Published online Oct 7, 2012. doi: 10.3748/wjg.v18.i37.5164
Revised: April 30, 2012
Accepted: May 5, 2012
Published online: October 7, 2012
Gastric cancer is the second most common cancer worldwide and the second most common cause of cancer-related deaths. Despite complete resection of gastric cancer and lymph node dissection, as well as improvements in chemotherapy and radiotherapy, there are still 700 000 gastric cancer-related deaths per year worldwide and more than 80% of patients with advanced gastric cancer die of the disease or recurrent disease within 1 year after diagnosis. None of the treatment modalities we have been applying today can influence the overall survival rates: at present, the overall 5-year relative survival rate for gastric cancer is about 28%. Cellular metaplasia due to chronic inflammation, injury and repair are the most documented processes for neoplasia. It appears that chronic inflammation stimulates tumor development and plays a critical role in initiating, sustaining and advancing tumor growth. It is also evident that not all inflammation is tumorigenic. Additional mutations can be acquired, and this leads to the cancer cell gaining a further growth advantage and acquiring a more malignant phenotype. Intestinalization of gastric units, which is called “intestinal metaplasia”; phenotypic antralization of fundic units, which is called “spasmolytic polypeptide-expressing metaplasia”; and the development directly from the stem/progenitor cell zone are three pathways that have been described for gastric carcinogenesis. Also, an important factor for the development of gastrointestinal cancers is peritumoral stroma. However, the initiating cellular event in gastric metaplasia is still controversial. Understanding gastric carcinogenesis and its precursor lesions has been under intense investigation, and our paper attempts to highlight recent progress in this field of cancer research.
- Citation: Gomceli I, Demiriz B, Tez M. Gastric carcinogenesis. World J Gastroenterol 2012; 18(37): 5164-5170
- URL: https://www.wjgnet.com/1007-9327/full/v18/i37/5164.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i37.5164
Cancer is a major public health problem and at the beginning of the 19th century, gastric cancer was the second most common cancer worldwide[1]. Every year there are 900 000 new cases and 700 000 gastric cancer-related deaths in the world[2]. Although chemotherapy improves life expectancy, and despite seemingly complete resection of gastric cancer (R0) via gastrectomy, more than 80% of patients with advanced gastric cancer die of the disease or recurrent disease within 1 year after diagnosis. This situation suggests that standard treatment protocols are ineffective in a considerable number of cases[3]. Thus, the understanding of the mechanism underlying the progression of gastric carcinoma is essential for the management of this disease.
A number of risk factors are known for gastric cancer (Table 1), but study results regarding some factors, especially salt intake, vitamin C, alcohol, occupational exposure to nitrosamines and inorganic dusts, have been inconsistent[4-9].
| Genetic factors | Environmental factors | Other factors |
| Sex | Helicobacter pylori | Gastric adenomas |
| Familial adenomatous polyposis | Epstein-Barr virus | Barrett’s esophagus |
| Hereditary nonpolyposis colorectal cancer (Lynch II) | Nitrites | Hamartomas |
| Genetic diffuse gastric cancer (E-cadherin - CDH1 mutation) | Excess alcohol ingestion | Ménétrier’s disease |
| Genetic polymorphisms for pro- and anti-inflammatory cytokines | High intake of salted, pickled, or smoked foods | Chronic atrophic gastritis |
| Polymorphisms for cell receptors of innate immune response | Low intake of fiber, fruits and vegetables | Gastric metaplasia |
| Peutz-Jeghers syndrome | Antioxidant consumption (especially ascorbic acid, carotenoids, folates and tocopherols) | Pernicious anemia |
| Tobacco smoking (adenocarcinoma of cardia) | Benign gastric ulcers | |
| Fundic gland polyps | ||
| Hyperplastic polyps | ||
| Gastric biopsy revealing high-grade dysplasia | ||
| History of subtotal gastrectomy (> 20 yr) |
The majority of gastric cancer patients have adenocarcinoma (90%); the remaining 10% have lymphoma or gastrointestinal stromal tumor. There are two general types of gastric adenocarcinoma: the intestinal type (50%) and the diffuse type (33%) according to the Lauren classification system[9]. The remaining 17% are mixed or unclassified type[10]. The intestinal type is more common and is more often located in the distal part of the stomach. In contrast, the diffuse type has a poorer prognosis; generally occurs in younger patients; and can occur anywhere in the stomach, but especially in the cardia. The intestinal type is frequently accompanied by liver metastasis, whereas because the diffuse type has an increased propensity for intra- and trans-mural spread, it has been associated with peritoneal dissemination and poorer prognosis[1]. The diffuse type of gastric cancer shows more poorly differentiated cells than the intestinal type[11]. Intestinal-type adenocarcinoma is preceded by metaplastic changes, whereas diffuse-type adenocarcinoma is thought to arise in normal gastric mucosa.
