Published online Oct 7, 2012. doi: 10.3748/wjg.v18.i37.5225
Revised: May 31, 2012
Accepted: August 4, 2012
Published online: October 7, 2012
AIM: To assess if software assisted-contrast-enhanced ultrasonography (CEUS) provides reproducible perfusion parameters of hepatic parenchyma in patients affected by chronic liver disease.
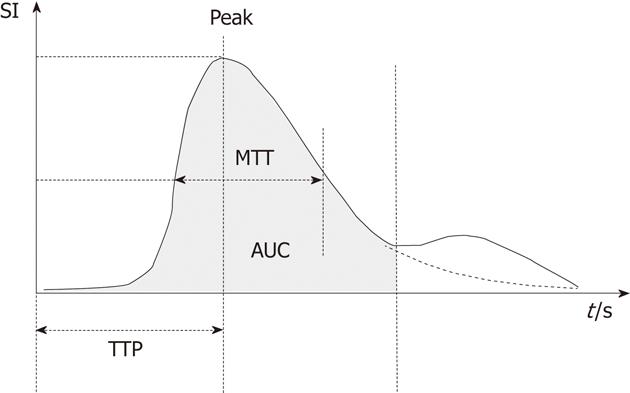
METHODS: Forty patients with chronic viral liver disease, with (n = 20) or without (n = 20) cirrhosis, and 10 healthy subjects underwent CEUS and video recordings of each examination were then analysed with Esaote’s Qontrast software. CEUS dedicated software Qontrast was used to determine peak (the maximum signal intensity), time to peak (TTP), region of blood value (RBV) proportional to the area under the time-intensity curve, mean transit time (MTT) measured in seconds and region of blood flow (RBF).
RESULTS: Qontrast-assisted CEUS parameters displayed high inter-observer reproducibility (κ coefficients of 0.87 for MTT and 0.90 TTP). When the region of interest included a main hepatic vein, Qontrast-calculated TTP was significantly shorter in cirrhotic patients (vs non-cirrhotics and healthy subjects) (71.0 ± 11.3 s vs 82.4 ± 15.6 s, 86.3 ± 20.3 s, P < 0.05). MTTs in the patients with liver cirrhosis were significantly shorter than those of controls (111.9 ± 22.0 s vs 139.4 ± 39.8 s, P < 0.05), but there was no significant difference between the cirrhotic and non-cirrhotic groups (111.9 ± 22.0 s vs 110.3 ± 14.6 s). Peak enhancement in the patients with liver cirrhosis was also higher than that observed in controls (23.9 ± 5.9 vs 18.9 ± 7.1, P = 0.05). There were no significant intergroup differences in the RBVs and RBFs.
CONCLUSION: Qontrast-assisted CEUS revealed reproducible differences in liver perfusion parameters during the development of hepatic fibrogenesis.
- Citation: Ridolfi F, Abbattista T, Busilacchi P, Brunelli E. Contrast-enhanced ultrasound evaluation of hepatic microvascular changes in liver diseases. World J Gastroenterol 2012; 18(37): 5225-5230
- URL: https://www.wjgnet.com/1007-9327/full/v18/i37/5225.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i37.5225
Changes in hepatic perfusion are a key feature of cirrhosis[1]. Capillarization of the sinusoids with loss of endothelial fenestration and increased tone of activated hepatic stellate cells (HSCs) increase the mechanical resistance to portal blood flow and augment hepatic vascular tone, leading to portal hypertension[2]. Alterations involving the microvascular bed of the liver are already evident during the precirrhotic stages of hepatic fibrogenesis. The ongoing liver damage induces the overexpression of angiogenic growth factors (such as vascular endothelial growth factor or platelet-derived growth factor)[3], which promote the persistence of inflammatory changes and fibrogenic tissue repair, facilitating the development of shunts between branches of the portal vein, the hepatic veins, and the hepatic artery within the newly formed fibrotic septa[2,4].
