunogenicity and follow-up. Aliment Pharmacol Ther. 2007;25:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Sangaré L, Manhart L, Zehrung D, Wang CC. Intradermal hepatitis B vaccination: a systematic review and meta-analysis. Vaccine. 2009;27:1777-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Bunupuradah T, Ananworanich J, Pancharoen C, Petoumenos K, Prasitsuebsai W, Wongngam W, Ubolyam S, Sriheara C, Lange J, Phanuphak P. Randomized study of intradermal compared to intramuscular hepatitis B vaccination in HIV-infected children without severe immunosuppression. Vaccine. 2011;29:2962-2967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Medeiros RH, Figueiredo AE, Poli-de-Figueiredo CE, d'Avila DO, de los Santos CA. Low response to intradermal hepatitis B vaccination in incident hemodialysis patients. J Bras Nefrol. 2011;33:45-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Sangfelt P, Uhnoo I, Reichard O, Weiland O. A low-dose intradermal hepatitis B vaccine programme in health-care workers and students is highly effective and cost saving: a retrospective follow-up survey in the clinical setting. Scand J Gastroenterol. 2008;43:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Leonardi S, Leggio T, Fischer A, Sciacca A, Musumeci S. Intradermal hepatitis B vaccination: efficacy and timing for a booster dose in infants at risk. Pediatr Infect Dis J. 1989;8:337. [PubMed] |
| 34. | Leonardi S, Musumeci S, Sciacca A, Greco D, Romano C. Reliability of intradermal vaccination against hepatitis B for accelerated prophylaxis. Pediatr Infect Dis J. 1990;9:520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Levin A. Dialysis: Intradermal HBV vaccination is preferable in non-responders. Nat Rev Nephrol. 2009;5:616-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Ghabouli MJ, Sabouri AH, Shoeibi N, Bajestan SN, Baradaran H. High seroprotection rate induced by intradermal administration of a recombinant hepatitis B vaccine in young healthy adults: comparison with standard intramuscular vaccination. Eur J Epidemiol. 2004;19:871-875. [PubMed] [DOI] [Full Text] |