Gastric adenocarcinoma can also be divided into two groups, known as "differentiated" and "undifferentiated", using the Nakamura classification system[12]. Intestinal-type adenocarcinoma is considered to be essentially equivalent to differentiated adenocarcinoma, as is diffuse-type equivalent to the undifferentiated adenocarcinoma. However, some cases of intestinal-type adenocarcinoma also arise from the gastric mucosa without intestinal metaplasia (IM). So based on the type of IM, some authors suggest that gastric cancer phenotypes can be classified into four groups depending on the marker combinations as: complete intestinal type, incomplete intestinal type, gastric type and unclassified type. Gastric-type differentiated adenocarcinomas can be distinguished from other types of differentiated adenocarcinomas on the basis of their increased malignant potential in the incipient phase of invasion and metastasis[13].
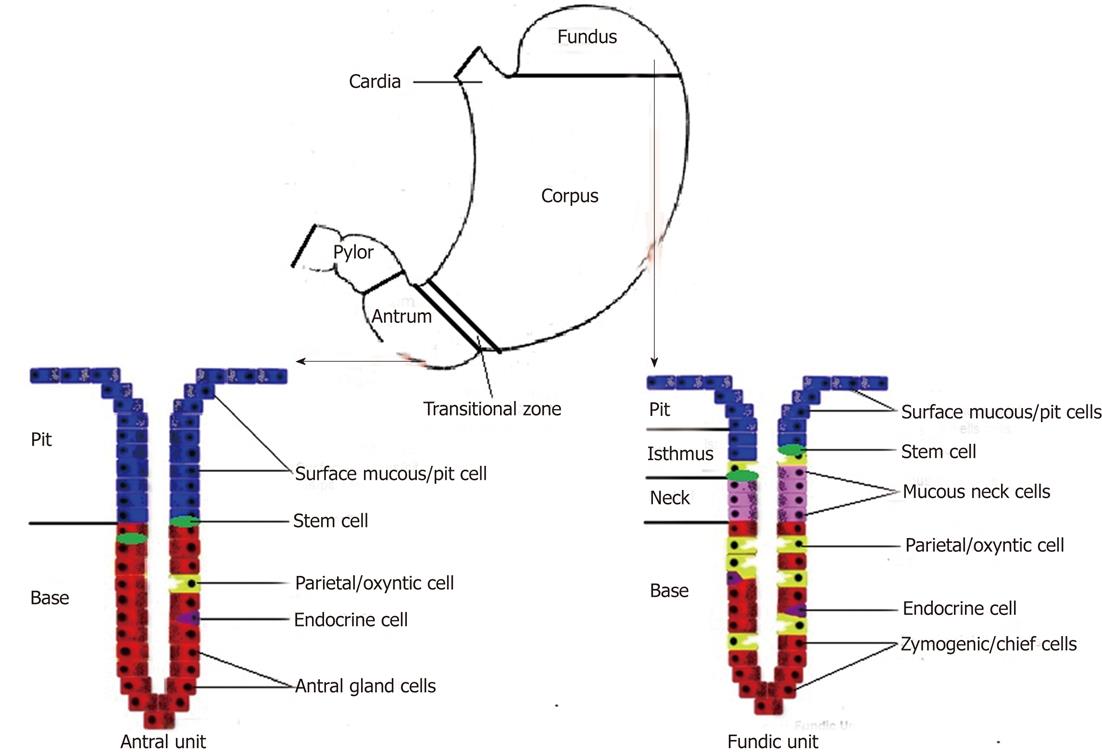
The mucous epithelium of the stomach represents a major barrier to the various noxious agents by means of intercellular tight junctions. This epithelium and its components are also vital for complex communications and physiological functions[14]. Histologically, the human gastric mucosa is divided into three regions: cardia, fundus-corpus and antrum-pylorus. Also, a transitional zone separates the stereotypic corpus and antral/pyloric epithelia and has features of each. The epithelium of these regions is composed of millions of glands that are surrounded by supporting stromal cells which are derived from mesenchyme. In the corpus, glands are long and composed of several epithelial cell types, including surface mucous foveolar cells (pit cells), acid-making oxyntic (parietal) cells, mucous neck cells (intermediate progenitor for chief cells), zymogenic (chief) cells, and hormone-secreting endocrine cells. In the antrum, the shorter glands are composed mainly of mucus-secreting cells and endocrine cells that secrete hormones such as gastrin and somatostatin. The stomach mesenchymal compartment surrounding the glands is less studied and little understood[15-18].