Computed tomography (CT) and magnetic resonance imaging (MRI) have both been used for quantitative and qualitative assessment of parenchymal perfusion in cirrhotic livers. CT studies revealed increased hepatic arterial flow and decreased mean transit times (MTT), as compared with values observed in control subjects[5]. In contrast, MRI with low molecular weight contrast material revealed increases in the MTT[6]. Use of these methods in clinical practice is limited, however, by the intensive post-processing required to obtain perfusion data, high costs, and the lack of standardized examination protocols[7].
B-mode ultrasonography with Doppler study of hepatic vessels is often the first-line imaging study for the work-up of patients with diffuse liver disease. The Doppler technique is used mainly to measure flow in macroscopic vessels and is not strictly related to the microcirculation of the liver[8]. Contrast-enhanced ultrasonography (CEUS) is performed after intravenous administration of a suspension of gas-filled microbubbles, which remain entirely within the intravascular space and thus act as a blood pool tracer. CEUS studies of perfusion in the liver parenchyma have focused mainly on the measurement of contrast-medium transit times (from the portal vein to the hepatic veins), which have proved to be significantly shorter in patients with cirrhosis (compared with non-cirrhotic patients with chronic liver disease)[9-11]. CEUS has also documented increased regional perfusion of the hepatic parenchyma in cirrhotic patients (compared with healthy subjects), and this increase displayed correlation with the degree of liver failure[12].
A major shortcoming of CEUS is its user-dependency. Qontrast™ (Esaote S.p.a., Florence, Italy) is a post-processing computational tool, which can be used with CEUS to obtain objective, quantitative parameters of microvascular damage in various organs, including the liver.
We examined patients with chronic liver disease using CEUS with Qontrast analysis of hepatic parenchymal perfusion. The aims of this study were to assess the reliability and reproducibility of software assisted-CEUS studies of hepatic parenchymal perfusion and to evaluate if the analysis of the parameters obtained could help to understand vascular changes developing during liver fibrogenesis.
The study protocol, which conformed to the guidelines outlined in the 1975 Declaration of Helsinki, was pre-approved by the institutional ethics committee. Written informed consent was obtained from all participants.
Participants were enrolled between March 2007 and December 2010. They included 10 control patients with no liver disease (as documented by self-reported history and blood chemistry data obtained during screening) and 40 patients consecutively seen by our staff for chronic viral liver disease. At the time of enrollment, all of these patients had been positive for hepatitis B surface antigen and/or anti-hepatitis C virus antibodies for at least 6 mo.
All participants underwent complete physical examinations, laboratory tests, and standard B-mode abdominal ultrasonography. Candidates were excluded if they presented any of the following: (1) sonographic evidence of focal liver lesions; (2) history of alcohol consumption of ≥ 20 g/d; and (3) current use of medication known to affect the intra- or extra-hepatic circulation.
The patients with chronic liver disease included 20 patients in whom the absence of cirrhosis had been confirmed by liver biopsy (non-cirrhotic group) and 20 others (cirrhotic group) with cirrhosis diagnosed by liver biopsy or on the basis of commonly accepted clinical criteria. The latter included a history of chronic liver disease together with portal hypertension manifested by two or more of the following: (1) endoscopic evidence of esophageal or gastric varices and/or portal hypertensive gastropathy; (2) hypersplenism (reflected by a white blood cell count of < 3500/mm3 and/or a platelet count of < 100 000 /mm3); (3) sonographic evidence of ascites; and (4) a hepatic venous pressure gradient (HVPG) of ≥ 12 mmHg[13,14]. The severity of the cirrhosis was rated with the Child-Pugh[15] and model for end-stage liver disease (MELD)[16] systems.
The liver biopsies used for patient classification had all been performed under sonographic guidance 1-3 mo prior to enrollment (mean: 1.8 mo; median: 1 mo). For the purposes of this study, all slides were independently reviewed by a single experienced liver pathologist, who was blinded to the patient’s clinical data and the results of his/her hepatic ultrasound examination. This examiner rated the presence of inflammation and fibrosis in each case with the Metavir scoring system[17].
