Published online Oct 7, 2012. doi: 10.3748/wjg.v18.i37.5240
Revised: June 11, 2012
Accepted: June 28, 2012
Published online: October 7, 2012
AIM: To determine the factors affecting mortality in patients who developed graft-versus-host disease (GvHD) after liver transplantation (LT).
METHODS: We performed a review of studies of GvHD following LT published in the English literature and accessed the PubMed, Medline, EBSCO, EMBASE, and Google Scholar databases. Using relevant search phrases, 88 articles were identified. Of these, 61 articles containing most of the study parameters were considered eligible for the study. Risk factors were first examined using a univariate Kaplan-Meier model, and variables with a significant association (P < 0.05) were then subjected to multivariate analyses using a Cox proportional-hazards model.
RESULTS: The 61 articles reported 87 patients, 58 male and 29 female, mean age, 40.4 ± 15.5 years (range: 8 mo to 74 years), who met the inclusion criteria for the present study. Deaths occurred in 59 (67.8%) patients, whereas 28 (32.2%) survived after a mean follow-up period of 280.8 ± 316.2 d (range: 27-2285 d). Among the most frequent symptoms were rash (94.2%), fever (66.6%), diarrhea (54%), and pancytopenia (54%). The average time period between LT and first symptom onset was 60.6 ± 190.1 d (range: 2-1865 d). The Kaplan-Meier analysis revealed that pancytopenia (42.8% vs 59.3%, P = 0.03), diarrhea (39.2% vs 61.0%, P = 0.04), age difference between the recipient and the donor (14.6 ± 3.1 years vs 22.6 ± 2.7 years, P < 0.0001), and time from first symptom occurrence to diagnosis or treatment (13.3 ± 2.6 mo vs 15.0 ± 2.3 mo, P < 0.0001) were significant factors affecting mortality, whereas age, sex, presence of rash and fever, use of immunosuppressive agents, acute rejection before GvHD, etiological causes, time of onset, and donor type were not associated with mortality risk. The Cox proportional-hazards model, determined that an age difference between the recipient and donor was an independent risk factor (P = 0.03; hazard ratio, 7.395, 95% confidence interval, 1.2-46.7).
CONCLUSION: This study showed that an age difference between the recipient and donor is an independent risk factor for mortality in patients who develop GvHD after LT.
- Citation: Akbulut S, Yilmaz M, Yilmaz S. Graft-versus-host disease after liver transplantation: A comprehensive literature review. World J Gastroenterol 2012; 18(37): 5240-5248
- URL: https://www.wjgnet.com/1007-9327/full/v18/i37/5240.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i37.5240
Graft-versus-host disease (GvHD) results from the reaction of donor immunocompetent cells against tissues of an immunosuppressed host[1-5]. GvHD is a well-known complication in patients who undergo allogeneic bone-marrow transplantation. However, few reports of GvHD after solid-organ transplantation include liver transplantation (LT)[6-9]. The reported incidence of this complication varies from 0.1% to 2%, with a mortality rate of > 75%; GvHD usually occurs between the second and sixth week after LT[5,10,11]. The clinical manifestations of GvHD following LT include fever, rash, diarrhea, and hematocytopenia, but the basic function of the transplanted liver is not affected[2,9,12,13]. The diagnosis of GvHD following LT can be difficult, as many of the clinical signs can be caused by drug reactions or viral infections including cytomegalovirus (CMV)[9]. Although a sizable number of modalities have been used to manage this disease, the most effective combination has not been determined.
The primary purpose of this study was to examine the existing literature on GvHD following LT. Thus, we conducted a thorough literature search regarding GvHD developing after LT using the PubMed, Medline, EBSCO, EMBASE, and Google Scholar databases from November 2011 to March 1988, when Burdick et al[14] presented the first study on GvHD following LT. The keywords we used for the search were “graft-versus-host disease,”“graft-versus-host disease after liver transplantation,”“graft-versus-host disease following liver transplantation,” and “graft-versus-host disease and solid-organ transplantation.” The reference lists of all articles introduced as reviews were checked to attain a wider search range. The search identified 88 article titles. More detailed information was requested through contact with the corresponding authors and/or the related journal editors for studies in which insufficient data were provided or in which full texts could not be accessed. Twenty-seven full-text articles were excluded from the study because the authors could not be reached, a case presentation was duplicated, or only a literature review was provided that did not include sufficient information for comparison with other studies. A total of 61 articles containing most of the parameters mentioned below were considered eligible for the study. One of the two cases presented by Schuchmann et al[15] was excluded because it was presented in another study. The study by Knox et al[16] was excluded because only pulmonary GvHD developed following LT. In the 61 studies for which full texts could be accessed, the following data were evaluated: age, sex, donor age, age difference between the recipient and the donor, blood group compatibility (identical or not), donor type (living/cadaveric), use of primary immunosuppressive medications (tacrolimus, cyclosporine, or azathiopurine), primary hepatic disease, time of onset (postoperative day), first manifestations (rash, fever, pancytopenia, thrombocytopenia, leukopenia, and diarrhea), time interval elapsed between the first manifestation and the diagnosis and/or treatment (d), re-transplantation, mortality, and follow-up. The aim of this literature search was to identify factors affecting the occurrence of mortality in post-transplantation GvHD. Thus, the patients were divided into a mortality group (n = 59) and a survival group (n = 28). Accordingly, symptoms such as fever, rash, and diarrhea were collected under the title of “first symptoms” after ruling out other possible causes. Similarly, pancytopenia, thrombocytopenia, or leukopenia that developed before the confirmation of the GvHD diagnosis were all collected under the title of “pancytopenia.” Symptoms or hematological disorders developing after commencement of treatment were left out of the former classifications. The time period between development of the first symptom associated with the disease and the transplantation was termed “time of onset.”