The human stomach mucosal tubular glands are further subdivided into foveolus, isthmus, neck and base regions. The gastric glands open into the bottom of the pits, on an average with 4 to 5 glands per pit. Fundic glands are quite straight, whereas antral glands are branched and coiled in their basal ends. Fundic and antral units (combination of a pit and a gland) differ very much in their cell characteristics and turnover rates (the human antral mucosa is known to have a much higher turnover rate). The gastric glands which contain ‘surface mucous’ cells and "mucous neck" cells (in the foveola), pepsinogen-secreting zymogenic (chief) cells (at the base of the glands), acid-secreting oxyntic (parietal) cells (at the base of the glands), and endocrine cells including the histamine-producing enterochromaffin-like (ECL) cells are located in the fundus; the zymogenic (chief) cells, oxyntic (parietal) cells and ECL cells are also found in the corpus of the stomach. The antral unit contains surface mucous foveolar cells, antral gland cells, endocrine cells (mainly gastrin-producing G-cells, but also EC and somatostatin-producing D cells), and occasional oxyntic cells. In the pylorus, the gastric glands contain many more mucinous cells, no zymogenic cells and few oxyntic cells (Figure 1)[19-23].
In addition, it should be noted that the subepithelial mesenchymal cells and their secreted basement membrane factors compose the lamina propria. This constitutes a structural support while regulating epithelial cell function and epithelial cell networks[24,25].
Some of the earliest observations in cancer biology as well as recent advances in molecular analyses contribute to our knowledge about the multistep process of gastric carcinogenesis[26-28].
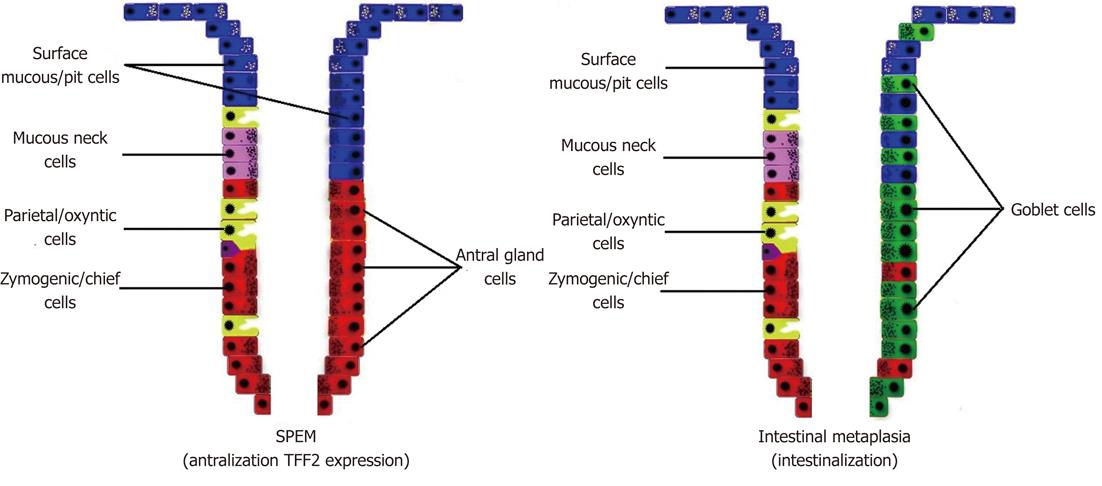
The gastrointestinal tract has rapid epithelial turnover and exposure to injury by infections and dietary toxins. These conditions create very high cancer prevalence. Intestinalization of gastric units, which is called "IM"; phenotypic antralization of fundic units, which is called "spasmolytic polypeptide-expressing metaplasia (SPEM)"; and the development directly from the stem/progenitor cell zone, are three pathways that have been described for gastric carcinogenesis[29-31].
Neoplasia can follow cellular metaplasia due to chronic inflammation, injury and repair[32]. This is the most documented process for gastric cancer[33-35]. An acceptable concept is that there are two corner-stones with regard to this process. Firstly, the initial observation of Rudolf Virchow in 1863 about leucocytes in neoplastic tissues and the connection between inflammation and cancer[36]; secondly, about 15 years ago, researchers’ evidence about the relationship between stomach cancer and infection by Helicobacter pylori (H. pylori) (isolated by Drs. Marshall and Warren in 1984[37]. Also, we must note that Epstein-Barr virus has been detected in stomach tissues in approximately 10% of gastric carcinoma cases[38].