All sonographic examinations were carried out by an experienced radiologist using a 3.5-MHz convex array transducer and a General Electric Logiq 9 scanner (Milwaukee, WI, United States) equipped with software for contrast imaging and color and power Doppler. Patients were examined after a fast of 6 h. After a standard B-mode scan, the second phase of the examination started, during which we evaluated perfusion within the hepatic parenchyma using a sonographic contrast agent composed of sulfur hexafluoride-filled microbubbles (Sonovue®, Bracco Spa, Milan, Italy). The probe was placed over the middle hepatic vein (at least 3 cm from the confluence) and surrounding tissue, where heartbeat artifacts were negligible. If an acceptable signal could not be obtained from this vein due to abdominal gas, the scan was made over the left or right hepatic vein. Tissue enhancement of these areas was recorded in a digital video format from 20 s before (baseline signal) to 130 s after the injection of a 2.5 mL bolus of Sonovue. Sonovue was injected manually into the antecubital vein at the rate of 1 mL/s, and the line was flushed with a 2.5-mL bolus of normal saline delivered at the same rate. Patients were instructed to breath gently during the procedure to minimize movement-related artifacts. Vital signs were monitored for 1 h after the examination, and patients were interviewed by phone 48 h after discharge to identify any adverse effects.
Video recordings were then analysed with the Qontrast software, which performs a full-map parametric analysis of perfusion within a selected set of frames in a specific region of interest (ROI). The loop of images is automatically processed after the tissue region and perfusion period have been defined; translational movements of the selected area can also be corrected. The area is then automatically aligned over all frames, and perfusion is analysed for points that continuously identify the moving tissue. Signal brightness is analysed separately at each point, and the optimal fitting curve is evaluated for each point.
In each patient, we evaluated two different ROIs. The first was a 25 cm2 area of parenchyma that included one of the main hepatic veins and surrounding tissue; the second was a smaller area (5 cm2) that included no major vessels. For each ROI, time-enhancement intensity curves were plotted with the Qontrast software, and the following parameters were generated (Figure 1): peak signal intensity (in dB) reached during the transit of the Sonovue bolus; time to peak (TTP) intensity, measured in seconds; regional blood volume (RBV), which is proportional to the area under the time-intensity curve; MTT (measured in seconds); and regional blood flow (RBF), which is the ratio of the RBV to MTT.
We evaluated interobserver and intraobserver variation in measurements of the MTT and TTP during CEUS. For the former assessment, two observers (one experienced physician the other one in training) independently and blindly reviewed the video recording of each examination. For the latter analysis, each video was re-examined by one of the observers (still blind) 2-3 mo after the original review. Concordant measurements were those that differed by no more than ± 1 s.
Data were expressed as group mean ± SD. Differences between the three groups (cirrhotic patients, non-cirrhotic patients, and controls) were evaluated with the Kruskall-Wallis test. A post-hoc t test was then used to evaluate differences between each group of participants. Differences in the proportion of male or female patients were assessed with the χ2 test. Kappa statistics were used to assess interobserver and intraobserver agreement in the calculation of the Qontrast parameters[18,19].
McGraw-Hill Primer Statistical Software (2nd edition, 1986) and MedCalc Statistical Software (version 11.6, 2011, Mariakerke, Belgium) were used for all analyses. Statistical significance was defined as P < 0.05.
The characteristics of the control group and the patients with chronic liver disease are shown in Table 1. Most of the patients with cirrhosis [diagnosed by liver biopsy (n = 4) or on the basis of clinical criteria (n = 16)] were Child-Pugh class A (n = 11), but classes B and C were also represented (5 and 4 patients, respectively). The mean MELD score for the cirrhotic subgroup was 10.3 ± 3.9. Eleven of these patients had ascites, 2 had signs of hepatic encephalopathy, esophageal varices were found in 11, and HVPG more than 12 mmHg was evident in 3 patients. There were no significant age differences between the three groups, but males were significantly more common in the subgroups with chronic liver disease.
| Group characteristics | Controls n = 10 | Patients with chronic liver disease | |
| Non-cirrhotic n = 20 | Cirrhoticn = 20 | ||
| Age (yr) | 48.5 ± 14.4 | 48.9 ± 12.4 | 57.7 ± 11.4 |
| Males, n (%) | 4 (40) | 12 (60)c | 17 (85)c |
| Viral etiology (HBV/HCV) | 0/0 | 2/18 | 4/16 |
| Necroinflammatory score | |||
| A0 | - | 3 | 0 |
| A1 | - | 10 | 0 |
| A2 | - | 7 | 3 |
| A3 | - | 0 | 1 |
| Fibrosis score | |||
| F0 | - | 5 | 0 |
| F1 | - | 6 | 0 |
| F2 | - | 4 | 0 |
| F3 | - | 5 | 0 |
| F4 | - | 0 | 4 |
| ALT (IU/L) | 22.3 ± 5.2 | 113.8 ± 89.1c | 76.9 ± 59.2c |
| Bilirubin (mg/dL) | 0.6 ± 0.1 | 0.7 ± 0.4 | 1.5 ± 0.9a |
| Albumin (g/dL) | 3.7 ± 0.3 | 4.1 ± 0.4 | 3 ± 0.8a |
| INR | 1.1 ± 0.1 | 1 ± 0.1 | 1.2 ± 0.2e |
Ultrasound and CEUS studies were successfully completed for all participants, and no adverse effects were observed during or after the procedure (as documented by phone interviews with patients).
The perfusion parameters generated by Qontrast analysis of the two ROIs examined is shown in Table 2: the first containing a hepatic vein and surrounding tissue, the second containing parenchyma alone, with no major vessels. In both cases, the MTTs in the patients with liver diseases were significantly shorter than those of controls, but there was no significant difference between the cirrhotic and non-cirrhotic groups. Analysis of the ROIs containing a hepatic vein (middle in 46 cases, right in 3, left in 1) revealed that TTPs in cirrhotic patients were significantly shorter than those of controls and of non-cirrhotic patients (P < 0.05). Similar findings emerged when we analyzed data obtained for the smaller ROIs although the differences in this case were not statistically significant. Peak enhancement in the patients with liver disease was also higher than that observed in controls, but again, this difference was significant only when the area analyzed included a main hepatic vein (P < 0.05). There were no significant intergroup differences in the RBVs, regardless of which ROI was considered. As for the RBF, the values observed in the chronic liver disease groups were appreciably (but not significantly) increased over those of controls, probably as a result of the shorter MTTs in these patients. This difference was also seen when the hepatic vein was not included in the ROI.
| Perfusion parameter | Controls | Non-cirrhotic patients | Cirrhotic patients | |||
| ROI with HV | ROI without HV | ROI with HV | ROI without HV | ROI with HV | ROI without HV | |
| TTP (s) | 86.3 ± 20.3 | 86.8 ± 24.9 | 82.4 ± 15.6 | 78.2 ± 12.0 | 71.0 ± 11.3a | 73.7 ± 17.8 |
| Peak (%) | 18.9 ± 7.1 | 18.5 ± 7.8 | 25.9 ± 7.8c | 23.1 ± 8.9 | 23.9 ± 5.9e | 25.5 ± 6.6 |
| RBV | 2828.7 ± 1720 | 2827.4 ± 1642.1 | 3402.3 ± 1515.1 | 2734.9 ± 1327.5 | 2809.3 ± 1111.6 | 3031.9 ± 1000.2 |
| RBF | 21.4 ± 9.5 | 21.4 ± 10.5 | 29.3 ± 10.5 | 25.6 ± 11.1 | 27.2 ± 7.9 | 28.8 ± 8.1 |
| MTT (s) | 139.4 ± 39.8 | 128.4 ± 37.8 | 110.3 ± 14.6c | 105.8 ± 17.1c | 111.9 ± 22.0c | 104.7 ± 24.4c |
Intraobserver agreement was calculated for MTT and TTP. Full agreement was considered when the two different analyses differed no more than ± 1 s. According to this finding, the agreement in reviewing Qontrast analysis for MTT and TTP by the same examiner was considered almost perfect: κ coefficient for MTT of 0.84 [95% confident interval (CI): 0.796-0.902] and for TTP of 0.92 (95% CI: 0.892-0.946). More interesting, the interobserver agreement for MTT and TTP by two examiners was found also to be almost perfect: κ coefficients for MTT of 0.87 (95% CI: 0.826-0.916) and for TTP of 0.90 (95% CI: 0.867-0.935).
CEUS represents the natural continuation of standard B mode ultrasound and Doppler studies, which are widely used for the diagnosis and follow-up of chronic liver disease. Compared with CT and MRI, ultrasound offers important advantages in terms of availability, safety, repeatability, and costs. We found CEUS of liver parenchyma to be safe and effective since we obtained a good quality digital video from each examination without any adverse effect observed. One of its main shortcomings is that it is highly operator dependent. We found Qontrast-assisted CEUS analysis of parenchymal perfusion to be highly reproducible. Intraobserver agreement was excellent (κ coefficient of 0.92) and, more interesting, interobserver agreement was almost perfect (κ coefficient: 0.90). Post-processing analysis of digitally recorded CEUS findings may thus be useful for standardizing this approach and improving its reproducibility.
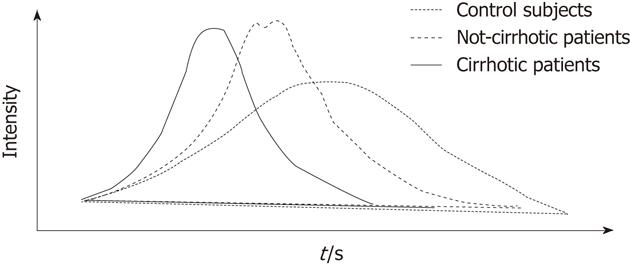
When analysis was restricted to ROIs containing no major vessels, the time-intensity curves in the healthy control group were generally flat with a late enhancement peak (86.8 ± 24.9 s) and long MTT (range: 91.4-192.1 s), findings that probably reflect an extensive vascular bed characterized by slow, continuous flow (Figure 2). By contrast, in patients with liver disease peak enhancement was higher and tended to occur earlier, and this pattern was more evident as the severity of the liver disease increased. From a quantitative point of view, this trend was reflected by MTTs in cirrhotic patients that were significantly shorter than those of the patients without liver disease. The steeper curve is an expression of faster, more concentrated flow of a volume of blood similar to that found in a healthy liver (the total hepatic blood volume is no different from that observed in the control group, as demonstrated by the RBVs). This picture (i.e., shorter MTTs associated with the same RBV) is fully compatible with sinusoid capillarization and increased activated HSC tone, which occur during the progression of chronic liver disease and can lead to high-velocity blood flow through the liver. When one of the major hepatic veins was included in the ROI, the TTP also decreased significantly across the three groups (controls > non-cirrhotics > cirrhotics). This picture could reflect the development of intrahepatic shunts that permit portal veins and branches of hepatic artery to cross cirrhotic areas, leading directly or indirectly into the central venous compartment[20].
Qontrast analysis indeed revealed more substantial differences between our patient subgroups when one of the hepatic veins was included in the ROI. The significant TTP shortening observed under these conditions is probably related in large part to the increasing presence of intra-hepatic shunts between branches of portal and/or hepatic artery and the hepatic veins. This is also the basis of the shortened hepatic vein arrival times documented in our cirrhotic patients (and those of other studies)[10,11,20] and also in patients with malignant liver disease[21].
The main limitation of our study is the small number of patients examined. Definitive conclusions on value of Qontrast analysis of CEUS data in diagnosing cirrhosis will have to be based on studies in much larger populations. In any case, use of this software does appear to increase the reproducibility of CEUS findings, and this could be useful for standardizing CEUS protocols and enhancing the comparability of findings obtained by different groups (that is for example a major weakness of Doppler examinations[22]).
Our initial experience with Qontrast-assisted CEUS studies of liver perfusion revealed clear differences between cirrhotic and non-cirrhotic patients with chronic liver disease and may serve as an incentive for further investigations. The reproducibility of Qontrast-assisted CEUS (although some training and experience are essential for optimal results) and its broad availability make it suitable for repeat examinations. Liver perfusion by CEUS could thus represent a valuable “non fibrotic-non invasive” tool to evaluate liver disease severity and to monitor the progression of chronic liver diseases.
The authors are grateful to Marian Everett Kent for editing passages of some versions of the manuscript and above all to the following registered nurses, whose support during the study was indispensable: Cinzia Bentivoglio, Filomena Mocciolella, Romina Patrizi, Laura Rossi Magi, Antonella Tarafino.
Changes in hepatic perfusion are a key feature that parallels the process of liver fibrogenesis developing during chronic liver diseases. These changes could be used to evaluate liver disease severity during the clinical follow up of chronic liver diseases.
Evaluation of liver disease severity by studies of perfusion in the liver parenchyma has been mainly performed by computer tomography or magnetic resonance imaging. These methods in clinical practice are limited, however, by the intensive post-processing required to obtain perfusion data, high costs, and the lack of standardized examination protocols. Contrast enhanced ultrasonography (CEUS) could represent the best way to assess liver perfusion due to its wide availability, low cost and safety of ultrasound.
Previous studies performed by CEUS to assess liver disease severity have been mainly focused on the measurement of contrast-medium transit times (mainly from the portal vein to the hepatic veins). A major shortcoming of CEUS is its user-dependency. In this study the authors performed a software-assisted CEUS: Qontrast™ (Esaote S.p.a., Florence, Italy) is a post-processing computational tool, which can be used with CEUS to obtain objective, quantitative parameters of microvascular damage in the liver. The authors demonstrated that software assisted CEUS studies of liver perfusion revealed clear differences between cirrhotic and non-cirrhotic patients with chronic liver disease. The reproducibility of Qontrast-assisted CEUS is quite high and could be useful for standardizing CEUS protocols and enhancing the comparability of findings obtained by different groups.
The authors’ initial experience with Qontrast-assisted CEUS studies of liver perfusion may serve as an incentive for further investigations. It is believed that liver perfusion by CEUS could represent a valuable “non fibrotic-non invasive” tool to evaluate liver disease severity and to monitor the progression of chronic liver diseases.
Liver perfusion: Alterations involving the microvascular bed of the liver are already evident during the pre-cirrhotic stages of hepatic fibrogenesis. Main features are capillarization of the sinusoids with loss of endothelial fenestration and increased tone of activated hepatic stellate cells leading to the increase of the mechanical resistance to portal blood flow and augment hepatic vascular tone. The development of shunts between branches of the portal vein, the hepatic veins, and the hepatic artery within the newly formed fibrotic septa are also a key event; CEUS: Performed after intravenous administration of a suspension of gas-filled microbubbles, which remain entirely within the intravascular space and thus act as a blood pool tracer. CEUS studies of perfusion can be applied to the liver to evaluate the times and the intensity of enhancement of liver parenchyma occurring after the contrast injection (i.e., the time-intensity curve of enhancement).
This is a good pivotal study in which the authors found time to peak contrast enhancement was significantly shorter in cirrhotic patients and contrast transit time was significantly shorter in the patients with liver diseases than those of controls. These results indicate that this new non-invasive method of analyzing hepatic vein transit time is useful for the prediction of liver disease progression.
Peer reviewers: Herwig R Cerwenka, Professor, Department of Surgery, Medical University of Graz, Auenbruggerplatz 29, Graz A-8036, Austria; Soeren Rafaelsen, MD, Department of Radiology, Vejle Hospital, Vejle 7100, Denmark; Fikri M Abu-Zidan, Professor, Department of Surgery, Faculty of Medicine, UAE University, PO Box 17666, Al-Ain, United Arab Emirates
S- Editor Wu X L- Editor A E- Editor Lu YJ
| 1. | Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655-1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2139] [Cited by in RCA: 2164] [Article Influence: 127.3] [Reference Citation Analysis (0)] |
| 2. | Bosch J. Vascular deterioration in cirrhosis: the big picture. J Clin Gastroenterol. 2007;41 Suppl 3:S247-S253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J Hepatol. 2010;53:976-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 4. | Ohnishi K, Chin N, Saito M, Tanaka H, Terabayashi H, Nakayama T, Iida S, Nomura F, Okuda K. Portographic opacification of hepatic veins and (anomalous) anastomoses between the portal and hepatic veins in cirrhosis--indication of extensive intrahepatic shunts. Am J Gastroenterol. 1986;81:975-978. [PubMed] |
| 5. | Chen ML, Zeng QY, Huo JW, Yin XM, Li BP, Liu JX. Assessment of the hepatic microvascular changes in liver cirrhosis by perfusion computed tomography. World J Gastroenterol. 2009;15:3532-3537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Hagiwara M, Rusinek H, Lee VS, Losada M, Bannan MA, Krinsky GA, Taouli B. Advanced liver fibrosis: diagnosis with 3D whole-liver perfusion MR imaging--initial experience. Radiology. 2008;246:926-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Taouli B, Ehman RL, Reeder SB. Advanced MRI methods for assessment of chronic liver disease. AJR Am J Roentgenol. 2009;193:14-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Bernatik T, Strobel D, Hahn EG, Becker D. Doppler measurements: a surrogate marker of liver fibrosis? Eur J Gastroenterol Hepatol. 2002;14:383-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Albrecht T, Blomley MJ, Cosgrove DO, Taylor-Robinson SD, Jayaram V, Eckersley R, Urbank A, Butler-Barnes J, Patel N. Non-invasive diagnosis of hepatic cirrhosis by transit-time analysis of an ultrasound contrast agent. Lancet. 1999;353:1579-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 163] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Ridolfi F, Abbattista T, Marini F, Vedovelli A, Quagliarini P, Busilacchi P, Brunelli E. Contrast-enhanced ultrasound to evaluate the severity of chronic hepatitis C. Dig Liver Dis. 2007;39:929-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Abbattista T, Ridolfi F, Ciabattoni E, Marini F, Bendia E, Brunelli E, Busilacchi P. Diagnosis of liver cirrhosis by transit-time analysis at contrast-enhanced ultrasonography. Radiol Med. 2008;113:860-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Berzigotti A, Nicolau C, Bellot P, Abraldes JG, Gilabert R, García-Pagan JC, Bosch J. Evaluation of regional hepatic perfusion (RHP) by contrast-enhanced ultrasound in patients with cirrhosis. J Hepatol. 2011;55:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Oberti F, Valsesia E, Pilette C, Rousselet MC, Bedossa P, Aubé C, Gallois Y, Rifflet H, Maïga MY, Penneau-Fontbonne D. Noninvasive diagnosis of hepatic fibrosis or cirrhosis. Gastroenterology. 1997;113:1609-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 272] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 14. | Bosch J, Garcia-Pagán JC, Berzigotti A, Abraldes JG. Measurement of portal pressure and its role in the management of chronic liver disease. Semin Liver Dis. 2006;26:348-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 15. | Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85. [PubMed] |
| 16. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3677] [Article Influence: 153.2] [Reference Citation Analysis (0)] |
| 17. | Rozario R, Ramakrishna B. Histopathological study of chronic hepatitis B and C: a comparison of two scoring systems. J Hepatol. 2003;38:223-229. [PubMed] |
| 18. | Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37:360-363. [PubMed] |
| 19. | Chien PF, Khan KS. Evaluation of a clinical test. II: Assessment of validity. BJOG. 2001;108:568-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Sugimoto H, Kaneko T, Hirota M, Tezel E, Nakao A. Earlier hepatic vein transit-time measured by contrast ultrasonography reflects intrahepatic hemodynamic changes accompanying cirrhosis. J Hepatol. 2002;37:578-583. [PubMed] |
| 21. | Rafaelsen SR, Jakobsen A. Contrast-enhanced ultrasound vs multidetector-computed tomography for detecting liver metastases in colorectal cancer: a prospective, blinded, patient-by-patient analysis. Colorectal Dis. 2011;13:420-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Sabbá C, Ferraioli G, Buonamico P, Mahl T, Taylor KJ, Lerner E, Albano O, Groszmann RJ. A randomized study of propranolol on postprandial portal hyperemia in cirrhotic patients. Gastroenterology. 1992;102:1009-1016. [PubMed] |