SPSS version 13.0 (SPSS, Inc., Chicago, IL, United States) was used for the statistical analysis. Data are presented as mean ± SD for continuous variables and as frequencies for categorical variables. The statistical significance of differences between groups was examined using Pearson’sχ2 test for categorical variables and the Student t-test for continuous variables. Risk factors for outcomes were first examined using a univariate Kaplan-Meier model, and variables with a significant association (P < 0.05) were then subjected to multivariate analyses using a Cox proportional-hazards model. All statistical tests were two-sided with a significance level of 0.05.
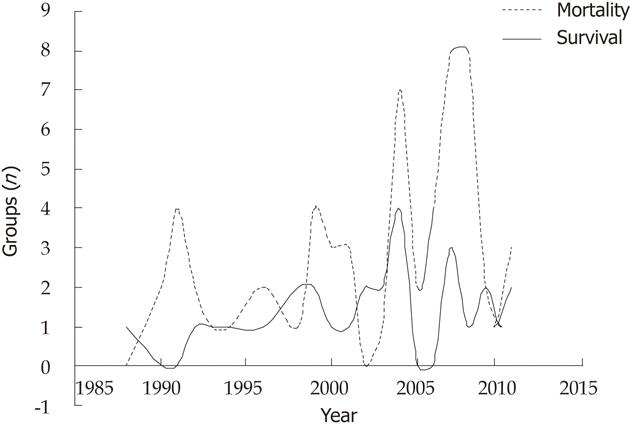
We retrospectively evaluated 61 studies that included 87 patients, 58 male and 29 female, with age range of 8 mo to 74 years (mean, 40.4 ± 15.5 years). There were 59 (67.8%) deaths, while 28 (32.2%) survived at a mean follow-up of 280.8 ± 316.2 d (range: 27-2285 d). In the Kaplan-Meier model, parameters such as pancytopenia (P = 0.03), diarrhea (P = 0.04), age difference between the recipient and the donor (P < 0.0001), and the time elapsed between development of the first symptoms and the diagnosis or treatment (P < 0.0001) were significant risk factors for mortality. The results of multivariate Cox proportional-hazards model analysis revealed that age difference was an independent and strong risk factor (P = 0.03; hazard ratio, 7.395, 95% confidence interval, 1.2-46.7). Kaplan-Meier mortality curves for patients with and without diarrhea and pancytopenia are presented in Figure 1. Demographic and statistical data for the mortality and survival groups is provided in Tables 1 and 2. The distribution of both groups by time since the first description of GvHD after LT is depicted in Figure 2. We noted that mortality rates peaked in some years and that cases in the survival group pursued a more stable course compared with those in the mortality group.
| Characteristics | Surviving (n = 28) | Dead (n = 59) | Total (n = 87) | Univariate analysis | Multivariate analysis |
| Age, yr | 38.7 ± 22.7 | 40.4 ± 15.5 | 40.4 ± 15.5 | 0.1 | |
| Sex | 0.8 | ||||
| Male | 20 (71.4) | 38 (64.4) | 58 (66.6) | ||
| Female | 8 (28.6) | 21 (35.6) | 29 (33.4) | ||
| Rash | 0.8 | ||||
| Present | 27 (96.4) | 55 (93.2) | 82 (94.2) | ||
| Absent | 1 (3.6) | 4 (6.8) | 5 (5.8) | ||
| Fever | 0.1 | ||||
| Present | 17 (60.7) | 41 (69.5) | 58 (66.7) | ||
| Absent | 11 (39.3) | 18 (30.5) | 29 (33.3) | ||
| Pancytopenia | 0.03 | 0.6 | |||
| Present | 12 (42.9) | 35 (59.3) | 47 (54) | ||
| Absent | 16 (57.1) | 24 (40.7) | 40 (46) | ||
| Diarrhea | 0.04 | 0.1 | |||
| Present | 11 (39.3) | 36 (61) | 47 (54) | ||
| Absent | 17 (60.7) | 23 (39) | 40 (46) | ||
| Acute rejection before GvHD | 0.4 | ||||
| Yes | 7 (25) | 8 (13.6) | 15 (17.3) | ||
| No | 21 (75) | 51 (86.4) | 72 (82.7) | ||
| Immunsuppressive agent | 0.5 | ||||
| Tacrolimus | 15 (53.6) | 25 (42.4) | 40 (46) | ||
| Cyclosporine | 10 (35.7) | 20 (33.9) | 30 (34.5) | ||
| Azathiopurine | 0 (0) | 1 (1.7) | 1 (1.1) | ||
| Un-noted | 3 (10.7) | 13 (22) | 16 (18.4) | ||
| Re-transplantation | 0.4 | ||||
| Yes | 2 (92.9) | 2 (3.4) | 4 (4.6) | ||
| No | 26 (7.1) | 57 (96.6) | 83 (95.4) | ||
| Time of onset (POD), d | 109 ± 64 | 38 ± 5 | 60.6 ± 190.11 | 0.4 | |
| Etiology | 0.3 | ||||
| Donor type | 0.2 | ||||
| Cadaveric | 16 (57.1) | 16 (27.1) | 32 (36.8) | ||
| Living | 2 (7.2) | 8 (13.6) | 10 (11.5) | ||
| Un-noted | 10 (35.7) | 35 (59.3) | 45 (51.7) | ||
| Age difference between recipient and donor, yr | 14.6 ± 3.1 | 22.6 ± 2.7 | 19.8 ± 13.2 | < 0.0001 | 0.031 |
| Blood group | 0.4 | ||||
| Identical | 16 (57.1) | 30 (51) | 46 (52.9) | ||
| Non-identical | 3 (10.7) | 3 (5) | 6 (6.9) | ||
| Un-noted | 9 (32.1) | 26 (44) | 35 (40.2) | ||
| Time between symptoms and diagnosis or first treatment, mo | 13.3 ± 2.6 | 15.0 ± 2.3 | 14.3 ± 14.3 | < 0.0001 | 0.1 |
| Etiology | Surviving (n = 28) | Dead (n = 59) | Total (n = 87) |
| ALD | 4 | 7 | 11 |
| ALD + HCC | 1 | 3 | 4 |
| ALD + HCV | 0 | 3 | 3 |
| HBV | 1 | 3 | 4 |
| HBV + HCC | 3 | 6 | 9 |
| HBV + HDV | 1 | 0 | 1 |
| HCV | 0 | 7 | 7 |
| HCV + HCC | 1 | 0 | 1 |
| HCC | 1 | 3 | 4 |
| PBC | 1 | 5 | 6 |
| PSC | 1 | 3 | 4 |
| PSC + HCC | 0 | 2 | 2 |
| Biliary atresia | 4 | 2 | 6 |
| Hemangioma | 0 | 1 | 1 |
| Cryptogenic | 4 | 5 | 9 |
| Acute failure | 3 | 4 | 7 |
| Autoimmune hepatitis | 1 | 1 | 2 |
| HAV | 1 | 0 | 1 |
| Policystic disease | 0 | 1 | 1 |
| Laennec's cirrhosis | 1 | 0 | 1 |
| Wilson disease | 0 | 1 | 1 |
| Langerhans' cell histiocytosis | 0 | 1 | 1 |
| NRH | 0 | 1 | 1 |
GvHD was first described by Billingham in 1966 as a reaction of the donor’s immunocompetent cells against the recipient’s cellular antigens[17,18]. The development of GvHD implies the fulfillment of three prerequisites: (1) a source of immunocompetent lymphocytes; (2) histocompatible antigenic differences between donor and host; and (3) inability of the host to reject donor lymphocytes[10,19-21]. This reaction occurs in as many as 80% of patients after bone-marrow transplantation. It has also been infrequently reported after transfusion of blood products or after solid-organ transplantation, such as pancreas-spleen, heart-lung, and liver[6,14,17,22,23]. The development of GvHD after solid-organ transplantation was first defined in 1984 by Starzl et al[24] in a patient undergoing a combined pancreas and splenic transplantation operation. GvHD developing after LT was first defined by Burdick et al[14] in 1988.
Although the exact mechanisms are still unclear, the three basic prerequisites mentioned above are also applicable to GvHD after LT. An estimated 109-1010 donor lymphocytes remain in the portal tracts and the parenchyma of a donor liver graft after flushing with cold preservative solution[18,21,25-27]. These T-cells are detectable in the peripheral blood and organs of patients during the first weeks after LT[18,20,25,26,28]. The donor lymphocytes colonize the recipient, recognize the host tissue antigens as foreign, and react against the host tissue. In other words, if the “balance of power” between the donor and recipient immune systems favors the donor, donor lymphocytes may be activated, leading to GvHD.
Although the exact incidence of GvHD following LT remains to be determined, various studies have cited rates of 0.1%-2%[27,29-32]. Yuksekkaya et al[11] reported that the incidence of GvHD was as high as 22.2% in patients whose donors were mismatched on at least one human leukocyte antigen (HLA) A and B antigens[11,33]. In our examination of 15 articles, we found that GvHD was evident in only 62 (0.06%) of 9492 patients undergoing LT, which was similar to frequencies reported previously[32-36].
GvHD has been reported after solid-organ transplantation with humoral and cellular presentations. The humoral type, also known as graft-versus-host hemolysis, is characterized by hemolysis and fever and occurs in patients transplanted with ABO-incompatible or non-identical grafts. The cellular type of GvHD occurs when immunocompetent donor lymphocytes originating from the transplanted liver undergo activation and clonal expansion, allowing them to mount a destructive cellular immune response against recipient tissues. The response is directed against the major histocompatibility complex and often results in severe multisystem disease with a high mortality rate[19,37-40].
GvHD responses can be classified as acute or chronic, depending on the timing and character of alloimmune activity[11,41,42]. Acute GvHD comprises all manifestations that occur during the first 100 d after transplantation, and chronic GvHD includes all manifestations that occur after 100 d[11,42-44]. However, multiple findings suggest that this may no longer be a suitably useful distinction. Acute GvHD lesions may be found after 100 d, whereas chronic GvHD lesions sometimes appear before 100 d. Acute GvHD histological findings can be found in biopsies performed after day 100, and lichenoid findings can be found in biopsies performed before day 100[42,44]. The number of days after transplant is an insufficient criterion to distinguish acute from chronic GvHD. Good clinical and pathological descriptions are needed. Chronic GvHD can occur as a progression of acute GvHD, as a recurrence following a disease-free interval, or without a history of acute GvHD. Each of these forms accounts for approximately one-third of cases.
The causes of GvHD following organ transplantation have not been clarified, but several risk factors have been implicated, including close HLA matching between the recipient and donor[18,27,45], blood transfusion prior to transplantation[11], immunosupressive treatment before transplantation[11,27], glucose intolerance[35], rejection before GvHD[4], autoimmune hepatitis[35], alcoholic liver disease[35], hepatocellular carcinoma (HCC)[27,35], re-transplantation[27], a large age discrepancy between donor (younger) and recipient (older)[25], recipient age > 65 years[18,27,45,46], and multiorgan transplantation[5,21,33,35,40]. Only two studies offered an evidence-based risk analysis with regard to the development of GvHD after LT. In a study by Smith et al[33], risk factors included recipient age ≥ 65 years, recipient-donor age difference ≥ 40 years, and close matching of the HLA types of the donor to those of the recipient. Chan et al[35] documented glucose intolerance, autoimmune hepatitis, alcoholic liver disease, HCC, and various combinations of these but not such parameters as age, sex, ischemia duration, HLA mismatch, or age differences as risk factors. Chan et al[35] argued that most of the risk factors they identified permitted patients to lapse into an immunosuppressive state, suggesting an inclination toward development of the disease before the LT operation. Our literature search showed that the most frequently encountered liver diseases in affected patients were HCC (23%) and alcoholic liver disease (20.7%). The suggestions by Chan et al[35] support our findings, but we lack confirming evidence.
The clinical presentation of GvHD following LT includes skin rash, fever, diarrhea and hematocytopenia[5,47]. Characteristically, the transplanted liver is not a target of GvHD after LT because both graft liver and immunocompetent cells responsible for GvHD are of donor origin[25,28,30]. The most frequently appearing symptoms in our search were rash (94.2%), fever (66.6%), diarrhea (54%), and pancytopenia (54%). Among these symptoms, pancytopenia (P = 0.03) and diarrhea (P = 0.04) were confirmed by univariate analysis to be risk factors affecting mortality. These results indicate that intestinal and bone-marrow involvement may give rise to severe complications.
The clinical symptoms of GvHD usually become apparent between 1 and 8 wk after LT, often after an initial uneventful recovery from surgery and discharge from the hospital[40,48]. Our literature review revealed that the first symptoms appear 60.6 ± 190.1 d (range: 2-1865 d) after the LT operation. Although the time interval was shorter in the non-surviving group, it was not among the risk factors for death (P = 0.4). Despite this result, we believe that the mortality in cases complicated with GvHD within the first month is much higher.
A diagnosis of GvHD after LT is based on the presence of clinical manifestations, a demonstration of chimerism, and histopathological evidence[29,42,47,49]. As the clinical presentation of GvHD is inconsistent, a high degree of suspicion is necessary to pursue a diagnosis. Any or all clinical symptoms mentioned above may be seen during the initial presentation of GvHD. A skin biopsy showing epidermal dyskeratosis with epithelial cell necrosis is highly suggestive but not pathognomonic for GvHD[29,39,40,48]. Chimerism can be established by various methods that examine the presence of donor cells in the recipient’s peripheral blood or various tissues[41,50]. These methods include serological HLA typing of peripheral blood, restriction fragment length polymorphism[28,29,51-53], and fluorescent in situ hybridization (FISH), which have been used to demonstrate chimerism in recipients with suspected GvHD after LT[28,34,48]. Chimerism at the tissue level has been shown by polymerase chain reaction, short tandem repeat analysis, and FISH techniques in the skin and bone marrow of patients with GvHD after LT[46,48,52,54,55]. Peripheral blood chimerism appears transiently in the majority of patients during the early postoperative period after LT, particularly in the first week, and rapidly declines by the third to fourth week post-transplant[39,45]. For this reason, chimerism may not be evident in the peripheral blood of patients with late-onset GvHD[32,56].
The differential diagnosis of GvHD after LT is frequently delayed because early symptoms are often non-specific. The differential diagnosis consists of (1) drug-induced skin reactions, including toxic epidermal necrolysis and mycophenolate mofetil toxicity; (2) viral exanthemas; (3) infectious enteritis, including CMV infection and Clostridium difficile colitis, and (4) organ rejection[13,41,57-59]. Many of the clinical signs of GvHD may also be seen with CMV infection. The presence of CMV in a patient with GvHD may complicate the appropriate diagnosis and delay treatment. A significant association between acute GvHD and CMV after transplant has been documented and may be related to pancytopenia resulting from bone-marrow depletion by attacking donor lymphocytes[9,11,34,39].
A rapid differential diagnosis and early implementation of treatment for GvHD following LT are two factors that affect survival. In contrast, studies showing that early treatment was not effective in the ultimate outcome have also been published[18,40]. Taylor et al[40] based their opinions on a literature search. They reported that early implementation of treatment did not produce a statistically significant difference in mortality. We found that the time interval between the appearance of first symptoms and definitive diagnosis and/or treatment, which ranged from 1 to 65 d, was a statistically significant predictor of death (P < 0.0001).
The evidence base for selecting the most appropriate therapy for established GvHD after LT is very limited; thus, treatment is largely empirical, although the extensive literature on managing acute GvHD after stem cell transplantation provides guidance[21,25,27,40]. A number of treatment modalities have been proposed based on the known pathophysiological mechanism of GvHD. However, as most of the treatment modalities are implemented in combinations, the optimal combination has not yet been identified. Moreover, some patients respond well to a decrease in the intensity of immunosuppressive treatment[38,55], or to replacement with another immunosuppressive agent[38,39,60,61], but good outcomes have also been reported using incremental doses of immunosuppressive drugs[52,57,58,62]. On the other hand, the literature has also reported the development of acute rejection in patients whose immunosuppressive drug dosage was decreased or the relevant medication was ceased; hence, switching to another medication may seem more reasonable than changing the dosage of the main immunosuppressive agent[31]. Each patient should be evaluated individually.
Among the most frequently administered treatment modalities for GvHD after LT mentioned in the literature are corticosteroid treatment[9,25,46,56], decrease/cessation/increase in or replacement of the immunosuppressive medication[22,26,52,55,62], and the use of antibodies directly targeting T lymphocytes, monoclonal antibodies targeting various receptors on the surfaces of lymphocytes, intravenous immunoglobulin[31,42,58,63,64] as an immune support, and antimicrobial treatments appropriate to suppress the infection[10,18].
Most of the experience regarding corticosteroid use in treating GvHD is based on the practices of hematopoietic stem cell transplantation[4]. The lympholytic and immunosuppressive effects of steroids, in addition to their potent anti-inflammatory characteristics, have provided justification for their widespread administration[4]. In our literature search, steroid treatment was instituted in 61 of 87 patients in whom GvHD developed after LT, whereas other treatment modalities were preferred in 21, and the remaining 5 patients were monitored for symptoms[19,38,65]. Death occurred in 43 patients on steroid treatment. Immunosuppressive treatment was re-administered upon development of acute rejection in two patients in whom the main immunosuppressive treatment was replaced by steroid treatment. Etanercept (Enbrel) therapy was commenced in one patient due to a failed response to steroid treatment, and a reduction in cyclosporine, and this approach yielded a successful outcome[45]. Most of the patients who experienced complications or a suboptimal response to treatment were administered various monoclonal antibodies or antagonist agents to T-lymphocytes[65-69]. The most commonly used drugs were the following: daclizumab (Zenapax)[21,29,32,68] and basiliximab (Simulect)[4,5,11,28,41], which bind to the CD25 subunit of interleukin (IL)-2 receptors on the surface of T-lymphocytes; muromonab (OKT3)[7,23,47,53], which binds to CD3 receptors on the surface of T-lymphocytes; alemtuzumab (Campath-1H)[50], which binds to CD52 receptors on the surface of mature lymphocytes; infliximab (Remicade)[30,32], which was developed against tumor necrosis factor-alpha; denileukin diftitox (Ontak)[39], which was developed by conjugation with diphtheria toxin for use against the IL-2 receptors on the surface of T-lymphocytes; and rituximab (Mabthera)[5], which binds to CD20 receptors on the surface of B lymphocytes. In addition to these agents, anti-thymocyte globulin (ATG)[26,28,66,67,69], effective directly on T-lymphocytes, and anti-lymphocyte globulin (ALG)[6,19,22] were also frequently utilized during treatment. In our literature analysis, we found that ATG, basiliximab, muromonab, ALG, daclizumab, infliximab, alemtuzumab, and rituximab and denileukin diftitox were administered in 25, 11, 7, 5, 4, 2, 1, 1, and 1 of the patients, respectively. Of these 57 patients, 13 were placed on monoclonal antibodies and/or T-lymphocyte antagonists as a first treatment modality, whereas steroids, immunosuppressive agents, and various combinations thereof were administered in 44 patients. Mortality rates did not differ among treatment conditions but were quite high in all treatment modalities, indicating that the most appropriate treatment modality has yet to be developed.
The prognosis for GvHD that develops after LT is rather poor, and mortality rates mentioned in the literature range from 75% to 91.6%[9,27,28,32,58]. The mortality rate observed in our literature analysis (67.8%) was lower than that reported in studies cited above. Nearly all patients died of multiple organ dysfunction syndrome, sepsis, or gastrointestinal bleeding despite significant antimicrobial and hematologic support. The only study evaluating mortality in GvHD after LT was a literature search conducted by Taylor et al[40] that included 51 cases. According to that study, rash and fever were identified as risk factors for mortality. We obtained different results (Table 1), which suggest that bone marrow (pancytopenia) and intestinal (diarrhea) involvement had a severe effect on mortality. However, the retrospective nature of this study, exclusion of some studies due to inadequate data, failure to obtain sufficient data regarding an HLA match, and the absence of a standardized treatment protocol were limiting factors. Such high rates of mortality despite any type of aggressive treatment revive the issue of protective precautions prior to LT.
Preventing GvHD among patients undergoing LT is an important issue. Depletion of T-lymphocytes from the liver before transplantation would eliminate the risk of GvHD. This could be achieved, at least in principal, by treating the cadaveric donor with ALG or by modifying the donor liver ex vivo by irradiation or perfusion with lytic monoclonal antibodies directed against a lymphocyte cell-surface protein[7,8]. However, whether these approaches can be justified is debatable, given the low incidence of GvHD after LT[40,47]. The donor’s immunoactive cells can be removed by sufficient perfusion of the graft by carefully removing perihepatic lymph nodes or through graft radiation[31]. Based on our LT experience, perfusion of grafts from living or cadaveric donors with University of Wisconsin (Viaspan) or histidine-tryptophan-ketoglutarate (Custodiol) solution, followed by lactated Ringer’s solution at 4 °C, has proved a fairy efficacious method to remove donor-related lymphocytes from graft material. Some authors believe that transfusion-associated GvHD can be prevented by irradiating blood products and avoiding the use of related donors. Therefore, limiting the application of blood products and using washed red blood cells, white blood cell-free plasma, or platelets could contribute to the prevention of GvHD[31]. We prefer to irradiate erythrocyte suspensions routinely before transfusion in patients who have undergone LT.
In conclusion, although GvHD is a rare complication of LT and the mortality rate remains very high, clinical features represent an important tool for early diagnosis. The prognosis remains poor and further research is needed to clarify the pathogenesis of GvHD and to provide new therapeutic agents for treating this condition effectively.
Graft-versus-host disease (GvHD) occurs when the donor’s immunocompetent cells react against the recipient’s cellular antigens. GvHD is a well-known complication in patients who undergo bone-marrow transplantation. However, few reports have described GvHD after liver or other solid organ transplantation. The prognosis for GvHD after liver transplantation (LT) is rather poor, and mortality rates mentioned in the literature range from 75% to 91.6%. Therefore, it is important to determine the factors that affect the prognosis of the disease.
The authors performed an extensive literature review regarding the development of GvHD after LT that included articles in the PubMed, Medline, EBSCO, EMBASE, and Google Scholar databases published between November 2011 and March 1988.
This study is the most extensive literature review examining factors affecting mortality in patients who develop GvHD after LT.
Univariate analyses showed that pancytopenia, diarrhea, an age difference between the recipient and donor, and time from first symptom occurrence to diagnosis or treatment were significant risk factors for mortality, and multivariate analysis demonstrated that an age difference between the recipient and donor was an independent risk factor for mortality.
GvHD can be divided into acute and chronic forms depending on the timing and character of alloimmune activity. The acute form comprises all manifestations that occur during the first 100 d after transplantation, and the chronic form includes all manifestations that occur after 100 d. GvHD can also be divided into humoral and cellular forms. The humoral form is characterized by hemolysis and fever and occurs in patients transplanted with ABO-incompatible or non-identical grafts. The cellular form occurs when immunocompetent donor lymphocytes originating from the transplanted liver undergo activation and clonal expansion, allowing them to mount a destructive cellular immune response against recipient tissues.
This is a good descriptive study in which authors determine the factors affecting mortality in patients who developed GvHD after LT. The results are interesting and showed that an age difference between the recipient and donor is an independent risk factor for mortality in patients who develop GvHD after LT.
Peer reviewer: Rui Tato Marinho, Professor, Department of Gastroenterology and Hepatology, Hospital Santa Maria, Rua Prof. Aires de Sousa, 1 r/c A, 1600-590 Lisboa, Portugal
S- Editor Gou SX L- Editor Cant MR E- Editor Xiong L
| 1. | DePaoli AM, Bitran J. Graft-versus-host disease and liver transplantation. Ann Intern Med. 1992;117:170-171. [PubMed] |
| 2. | Sudhindran S, Taylor A, Delriviere L, Collins VP, Liu L, Taylor CJ, Alexander GJ, Gimson AE, Jamieson NV, Watson CJ. Treatment of graft-versus-host disease after liver transplantation with basiliximab followed by bowel resection. Am J Transplant. 2003;3:1024-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Pollack MS, Speeg KV, Callander NS, Freytes CO, Espinoza AA, Esterl RM, Abrahamian GA, Washburn WK, Halff GA. Severe, late-onset graft-versus-host disease in a liver transplant recipient documented by chimerism analysis. Hum Immunol. 2005;66:28-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Perri R, Assi M, Talwalkar J, Heimbach J, Hogan W, Moore SB, Rosen CB. Graft vs. host disease after liver transplantation: a new approach is needed. Liver Transpl. 2007;13:1092-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Mawad R, Hsieh A, Damon L. Graft-versus-host disease presenting with pancytopenia after en bloc multiorgan transplantation: case report and literature review. Transplant Proc. 2009;41:4431-4433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Bhaduri BR, Tan KC, Humphreys S, Williams R, Donaldson P, Vergani D, Mowat AP, Mieli-Vergani G. Graft-versus-host disease after orthotopic liver transplantation in a child. Transplant Proc. 1990;22:2378-2380. [PubMed] |
| 7. | Sanchez-Izquierdo JA, Lumbreras C, Colina F, Martinez-Laso J, Jiménez C, Gómez R, García I, Alvarez M, Arnaiz-Villena A, Moreno E. Severe graft versus host disease following liver transplantation confirmed by PCR-HLA-B sequencing: report of a case and literature review. Hepatogastroenterology. 1996;43:1057-1061. [PubMed] |
| 8. | Soejima Y, Shimada M, Suehiro T, Hiroshige S, Gondo H, Takami A, Yasue S, Maehara Y. Graft-versus-host disease following living donor liver transplantation. Liver Transpl. 2004;10:460-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Sun B, Zhao C, Xia Y, Li G, Cheng F, Li J, Zhang F, Wang X. Late onset of severe graft-versus-host disease following liver transplantation. Transpl Immunol. 2006;16:250-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Guo ZY, He XS, Wu LW, Zhu XF, Ju WQ, Wang DP, You S, Ma Y, Wang GD, Huang JF. Graft-verse-host disease after liver transplantation: a report of two cases and review of literature. World J Gastroenterol. 2008;14:974-979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Yuksekkaya HA, Arikan C, Tumgor G, Aksoylar S, Kilic M, Aydogdu S. Late-onset graft-versus-host disease after pediatric living-related liver transplantation for Langerhans cell histiocytosis. Pediatr Transplant. 2011;15:E105-E109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Lehner F, Becker T, Sybrecht L, Lück R, Schwinzer R, Slateva K, Blasczyk R, Hertenstein B, Klempnauer J, Nashan B. Successful outcome of acute graft-versus-host disease in a liver allograft recipient by withdrawal of immunosuppression. Transplantation. 2002;73:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Schrager JJ, Vnencak-Jones CL, Graber SE, Neff AT, Chari RS, Wright KJ, Pinson CW, Stewart JH, Gorden DL. Use of short tandem repeats for DNA fingerprinting to rapidly diagnose graft-versus-host disease in solid organ transplant patients. Transplantation. 2006;81:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Burdick JF, Vogelsang GB, Smith WJ, Farmer ER, Bias WB, Kaufmann SH, Horn J, Colombani PM, Pitt HA, Perler BA. Severe graft-versus-host disease in a liver-transplant recipient. N Engl J Med. 1988;318:689-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 135] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Schuchmann M, Meyer RG, Distler E, von Stebut E, Kuball J, Schnürer E, Wölfel T, Theobald M, Konur A, Gregor S. The programmed death (PD)-1/PD-ligand 1 pathway regulates graft-versus-host-reactive CD8 T cells after liver transplantation. Am J Transplant. 2008;8:2434-2444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Knox KS, Behnia M, Smith LR, Vance GH, Busk M, Cummings OW, Kwo PY, Wilkes DS. Acute graft-versus-host disease of the lung after liver transplantation. Liver Transpl. 2002;8:968-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Triulzi DJ, Nalesnik MA. Microchimerism, GVHD, and tolerance in solid organ transplantation. Transfusion. 2001;41:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Chaib E, Silva FD, Figueira ER, Lima FR, Andraus W, D'Albuquerque LA. Graft-versus-host disease after liver transplantation. Clinics (Sao Paulo). 2011;66:1115-1118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Roberts JP, Ascher NL, Lake J, Capper J, Purohit S, Garovoy M, Lynch R, Ferrell L, Wright T. Graft vs. host disease after liver transplantation in humans: a report of four cases. Hepatology. 1991;14:274-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Collins RH, Cooper B, Nikaein A, Klintmalm G, Fay JW. Graft-versus-host disease in a liver transplant recipient. Ann Intern Med. 1992;116:391-392. [PubMed] |
| 21. | Wang B, Lu Y, Yu L, Liu C, Wu Z, Liu X. Diagnosis and treatment for graft-versus-host disease after liver transplantation: two case reports. Transplant Proc. 2007;39:1696-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Marubayashi S, Matsuzaka C, Takeda A, Costa MM, Jamieson NV, Joysey V, Calne RY. Fatal generalized acute graft-versus-host disease in a liver transplant recipient. Transplantation. 1990;50:709-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Redondo P, España A, Herrero JI, Quiroga J, Cienfuegos JA, Azanza JR, Prieto J. Graft-versus-host disease after liver transplantation. J Am Acad Dermatol. 1993;29:314-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Starzl TE, Iwatsuki S, Shaw BW, Greene DA, Van Thiel DH, Nalesnik MA, Nusbacher J, Diliz-Pere H, Hakala TR. Pancreaticoduodenal transplantation in humans. Surg Gynecol Obstet. 1984;159:265-272. [PubMed] |
| 25. | Cho EH, Suh KS, Yang SH, Lee HW, Cho JY, Cho YB, Yi NJ, Lee KU. Acute graft versus host disease following living donor liver transplantation: first Korean report. Hepatogastroenterology. 2007;54:2120-2122. [PubMed] |
| 26. | Chinnakotla S, Smith DM, Domiati-Saad R, Agura ED, Watkins DL, Netto G, Uemura T, Sanchez EQ, Levy MF, Klintmalm GB. Acute graft-versus-host disease after liver transplantation: role of withdrawal of immunosuppression in therapeutic management. Liver Transpl. 2007;13:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Kohler S, Pascher A, Junge G, Sauer IM, Nagy M, Schönemann C, Koch M, Neumann U, Pratschke J, Neuhaus P. Graft versus host disease after liver transplantation - a single center experience and review of literature. Transpl Int. 2008;21:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Meves A, el-Azhary RA, Talwalkar JA, Moore SB, Brewer JD, Motsonelidze C, McNallan KT, Reed AM, Rosen CB. Acute graft-versus-host disease after liver transplantation diagnosed by fluorescent in situ hybridization testing of skin biopsy specimens. J Am Acad Dermatol. 2006;55:642-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Lu Y, Wu LQ, Zhang BY, Cao JY. Graft-versus-host disease after liver transplantation: successful treatment of a case. Transplant Proc. 2008;40:3784-3786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Piton G, Larosa F, Minello A, Becker MC, Mantion G, Aubin F, Deconinck E, Hillon P, Di Martino V. Infliximab treatment for steroid-refractory acute graft-versus-host disease after orthotopic liver transplantation: a case report. Liver Transpl. 2009;15:682-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Gao PJ, Leng XS, Wang D, Li GM, Huang L, Gao J, Zhu JY. Graft versus host disease after liver transplantation: a case report. Front Med China. 2010;4:469-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Xu X, Ling Q, Wei Q, Wang K, Zhou B, Zhuang L, Zhou L, Zheng S. Korean red ginseng: a new approach for the treatment of graft-versus-host disease after liver transplantation. Transplant Proc. 2011;43:2651-2655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Smith DM, Agura E, Netto G, Collins R, Levy M, Goldstein R, Christensen L, Baker J, Altrabulsi B, Osowski L. Liver transplant-associated graft-versus-host disease. Transplantation. 2003;75:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Nemoto T, Kubota K, Kita J, Shimoda M, Rokkaku K, Tagaya N, Fujiwara T, Sunakawa M. Unusual onset of chronic graft-versus-host disease after adult living-related liver transplantation from a homozygous donor. Transplantation. 2003;75:733-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Chan EY, Larson AM, Gernsheimer TB, Kowdley KV, Carithers RL, Reyes JD, Perkins JD. Recipient and donor factors influence the incidence of graft-vs.-host disease in liver transplant patients. Liver Transpl. 2007;13:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Hassan G, Khalaf H, Mourad W. Dermatologic complications after liver transplantation: a single-center experience. Transplant Proc. 2007;39:1190-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Aziz H, Trigo P, Lendoire J, Bianco G, Saúl J, Braslavsky G, Kien M, Zylberman M, Cueto G, Imventarza O. Successful treatment of graft-vs-host disease after a second liver transplant. Transplant Proc. 1998;30:2891-2892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Pinna AD, Weppler D, Berho M, Masetti M, DeFaria W, Kato T, Thompson J, Ricordi C, Tzakis AG. Unusual presentation of graft-versus-host disease in pediatric liver transplant recipients: evidence of late and recurrent disease. Pediatr Transplant. 1999;3:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Hanaway MJ, Buell JF, Musat AI, Kalayoglu M. Graftversus-host disease in solid organ transplantation. Graft. 2001;4:205-208. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 40. | Taylor AL, Gibbs P, Bradley JA. Acute graft versus host disease following liver transplantation: the enemy within. Am J Transplant. 2004;4:466-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 41. | Taylor AL, Gibbs P, Sudhindran S, Key T, Goodman RS, Morgan CH, Watson CJ, Delriviere L, Alexander GJ, Jamieson NV. Monitoring systemic donor lymphocyte macrochimerism to aid the diagnosis of graft-versus-host disease after liver transplantation. Transplantation. 2004;77:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Walling HW, Voigt MD, Stone MS. Lichenoid graft vs. host disease following liver transplantation. J Cutan Pathol. 2004;31:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Schmuth M, Vogel W, Weinlich G, Margreiter R, Fritsch P, Sepp N. Cutaneous lesions as the presenting sign of acute graft-versus-host disease following liver transplantation. Br J Dermatol. 1999;141:901-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Peñas PF, Fernández-Herrera J, García-Diez A. Dermatologic treatment of cutaneous graft versus host disease. Am J Clin Dermatol. 2004;5:403-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Thin L, Macquillan G, Adams L, Garas G, Seow C, Cannell P, Augustson B, Mitchell A, Delriveire L, Jeffrey G. Acute graft-versus-host disease after liver transplant: novel use of etanercept and the role of tumor necrosis factor alpha inhibitors. Liver Transpl. 2009;15:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Schöniger-Hekele M, Müller C, Kramer L, Dauber E, Mayr WR, Wrba F, Rockenschaub S, Mühlbacher F. Graft versus host disease after orthotopic liver transplantation documented by analysis of short tandem repeat polymorphisms. Digestion. 2006;74:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Paizis G, Tait BD, Kyle P, Angus PW, Grigg AP. Successful resolution of severe graft versus host disease after liver transplantation correlating with disappearance of donor DNA from the peripheral blood. Aust N Z J Med. 1998;28:830-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Kanehira K, Riegert-Johnson DL, Chen D, Gibson LE, Grinnell SD, Velgaleti GV. FISH diagnosis of acute graft-versus-host disease following living-related liver transplant. J Mol Diagn. 2009;11:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Au WY, Lo CM, Hawkins BR, Ma ES, Lie AK, Kwong YL. Evans' syndrome complicating chronic graft versus host disease after cadaveric liver transplantation. Transplantation. 2001;72:527-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Kuball J, Theobald M, Ferreira EA, Hess G, Burg J, Maccagno G, Barreiros AP, Lüth S, Schimanski CC, Schuchmann M. Control of organ transplant-associated graft-versus-host disease by activated host lymphocyte infusions. Transplantation. 2004;78:1774-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Cattral MS, Langnas AN, Wisecarver JL, Harper JC, Rubocki RJ, Bynon JS, Fox IJ, Heffron TG, Shaw BW. Survival of graft-versus-host disease in a liver transplant recipient. Transplantation. 1994;57:1271-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Whitington PF, Rubin CM, Alonso EM, McKeithan TW, Anastasi J, Hart J, Thistlethwaite JR. Complete lymphoid chimerism and chronic graft-versus-host disease in an infant recipient of a hepatic allograft from an HLA-homozygous parental living donor. Transplantation. 1996;62:1516-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 53. | Burt M, Jazwinska E, Lynch S, Kerlin P, Gill D, Steadman C, Jonsson J, Strong R, Powell E. Detection of circulating donor deoxyribonucleic acid by microsatellite analysis in a liver transplant recipient. Liver Transpl Surg. 1996;2:391-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | Mazzaferro V, Andreola S, Regalia E, Poli F, Doci R, Bozzetti F, Gennari L. Confirmation of graft-versus-host disease after liver transplantation by PCR HLA-typing. Transplantation. 1993;55:423-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Hahn AB, Baliga P. Rapid method for the analysis of peripheral chimerism in suspected graft-versus-host disease after liver transplantation. Liver Transpl. 2000;6:180-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Romagnuolo J, Jewell LD, Kneteman NM, Bain VG. Graft-versus-host disease after liver transplantation complicated by systemic aspergillosis with pancarditis. Can J Gastroenterol. 2000;14:637-640. [PubMed] |
| 57. | Joseph JM, Mosimann F, Tiercy JM, Roux E, Cerottini JP, Gillet M, Aubert V. PCR confirmation of microchimerism and diagnosis of graft versus host disease after liver transplantation. Transpl Int. 1999;12:468-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Wu Z, Shi W. Rash as the first manifestation of acute graft-versus-host disease after orthotopic liver transplantation. Eur J Dermatol. 2011;21:997-998. [PubMed] |
| 59. | Whalen JG, Jukic DM, English JC. Rash and pancytopenia as initial manifestations of acute graft-versus-host disease after liver transplantation. J Am Acad Dermatol. 2005;52:908-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 60. | Merhav HJ, Landau M, Gat A, Gazit E, Baratz M, Bialy-Golan A, Konikof F, Bril S, Nakache R. Graft versus host disease in a liver transplant patient with hepatitis B and hepatocellular carcinoma. Transplant Proc. 1999;31:1890-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 61. | Dunn SP, Krueger LJ, Butani L, Punnett H. Late onset of severe graft-versus-host disease in a pediatric liver transplant recipient. Transplantation. 2001;71:1483-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Comenzo RL, Malachowski ME, Rohrer RJ, Freeman RB, Rabson A, Berkman EM. Anomalous ABO phenotype in a child after an ABO-incompatible liver transplantation. N Engl J Med. 1992;326:867-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Shimizu T, Hayashi M, Inoue Y, Komeda K, Asakuma M, Hirokawa F, Iwamoto M, Miyamoto Y, Yonetani N, Saji H. Acute graft-versus-host disease after living donor liver transplantation with donor-dominant one-way human leukocyte antigen matching at two Loci. Transplantation. 2010;89:1164-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | Kriss M, Feliciano J, Fryer J, Mehta J, Levitsky J. Haploidentical hematopoietic stem cell transplantation for graft-versus-host disease after liver transplantation. Blood. 2011;118:3448-3449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 65. | Kiuchi T, Harada H, Matsukawa H, Kasahara M, Inomata Y, Uemoto S, Asonuma K, Egawa H, Maruya E, Saji H. One-way donor-recipient HLA-matching as a risk factor for graft-versus-host disease in living-related liver transplantation. Transpl Int. 1998;11 Suppl 1:S383-S384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 66. | Au WY, Ma SK, Kwong YL, Ng IO, Hawkins BR, Wan TS, Liu CL, Fan ST, Lo CM. Graft-versus-host disease after liver transplantation: documentation by fluorescent in situ hybridisation and human leucocyte antigen typing. Clin Transplant. 2000;14:174-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Hara H, Ohdan H, Tashiro H, Itamoto T, Tanaka Y, Mizunuma K, Tokita D, Onoe T, Ito R, Asahara T. Differential diagnosis between graft-versus-host disease and hemophagocytic syndrome after living-related liver transplantation by mixed lymphocyte reaction assay. J Invest Surg. 2004;17:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 68. | Riñón M, Maruri N, Arrieta A, Fernández JR, Ortiz de Urbina J, García Masdevall MD. Selective immunosuppression with daclizumab in liver transplantation with graft-versus-host disease. Transplant Proc. 2002;34:109-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Neumann UP, Kaisers U, Langrehr JM, Müller AR, Blumhardt G, Bechstein WO, Lobeck H, Riess H, Zimmermann R, Neuhaus P. Fatal graft-versus-host-disease: a grave complication after orthotopic liver transplantation. Transplant Proc. 1994;26:3616-3617. [PubMed] |