Beginning with some of the earliest observations in cancer biology, it appears that chronic inflammation stimulates tumor development and plays a critical role in initiating, sustaining and advancing tumor growth[39,40]. Direct effect of the viral pathogens on neoplastic transformation of epithelial cells has been shown; however, it is also evident that not all inflammation is tumorigenic[41]. It can be suggested that either the tumor alters the immune response by reactive oxygen species and cytokines or chronic inflammation plays a primary role in transforming tissue cells (especially mentioned in “stem cell theory”) into tumor cells. In the acute phase of inflammation, the release of endogenous reactive oxygen and nitrogen species (O2-, H2O2, NO, OH, ONOO-, HOCl) from such innate immune cells as macrophages and leukocytes plays an important role in the elimination of pathogens[42]. However, when present chronically, this can induce DNA damage in proliferating cells. In addition, it is also possible for other bacteria to colonize the stomach and additionally trigger carcinogenesis by gastric atrophy (result of chronic inflammation) which represents a loss of gastric glands and associated lower acidity of gastric juice[43,44]. Hypoacidity associated with H. pylori infection induces gastric mucosal atrophy to advance multistage carcinogenesis in the stomach. Interleukin (IL)-1β, IL-6, IL-8, tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) are elevated in gastric mucosa with H. pylori infection. Gastrin is upregulated and acid secretion from parietal cells is inhibited mainly due to pro-inflammatory cytokines IL-1β and TNF-α[45,46]. TNF-α and IL-1β are essential in the initiation of chronic inflammation. Recent works have shown that IL-1β overexpression, in the absence of Helicobacter infection, is sufficient to cause gastric cancer and it is one of the essential proinflammatory cytokines modulated during H. pylori infection that directs the mucosa toward atrophy, metaplasia, and neoplastic transformation[47-49]. Another important point that should be added is that H. pylori has been consistently associated with higher risk of gastric noncardia cancer. The inverse association of H. pylori with gastric cardia cancer or esophageal adenocarcinoma has been shown in several studies, especially in Western populations[50]. Furthermore, mast cells in particular play an important role in attracting inflammatory cells by releasing inflammatory mediators. Monocytes differentiate into macrophages, and become activated in response to local chemokine and cytokine interactions[51]. Also, the correlation between tumor-associated macrophage abundance and poor prognosis has been shown[52]. Furthermore, macrophage-deficient mice display reduced progression of tumors to a more malignant phenotype[53]. Recently, direct evidence has also linked IL-6 to inflammation-mediated tumor initiation and proliferation in colon cancer[54]. IL-6 can inhibit dendritic cell maturation and, together with the NF-κB-activating cytokines IL-1 and TNF, can promote tumor progression. Cytokines also affect cell death and cell cycle pathways[55,56]. TNF-α is produced mainly by macrophages. It is also produced by tumor cells. TNF-α is associated with tissue destruction and plays a role in destroying tumor blood supply. However, if it is produced chronically, it can act as a tumor promoter by contributing to tissue remodeling and stromal development[57,58]. Nuclear factor (NF)-κB and STAT3 pathways have emerged as key regulators of the release of these pro-inflammatory cytokines, and important mediators of both tumor proliferation and persistence of chronic inflammation. The activation of these pathways results in further cytokine release[57,59,60].
Activation of the innate immune system is followed by the adaptive immune response. Th1 response and its accompanying mediators (IFN-γ) are not only necessary for Helicobacter-induced inflammation but also for the development of atrophy or metaplasia and SPEM; however a Th2 response and its mediators (i.e., IL-4) appear to be protective. The presence of a Th1, rather than a Th2, immune response is also associated with better survival in gastric cancer patients[36].
Although the subsequent pathways are different, chronic inflammation is the first step in both the intestinal and the diffuse type of gastric cancer. While the intestinal type has a sequence of multifocal atrophic gastritis, IM and dysplasia, which advances to carcinoma, the diffuse type tends to be primarily genetic in origin[61,62]. The progress from IM to gastric cancer has a wide range of molecular alterations affecting transcription factors, such as CDX1 and CDX2, telomerases, microsatellite instability, mutations of p53 protein, overexpression of COX-2, cyclin D2, and decreased expression of p27[63]. The next step is gastric dysplasia. During the progression of normal tissue through the metaplasia-dysplasia sequence, there are mutations in genes including p53, also loss of heterozygosity of the adenomatous polyposis coli gene, overexpression of the antiapoptotic gene bcl-2 and a mixture of polyploidy and aneuploidy[63].
Inflammation also plays an important role in the ability of tumor cells to invade and metastasize. The ability of epithelial tumor cells which metastasize to express specific chemokine receptors has been shown[64]. Paracrine secretion of pro-inflammatory cytokines (i.e., IL-1β, IL-6, TNF-α) and certain autocrine cytokine production support this process[65]. During the later stages, additional mutations can be acquired, and this leads to the cancer cell gaining a further growth advantage and acquiring a more malignant phenotype[66,67].
In recent studies investigators have found out that K-ras activation resulted in an inflammatory response and enhanced the expression of COX-2 in the glandular stomach. COX2 is upregulated in the gastric epithelium and in the infiltrating inflammatory cells in the stomach during gastritis[68-70]. Furthermore, it has been shown that sulindac, a nonsteroidal anti-inflammatory drug, suppresses the progression of gastric cancer in mice[71]. Hence, a K-ras activation-induced inflammatory response may facilitate the formation of IM and promote the progression of gastric cancer.
SPEM is associated more commonly with gastric cancer than IM[72,73]. It can be defined as a corpus lesion. Nevertheless, IM and SPEM often occur together[74,75]. Increase in mucus and loss of mature parietal and chief cells in humans correlates with SPEM (Figure 2)[73]. SPEM is characterized by expression of TFF2 (spasmolytic polypeptide) which is normally a product of mucous neck cells and antral gland cells[72]. SPEM also arises from a second proliferative zone at the bases of metaplastic fundic units, either by transdifferentiation of chief cells or activation of an unknown basal crypt progenitor[76,77]. However, it is not clear whether these cells are related to the gastric progenitor cells[78].
It must be noted that an important factor for the development of gastrointestinal cancers is peritumoral stroma. Activated fibroblasts within the stroma can help to create an environment containing vessels and infiltrating inflammatory cells and it is the interaction between these different cell types which is permissive of tumor growth, angiogenesis, and invasion[79-81].
The question that must be answered is: what is the initiating cellular event in gastric metaplasia? The interpretation that the metaplasia is an intermediate step in the development of gastric cancer may be facile, because different types of IM have different degrees of association with malignancy, and early stage gastric cancers can arise in nonintestinalized epithelium[82-84]. Investigators have reported that solid cancers might originate from differentiated cells and they have reported the possible existence of cancer stem cells (CSCs) or tumor initiating cells in solid malignant tumors[85,86]. However, based on the assessment of the differentiation status of tumor cells, they appear to deviate little from their normal progenitors and to show similar differentiation programs. Studies on tissues undergoing continuous cell renewal suggest that cancer cells may originate from a stem cell compartment[87]. The origin of human gastric CSCs has yet to be elucidated, but data obtained from a mouse model of Helicobacter-induced gastric cancer have implicated bone marrow-derived cells as a potential candidate. Further studies focusing on the identification and characterization of CSCs in gastric cancer may lead to novel diagnostic and therapeutic tools, dramatically improving the prognosis of gastric cancer patients.
Peer reviewers: Dr. Wael El-Rifai, Department of Surgery, Vanderbilt University Medical Center, 1255 MRB-IV Light Hall, 2215 Garland Ave, Nashville, TE 37232, United States; Shogo Kikuchi, Professor, Department of Public Health, Aichi Medical University School of Medicine, 21 Karimata, Yazako, Nagakute-cho, Aichi 480-1195, Japan
S- Editor Gou SX L- Editor Logan S E- Editor Xiong L
| 1. | Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37 Suppl 8:S4-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1370] [Cited by in RCA: 1370] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 2. | Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 604] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 3. | Pelucchi C, Tramacere I, Bertuccio P, Tavani A, Negri E, La Vecchia C. Dietary intake of selected micronutrients and gastric cancer risk: an Italian case-control study. Ann Oncol. 2009;20:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Kato S, Tsukamoto T, Mizoshita T, Tanaka H, Kumagai T, Ota H, Katsuyama T, Asaka M, Tatematsu M. High salt diets dose-dependently promote gastric chemical carcinogenesis in Helicobacter pylori-infected Mongolian gerbils associated with a shift in mucin production from glandular to surface mucous cells. Int J Cancer. 2006;119:1558-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Chen SY, Liu TY, Shun CT, Wu MS, Lu TH, Lin JT, Sheu JC, Santella RM, Chen CJ. Modification effects of GSTM1, GSTT1 and CYP2E1 polymorphisms on associations between raw salted food and incomplete intestinal metaplasia in a high-risk area of stomach cancer. Int J Cancer. 2004;108:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Sjödahl K, Jia C, Vatten L, Nilsen T, