Published online Oct 21, 2012. doi: 10.3748/wjg.v18.i39.5551
Revised: May 18, 2012
Accepted: May 26, 2012
Published online: October 21, 2012
AIM: To clarify differences in mucin phenotype, proliferative activity and oncogenetic alteration among subtypes of colorectal laterally spreading tumor (LST).
METHODS: LSTs, defined as superficial elevated lesions greater than 10 mm in diameter with a low vertical axis, were macroscopically classified into two subtypes: (1) a granular type (Gr-LST) composed of superficially spreading aggregates of nodules forming a flat-based lesion with a granulonodular and uneven surface; and (2) a non-granular type (NGr-LST) with a flat smooth surface and an absence of granulonodular formation. A total of 69 LSTs, comprising 36 Gr-LSTs and 33 NGr-LSTs, were immunohistochemically stained with MUC2, MUC5AC, MUC6, CD10 (markers of gastrointestinal cell lineage), p53, β-catenin and Ki-67 antibodies, and examined for alteration in exon 1 of v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) and exon 15 of v-raf murine sarcoma viral oncogene homologue B1 (BRAF) by polymerase chain reaction followed by direct sequencing.
RESULTS: Histologically, 15 Gr-LST samples were adenomas with low-grade dysplasia (LGD), 12 were high-grade dysplasia (HGD) and 9 were adenocarcinomas invading the submucosa (INV), while 12 NGr-LSTs demonstrated LGD, 14 HGD and 7 INV. In the proximal colon, MUC5AC expression was significantly higher in the Gr-type than the NGr-type. MUC6 was expressed only in NGr-LST. MUC2 or CD10 did not differ. P53 expression demonstrated a significant stepwise increment in progression through LGD-HGD-INV with both types of LST. Nuclear β-catenin expression was significantly higher in the NGr-type. Ki-67 expression was significantly higher in the Gr-type in the lower one third zone of the tumor. In proximal, but not distal colon tumors, the incidence of KRAS provided mutation was significantly higher in the Gr-type harboring a specific mutational pattern (G12V). BRAF mutations (V600E) were detected only in two Gr-LSTs.
CONCLUSION: The two subtypes of LST, especially in the proximal colon, have differing phenotypes of gastrointestinal cell lineage, proliferation and activation of Wnt/β-catenin or RAS/RAF/extracellular signal-regulated kinase signaling.
-
Citation: Nakae K, Mitomi H, Saito T, Takahashi M, Morimoto T, Hidaka Y, Sakamoto N, Yao T, Watanabe S. MUC5AC/β-catenin expression and
KRAS gene alteration in laterally spreading colorectal tumors. World J Gastroenterol 2012; 18(39): 5551-5559 - URL: https://www.wjgnet.com/1007-9327/full/v18/i39/5551.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i39.5551
Colorectal cancer (CRC) is considered to arise from an adenoma precursor, and a model for genetic alterations in the adenoma-carcinoma sequence has been proposed [1,2]. In this model, an adenomatous polyposis coli (APC) gene mutation occurs at the earliest stage, followed by a v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation, as well as a change in p53. Along this sequence, the Wnt/APC/β-catenin and RAS/RAF/extracellular signal-regulated kinase (ERK) signaling pathways also play important roles[3-7]. Previous studies have primarily considered protruded adenomatous polyps as the most likely precursor of CRC[1,2]. However, recent advances have led to changes in the diagnosis of early colorectal tumors, which can now be morphologically divided into three groups: classical protruded tumors, depressed tumors, and laterally spreading tumors (LSTs)[8,9]. The latter two are possible candidates for alternative pathways of colorectal tumorigenesis. LSTs are considered to be less invasive, as neoplastic cells tend to spread along the surface of the lumen, and are usually categorized into two subtypes: granular type (Gr-LST) and flat- or non-granular type (NGr-LST); NGr-LSTs were more often associated with submucosal invasion compared to Gr-LSTs[9,10]. An earlier report demonstrated unique cell kinetics in LSTs[11]. Subsequent studies also evaluated alteration of APC[12] or β-catenin[12-15] for Wnt/APC/β-catenin, mutation of KRAS[11,12,14,16-19] or v-raf murine sarcoma viral oncogene homologue B1 (BRAF)[12,14,19] for RAS/RAF/ERK and mutation of phosphoinositide-3-kinase (PI3K) catalytic-α polypeptide[12,19] for the PI3K/AKT signaling pathway in LSTs. In addition, promoter methylation of CpG islands including its methylator phenotype (CIMP) in association with other genetic alterations has been shown to make important contributions to LST development[14,18].
In an earlier study, de novo appearance of gastric mucin genes MUC5AC and MUC6, which are present in surface foveolar cells and mucous neck cells of the oxyntic mucosa and antral-type glands in the stomach[20], was shown in colonic adenomas[21]. Altered expression of MUC2 as an intestinal apomucin known to be expressed in goblet cells[22], and CD10 expressed on the brush borders of intestinal epithelial cells[23], occurred during progression of adenoma to early adenocarcinoma state[24-26]. While considerable effort has been made to identify gene mutations and alteration of gastrointestinal phenotypes in conventional colorectal tumors, less attention has been paid to LSTs. Therefore, in the present study, we focused on differences in expression of MUC2, MUC5AC, MUC6 and CD10, p53 alteration, nuclear translocation of β-catenin, cell proliferation, and KRAS/BRAF mutations in morphologically different LST subtypes.
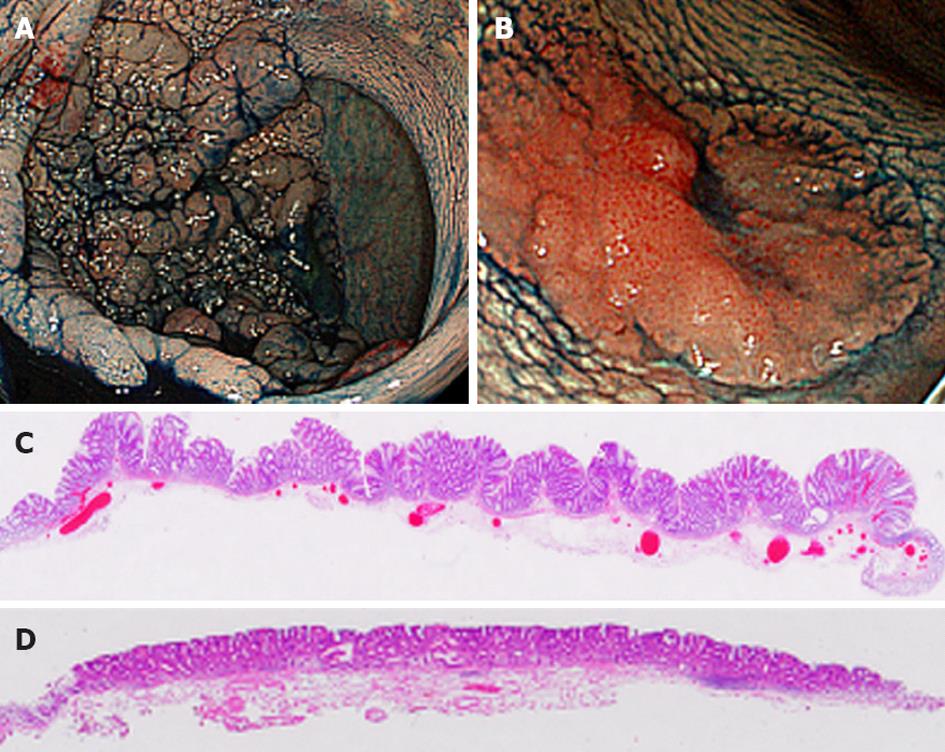
LSTs are defined as superficial elevated lesions greater than 10 mm in diameter with a low vertical axis[8-10] and are macroscopically classified into two subtypes: (1) Gr-LSTs composed of superficially spreading aggregates of nodules forming flat based lesions with a granulonodular and uneven surface (Figure 1A and C); and (2) NGr-LSTs with flat smooth surfaces and an absence of granulonodular formation (Figure 1B and D).
The material for our study was a series of 69 LSTs (from 69 patients) resected endoscopically or surgically at Jundendo University Hospital (Tokyo, Japan) between May 2008 and July 2011. Patients with familial polyposis coli, hereditary non-polyposis colorectal carcinoma, multiple colorectal carcinomas, or inflammatory bowel disease were not included in the analysis. Proximal LSTs were classified as tumors proximal to the splenic flexure and the remaining tumors were defined as distal. Resected specimens were fixed in 15% formalin and processed for embedding in paraffin wax according to routine procedures and then sections were cut and stained with hematoxylin and eosin (H and E). This study was approved by the Institutional Review Board and the ethical committee of our hospital.
All slides stained with H and E were reviewed by two experienced gastrointestinal pathologists (Mitomi H, Yao T) independently. According to the World Health Organization classification[27], adenoma was classified as tubular, tubulovillous or villous type with low grade dysplasia (LGD) or high grade dysplasia (HGD). Intramucosal carcinoma and carcinoma in situ were included in adenoma with HGD. Adenocarcinoma invading submucosa (INV) was classified as well, moderately or poorly differentiated. The subjects were classified according to the most advanced lesion identified. Interobserver variation was resolved by reevaluation and discussion to reach consensus.
Briefly, 4 μm thick tissue serial sections were dewaxed in xylene and rehydrated, then endogenous peroxidase activity was blocked by incubation in 3% hydrogen peroxidase. For heat-induced antigen retrieval, sections were transferred to citrate buffer (10 mmol/L, pH 6.0) and heated at 100 °C in a water bath. The primary antibodies employed were MUC2 (Ccp58, 1:100 dilution; Leica Biosystems/Novocastra Laboratories, Newcastle Upon Tyne, UK), MUC5AC (CLH2, 1:100 dilution; Novocastra Laboratories), MUC6 (CLH5, 1:100 dilution; Novocastra Laboratories), CD10 (56C6, 1:100 dilution; Novocastra Laboratories), p53 (PAb 1801, 1:100; Leica Biosystems/Novocastra Laboratories), β-catenin (14/Beta-Catenin, dilution 1:100, BD Biosciences, San Diego, CA), and Ki-67 (MIB-1, 1:100; DAKO, Glostrup, Denmark), with incubation for 120 min at room temperature. Immunostaining was performed using the streptavidin-biotin-peroxidase complex method using a Histofine SAB-PO Kit (Nichirei Corp., Tokyo, Japan). The chromogen was 3,3’-diaminobenzidine and the sections were counterstained lightly with Mayer’s hematoxylin to facilitate recognition of structures.
Distinct cytoplasmic staining for MUC2, MUC5AC, MUC6 and apical staining for CD10 were considered positive, as was nuclear staining for p53 and Ki-67, regardless of the staining intensity. Expression of β-catenin, which generally showed an inverse relationship between membranous and nuclear (with cytoplasmic) reactivity, was evaluated only with respect to nuclear localization in this study. Immunostaining for all markers was evaluated by two of the authors (Mitomi H, Nakae K) independently, without prior knowledge of clinicopathological data. Discrepancies were resolved by re-evaluation to reach consensus.
Immunoreactive scores (IRSs) for MUC2, MUC5AC, MUC6 and CD10 were classified into five grades: 0 points, positive cells < 5% of tumor area; 1 point, 5%-24%; 2 points, 25%-49%; 3 points, 50%-74%; 4 points, ≥ 75%. IRSs for p53 and nuclear β-catenin were also classified into five grades: 0 points, positive nuclei < 5% of tumor cells; 1 point, 5%-24%; 2 points, 25%-49%; 3 points, 50%-74%; 4 points, ≥ 75%. For topological evaluation of the Ki-67 labeling index (LI; %), tumor glands in the lamina propria were separated into three equal zones (upper, middle and lower thirds), and the number of immunoreactive nuclei per approximately 300 tumor cells were counted in each zone (for a total of approximately 1000 cells in whole glands).
Mutation analysis for KRAS and BRAF were performed using genomic DNA derived from formalin-fixed paraffin-embedded tissue of 29 Gr-LSTs (10 LGD, 10 HGD and 9 INV) and 27 NGr-LSTs (10 LGD, 10 HGD and 7 INV). Mutations were examined in exon 1 of KRAS and exon 15 of BRAF by polymerase chain reaction followed by direct sequencing. The primer sequences in this study were as previously described[28].
All statistical analysis were carried out using StatView for Windows Version 5.0 (SAS Institute Inc., Cary, NC, United States). IRSs and LI are presented as mean ± SD. Continuous data were compared with the Mann-Whitney U test. Categorical analysis of variables was performed using either the χ2 test (with Yates’ correction) or the Fisher’s exact test, as appropriate. Correlations among expression levels of the encoded proteins were assessed with the Spearman’s rank correlation coefficient. A P value < 0.05 was considered statistically significant, with classification into two grades: P < 0.05 and P < 0.01.
The clinicopathological characteristics of the Gr-LSTs (15 LGD, 12 HGD, 9 INV) and NGr-LSTs (12 LGD, 14 HGD, 7 INV) are shown in Table 1. Gr-LSTs were located equally in proximal and distal colons, whereas NGr-LSTs were more frequently found in the distal colon, but without reaching statistical significance. Gr-LSTs were significantly larger than NGr-LSTs. Histologically, 12 out of 13 (92%) tubulovillous adenomas with HGD were Gr-LSTs, whereas all of the 13 tubular adenomas with HGD were NGr-LSTs. All NGr-INVs and 7 out of 9 (78%) Gr-INVs were well differentiated adenocarcinomas. The two other Gr-INVs were an adenocarcinoma coexisting with well and moderately differentiated histology, and a mixed well and poorly differentiated type adenocarcinoma.
| Variable | Gr-type (n = 36) | NGr-type (n = 33) |
| Age (yr) | 68.5 ± 10.9 (34-88) | 69.0 ± 10.3 (44-85) |
| Sex | ||
| Male | 21 | 23 |
| Female | 15 | 10 |
| Location | ||
| Proximal colon | 18 | 12 |
| Distal colon | 18 | 21 |
| Size of tumor (mm) | 35.3 ± 17.9 (12-80)b | 23.0 ± 9.1 (10-50)b |
| Histology | ||
| LGD | 15 | 12 |
| Tubular type | 11 | 11 |
| Tubulovillous type | 4 | 1 |
| HGD | 12 | 14 |
| Tubular typed | 0 | 13 |
| Tubulovillous typed | 12 | 1 |
| INV | 9 | 7 |
| Well differentiated type | 7 | 7 |
| Others | 2 | 0 |
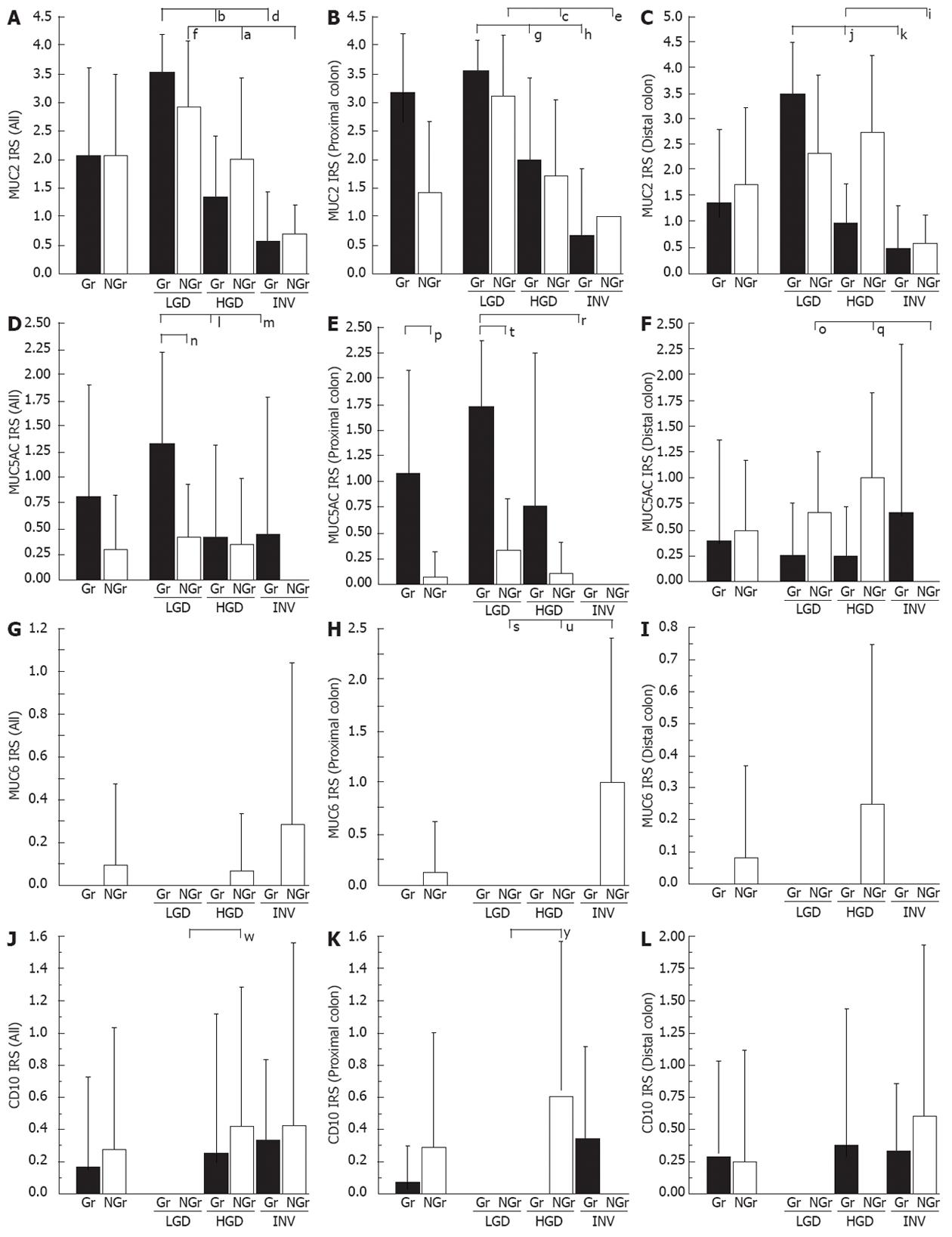
For the MUC2 IRS (Figure 2A-C), significant stepwise decrement was evident in progression through LGD - HGD-INV in Gr- and NGr-LSTs. For the MUC5AC IRS (Figure 2D-F), the Gr-type value was significantly higher than that of the NGr-type, in the proximal colon. MUC6 was expressed only in NGr-LSTs (Figure 2G-I). A low extent of CD10 expression was detected in HGD and INV, but not LGD of both Gr- and NGr-LSTs (Figure 2J-L).
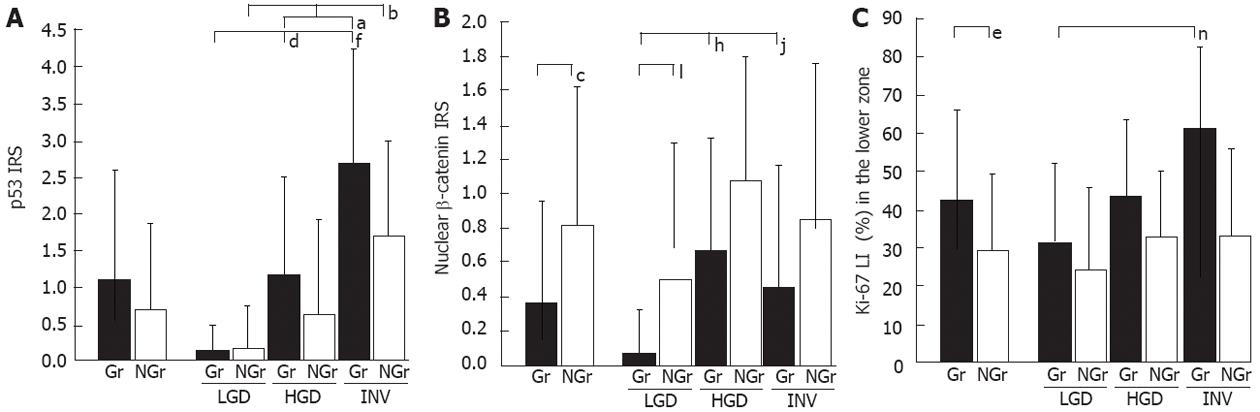
For the p53 IRS (Figure 3A), a stepwise increment in progression was noted through LGD to INV in Gr- and NGr-LSTs, which was statistically significant. For the nuclear β-catenin IRS (Figure 3B), the NGr-type value was significantly higher than the Gr-type. A similar trend for p53 and nuclear β-catenin IRSs was observed in proximal and distal LSTs (data not shown). For the Ki-67 LI (%) in the lower zone (Figure 3C), the Gr-type value was significantly higher than that of the NGr-type. A similar trend without statistical significance was found in the upper and middle zones (data not shown).
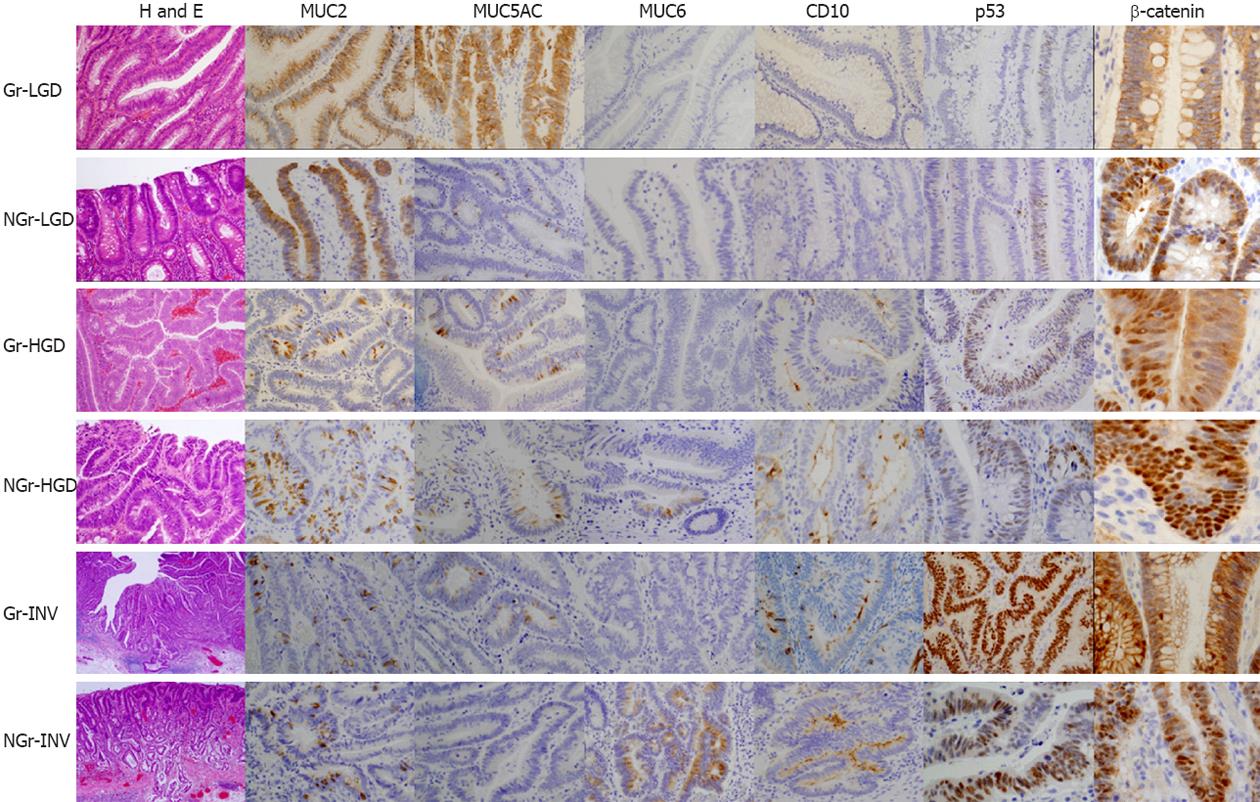
Representative H and E features and expression of MUC2, MUC5AC, MUC6, CD10, p53 and β-catenin are illustrated in Figure 4.
Data for correlations among IRS are given in Table 2. Significant inverse relationships were found between p53 and MUC2 or MUC5AC. In addition, inverse associations were shown between nuclear β-catenin and MUC2 in NGr-LSTs, or MUC5AC in Gr-LSTs. Reciprocally, inverse associations were noted between Ki-67 and MUC2 in Gr-LSTs, or MUC5AC in NGr-LSTs.
| MUC5AC | MUC6 | CD10 | p53 | Nuclearβ-catenin | Ki-67 | ||||||
| Gr-type | NGr-type | Gr-type | NGr-type | Gr-type | NGr-type | Gr-type | NGr-type | Gr-type | NGr-type | Gr-type | |
| MUC2 | 0.550b | 0.486b | NS | NS | -0.417a | NS | -0.549a | -0.450a | NS | 0.413a | -0.397a |
| MUC5AC | Blank | NS | NS | NS | NS | -0.430a | -0.368a | -0.334a | NS | NS | |
| MUC6 | Blank | Blank | NS | NS | NS | NS | NS | NS | NS | ||
| CD10 | Blank | Blank | Blank | NS | NS | NS | NS | NS | |||
| p53 | Blank | Blank | Blank | Blank | NS | NS | 0.514b | ||||
| Nuclear β-catenin | Blank | Blank | Blank | Blank | Blank | NS | |||||
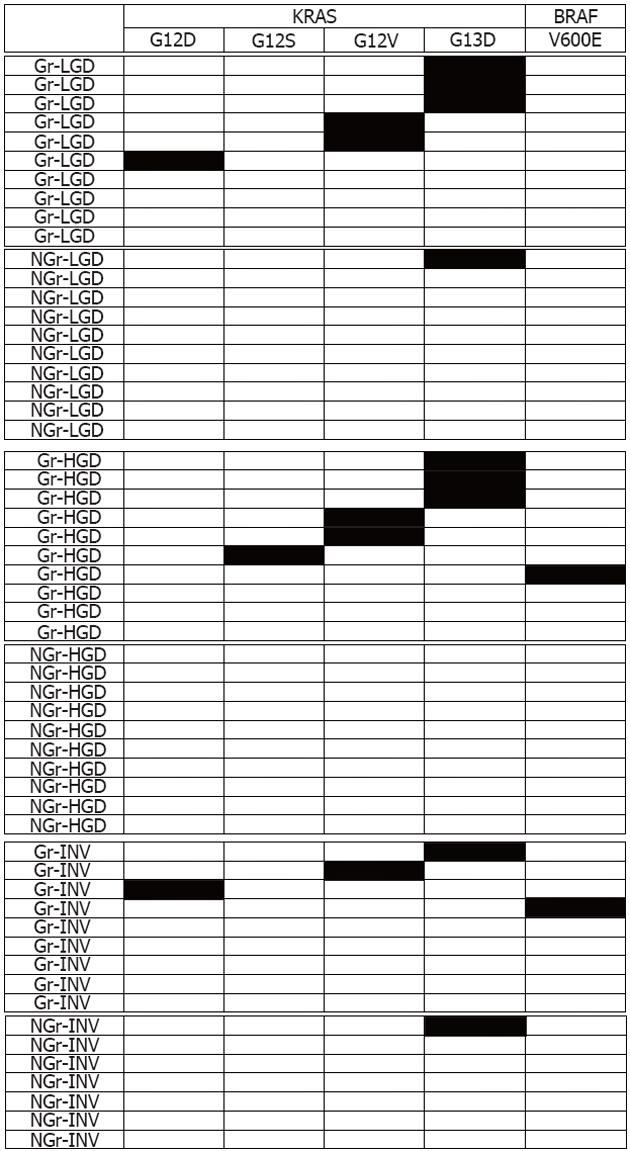
The results for KRAS and BRAF mutations analyzed are shown in Table 3. In proximal colon lesions, the incidence of KRAS mutations was significantly higher in Gr-LSTs (69%) than in NGr-LSTs (6%; P < 0.001). BRAF mutations were only detected in distal Gr-LSTs. In Gr-LSTs, 7 out of 29 (24%) tumors showed mutations from wild-type GGC (glycine) to GAC (aspartic acid) in codon 13 of the KRAS gene, and 5 (17%) tumors harbored mutations from GGT (glycine) to GTT (valine) in codon 12. In NGr-LSTs, 2 out of 27 (7%) tumors had mutations from GGC (glycine) to GAC (aspartic acid) in codon 13 of the KRAS gene. BRAF mutations were GTG (valine) to GAG (glutamic acid) in codon 600. A schematic representation of KRAS and BRAF mutational patterns is shown in Figure 5.
| Type of tumors | Number (%) of mutated samples | ||||
| All | Proximal colon | ||||
| n | KRAS | BRAF | n | KRAS | |
| Gr-type | 29 | 15 (52)a | 2 (7) | 13 | 9 (69)c |
| LGD | 10 | 6 (60) | 0 (0) | 7 | 6 (86)d,e |
| HGD | 10 | 6 (60)b | 1 (10) | 3 | 2 (67) |
| INV | 9 | 3 (33) | 1 (11) | 3 | 1 (33) |
| NGr-type | 27 | 2 (7)a | 0 (0) | 18 | 1 (6)c |
| LGD | 10 | 1 (10) | 0 (0) | 8 | 1 (13)d |
| HGD | 10 | 0 (0)b | 0 (0) | 8 | 0 (0) |
| INV | 7 | 1 (14) | 0 (0) | 2 | 0 (0) |
| Total | 56 | 17 (30) | 2 (4) | 30 | 10 (33) |
Since there were only two cases of NGr-LST harbored mutations of KRAS, and neither had BRAF mutations, we analyzed only Gr-LSTs with respect to the relationship of KRAS or BRAF mutations with clinicopathological parameters (age, sex, location, size of tumor, and dysplastic grade) and IRSs of the encoded proteins. No significant link was observed with clinicopathological parameters. The MUC5AC IRS was significantly higher in mutated Gr-LSTs compared to non-mutated tumors (1.2 ± 1.3 vs 0.2 ± 0.6, P < 0.05). No significant differences were found in other IRSs (data not shown).
Only a few reports are available on the phenotypic expression of gastrointestinal cell lineage with immunohistochemistry of MUC2, MUC5AC, MUC6 and CD10 in relation to colorectal adenoma-carcinoma sequence[24-26]. In proximal colon lesions, MUC5AC expression was significantly higher in Gr-LSTs than NGr-LSTs. Expression of MUC5AC or HGM was increased in adenoma with villous histology or polypoid growth[21,24,25]. Enhanced MUC6 immunoreactivity was reported to be exhibited in large adenomas[21]. In the current study, MUC6 was expressed only in NGr-LSTs, suggesting it as a marker of a morphologically distinct tumor. The molecular mechanisms responsible for the aberrant expression of gastric mucin, MUC5AC and MUC6 in colorectal tumors are still unclear and may be due to changes in transcriptional regulation.
It was previously reported that none of the four NGr-LSTs harbored p53 mutations, whereas 7 out of 24 (29%) Gr-LSTs were positive[16]. We have shown that no significant differences in p53 expression, along with an inverse relation of p53 to MUC2 or MUC5AC expression in two types of LSTs. An experimental study identified MUC2 expression as increased along with induction of wild-type p53 in carcinoma cell lines in vitro, and potential p53-binding sites in the MUC2 promoter, which contributes to stimulation of promoter activity[29]. P53 overexpression as an indirect sign of loss of functional p53 by its mutation is possibly related to the down-regulation of MUC2 expression in colorectal LST. A detailed study on the relation of p53 to the altered mucin expression appears warranted.
We have documented high nuclear β-catenin expression in NGr-LSTs in line with a previous study[15]. In an analysis of LSTs, no mutation of β-catenin exon 3 was found, while LOH at 5q (APC locus) was more frequently detected in NGr-LSTs than in Gr-LSTs[12]. To date, there is only one case report of NGr-LST harboring interstitial deletion of β-catenin exon 3[13]. Nuclear accumulation of β-catenin has been observed in tumors with mutations in the β-catenin or APC gene[3]. Taken together, the available data suggest that activation of Wnt/APC/β-catenin signaling in NGr-LSTs is due primarily to alterations in the APC gene. Furthermore, inverse associations were shown between nuclear β-catenin and MUC2 or MUC5AC in LSTs. Experimental studies have demonstrated that abrogation of MUC2 in tumors of the rat colon is related to nuclear β-catenin localization and its mutation[30,31]. Interaction of mucin core protein with Wnt/APC/β-catenin signaling may have some role in the progression of LSTs.
Ki-67, considered to be a reliable indicator for accurately assessing growth fraction, is increased with a shift in the proliferative zone toward the upper compartment in LSTs[11]. In the current study of Ki-67, a similar upward shift was detected in Gr- and NGr-LSTs. Furthermore, higher proliferation in the lower compartment was more apparent in Gr-LSTs, which may explain the morphologic variation in LSTs. We also found an inverse association between tumor cell proliferation (Ki-67) and MUC2 in LST. This is in line with the fact that decreased in vivo expression of MUC2 is related to colon carcinogenesis, accompanied by increased cell proliferation[32].
We have shown that, in proximal colon, the incidence of KRAS mutation was significantly higher in Gr-LST (69%) than NGr-LST (6%), with a relatively frequent and specific pattern in Gr-type for G12V, as it was for G12C in another report[16]. Previously reported incidences of KRAS mutation were 21%-83% in Gr-LST and 17%-26% in NGr-LST[12,16,17-19]. In the current study, BRAF mutations (V600E) were only detected in two Gr-LSTs. Gr-LSTs, particularly those located in the proximal colon, exhibited frequent KRAS mutations and high CIMP[18]. BRAF mutations are often characteristic of CIMP-high/microsatellite instability-high colorectal cancer[6], and are infrequent in LST[12,14,19]. These facts suggest that proximal Gr-LST is a possible candidate for early lesions of CIMP-high/microsatellite stable cancer. Furthermore, MUC5AC expression was significantly higher in KRAS mutated Gr-LSTs than in non-mutated tumors. Aberrant MUC5AC expression is thought to be related to KRAS mutations in experimental colon carcinogenesis[31]. In vitro, upregulation of MUC5AC may occur through concomitant activation of the EGFR/RAS/RAF/ERK signaling pathway and Sp1 binding to the gene promoter[33]. We therefore hypothesize that ERK signal activation induced by mutated RAS in relation to aberrant gastric mucin expression may play in a role in the development and progression of Gr-LSTs in the proximal colon.
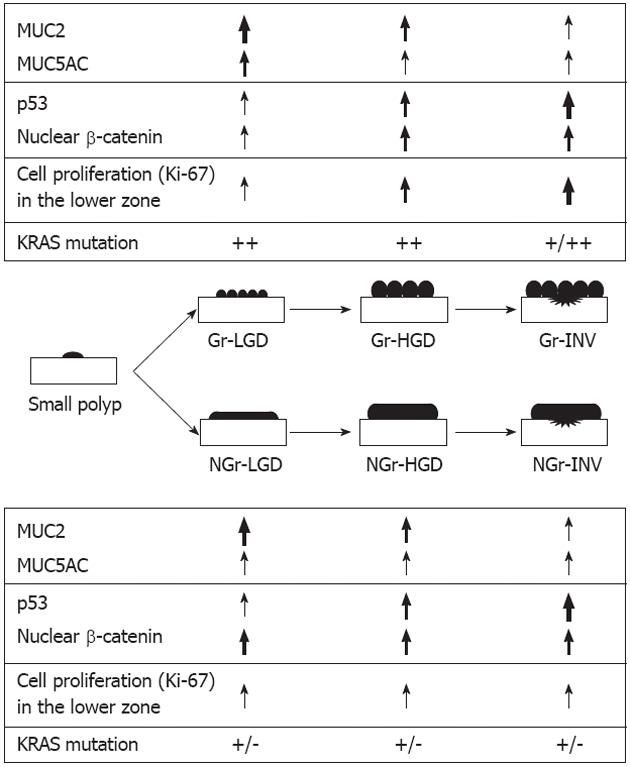
In conclusion, as summarized schematically in Figure 6, the two subtypes of LST have differing mucin phenotypic expression, proliferative activity, and activation of Wnt/β-catenin or RAS/RAF/ERK signaling in progression from adenomas through to invasive carcinomas.
We thank Hiroshi Abe and Keiko Mitani, Department of Human Pathology, Juntendo University School of Medicine, for their expert technical assistance.
Laterally spreading tumors (LSTs) in the colorectum are usually categorized into two subtypes: granular (Gr-LST) and flat- or non-granular types (NGr-LST). While considerable effort has been made to identify gene mutations and alteration of gastrointestinal phenotypes in conventional colorectal tumors, less attention has been paid to LSTs.
The authors focused on differences in expression of MUC2, MUC5AC, MUC6 and CD10, p53 alteration, nuclear translocation of β-catenin, cell proliferation, and v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS)/v-raf murine sarcoma viral oncogene homologue B1 mutations in morphologically different LST subtypes.
The authors showed that the two types of LSTs have different phenotypes, particularly with respect to MUC5AC (expression greater in Gr- vs NGr- types) and MUC6 (only expressed in NGr-type). They showed a higher nuclear β-catenin expression in NGr-type, and Ki-67 was much more prevalent in the Gr-type. Finally, the incidence of KRAS mutations was much more frequent in Gr-LST.
The subtypes of LSTs may be different candidates for alternative pathways of colorectal tumorigenesis. The results of the study represent a further impact on research in colorectal carcinogenesis.
This is a good descriptive study in which the authors clarify differences in mucin phenotype, proliferative activity and oncogenetic alteration among subtypes of LST. The results are interesting and suggest that they are different candidates for alternative pathways of colorectal carcinogenesis.
Peer reviewer: Dr. Inti Zlobec, PhD, Institute for Pathology, University Hospital Basel, Schoenbeinstrasse 40, CH-4031 Basel, Switzerland
S- Editor Lv S L- Editor Rutherford A E- Editor Lu YJ
| 1. | Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4616] [Cited by in RCA: 4464] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 2. | Cho KR, Vogelstein B. Genetic alterations in the adenoma--carcinoma sequence. Cancer. 1992;70:1727-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Wong NA, Pignatelli M. Beta-catenin--a linchpin in colorectal carcinogenesis? Am J Pathol. 2002;160:389-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 265] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 4. | Samowitz WS, Powers MD, Spirio LN, Nollet F, van Roy F, Slattery ML. Beta-catenin mutations are more frequent in small colorectal adenomas than in larger adenomas and invasive carcinomas. Cancer Res. 1999;59:1442-1444. [PubMed] |
| 5. | Wong SC, Lo ES, Lee KC, Chan JK, Hsiao WL. Prognostic and diagnostic significance of beta-catenin nuclear immunostaining in colorectal cancer. Clin Cancer Res. 2004;10:1401-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Kambara T, Simms LA, Whitehall VL, Spring KJ, Wynter CV, Walsh MD, Barker MA, Arnold S, McGivern A, Matsubara N. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53:1137-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 566] [Article Influence: 27.0] [Reference Citation Analysis (1)] |
| 7. | Nagasaka T, Sasamoto H, Notohara K, Cullings HM, Takeda M, Kimura K, Kambara T, MacPhee DG, Young J, Leggett BA. Colorectal cancer with mutation in BRAF, KRAS, and wild-type with respect to both oncogenes showing different patterns of DNA methylation. J Clin Oncol. 2004;22:4584-4594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy. 1993;25:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 553] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 9. | Hurlstone DP, Sanders DS, Cross SS, Adam I, Shorthouse AJ, Brown S, Drew K, Lobo AJ. Colonoscopic resection of lateral spreading tumours: a prospective analysis of endoscopic mucosal resection. Gut. 2004;53:1334-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 204] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Uraoka T, Saito Y, Matsuda T, Ikehara H, Gotoda T, Saito D, Fujii T. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut. 2006;55:1592-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 11. | Sada M, Mitomi H, Igarashi M, Katsumata T, Saigenji K, Okayasu I. Cell kinetics, p53 and bcl-2 expression, and c-Ki-ras mutations in flat-elevated tubulovillous adenomas and adenocarcinomas of the colorectum: comparison with polypoid lesions. Scand J Gastroenterol. 1999;34:798-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Sugimoto T, Ohta M, Ikenoue T, Yamada A, Tada M, Fujishiro M, Ogura K, Yamaji Y, Okamoto M, Kanai F. Macroscopic morphologic subtypes of laterally spreading colorectal tumors showing distinct molecular alterations. Int J Cancer. 2010;127:1562-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Nosho K, Yamamoto H, Mikami M, Takahashi T, Adachi Y, Endo T, Hirata K, Imai K, Shinomura Y. Laterally spreading tumour in which interstitial deletion of beta-catenin exon 3 was detected. Gut. 2005;54:1504-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Takahashi T, Nosho K, Yamamoto H, Mikami M, Taniguchi H, Miyamoto N, Adachi Y, Itoh F, Imai K, Shinomura Y. Flat-type colorectal advanced adenomas (laterally spreading tumors) have different genetic and epigenetic alterations from protruded-type advanced adenomas. Mod Pathol. 2007;20:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Hashimoto S, Higaki S, Amano A, Harada K, Nishikawa J, Yoshida T, Okita K, Sakaida I. Relationship between molecular markers and endoscopic findings in laterally spreading tumors. J Gastroenterol Hepatol. 2007;22:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Noro A, Sugai T, Habano W, Nakamura S. Analysis of Ki-ras and p53 gene mutations in laterally spreading tumors of the colorectum. Pathol Int. 2003;53:828-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Mukawa K, Fujii S, Takeda J, Kitajima K, Tominaga K, Chibana Y, Fujita M, Ichikawa K, Tomita S, Ono Y. Analysis of K-ras mutations and expression of cyclooxygenase-2 and gastrin protein in laterally spreading tumors. J Gastroenterol Hepatol. 2005;20:1584-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Hiraoka S, Kato J, Tatsukawa M, Harada K, Fujita H, Morikawa T, Shiraha H, Shiratori Y. Laterally spreading type of colorectal adenoma exhibits a unique methylation phenotype and K-ras mutations. Gastroenterology. 2006;131:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Kaji E, Kato J, Suzuki H, Akita M, Horii J, Saito S, Higashi R, Ishikawa S, Kuriyama M, Hiraoka S. Analysis of K-ras, BRAF, and PIK3CA mutations in laterally-spreading tumors of the colorectum. J Gastroenterol Hepatol. 2011;26:599-607. [PubMed] [DOI] [Full Text] |
| 20. | Ho SB, Niehans GA, Lyftogt C, Yan PS, Cherwitz DL, Gum ET, Dahiya R, Kim YS. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 1993;53:641-651. [PubMed] |
| 21. | Bartman AE, Sanderson SJ, Ewing SL, Niehans GA, Wiehr CL, Evans MK, Ho SB. Aberrant expression of MUC5AC and MUC6 gastric mucin genes in colorectal polyps. Int J Cancer. 1999;80:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Ho SB, Roberton AM, Shekels LL, Lyftogt CT, Niehans GA, Toribara NW. Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology. 1995;109:735-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 210] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Trejdosiewicz LK, Malizia G, Oakes J, Losowsky MS, Janossy G. Expression of the common acute lymphoblastic leukaemia antigen (CALLA gp100) in the brush border of normal jejunum and jejunum of patients with coeliac disease. J Clin Pathol. 1985;38:1002-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Takata M, Yao T, Nishiyama KI, Nawata H, Tsuneyoshi M. Phenotypic alteration in malignant transformation of colonic villous tumours: with special reference to a comparison with tubular tumours. Histopathology. 2003;43:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Koga Y, Yao T, Hirahashi M, Kumashiro Y, Ohji Y, Yamada T, Tanaka M, Tsuneyoshi M. Flat adenoma-carcinoma sequence with high-malignancy potential as demonstrated by CD10 and beta-catenin expression: a different pathway from the polypoid adenoma-carcinoma sequence. Histopathology. 2008;52:569-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Bu XD, Li N, Tian XQ, Li L, Wang JS, Yu XJ, Huang PL. Altered expression of MUC2 and MUC5AC in progression of colorectal carcinoma. World J Gastroenterol. 2010;16:4089-4094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Hamilton SR, Bosman FT, Boffetta P, Ilyas M, Morreau H. Carcinoma of the colon and rectum. World Health Organization classification of tumours of the digestive system. Lyon: IARC Press 2010; 134-146. |
| 28. | Imamhasan A, Mitomi H, Saito T, Arakawa A, Yao T. Clear cell variant of squamous cell carcinoma originating in the esophagus: report of a case with immunohistochemical and oncogenetic analyses. Pathol Int. 2012;62:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Ookawa K, Kudo T, Aizawa S, Saito H, Tsuchida S. Transcriptional activation of the MUC2 gene by p53. J Biol Chem. 2002;277:48270-48275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Femia AP, Bendinelli B, Giannini A, Salvadori M, Pinzani P, Dolara P, Caderni G. Mucin-depleted foci have beta-catenin gene mutations, altered expression of its protein, and are dose- and time-dependent in the colon of 1,2-dimethylhydrazine-treated rats. Int J Cancer. 2005;116:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Femia AP, Tarquini E, Salvadori M, Ferri S, Giannini A, Dolara P, Caderni G. K-ras mutations and mucin profile in preneoplastic lesions and colon tumors induced in rats by 1,2-dimethylhydrazine. Int J Cancer. 2008;122:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 715] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 33. | Perrais M, Pigny P, Copin MC, Aubert JP, Van Seuningen I. Induction of MUC2 and MUC5AC mucins by factors of the epidermal growth factor (EGF) family is mediated by EGF receptor/Ras/Raf/extracellular signal-regulated kinase cascade and Sp1. J Biol Chem. 2002;277:32258-32267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 221] [Article Influence: 9.6] [Reference Citation Analysis (0)] |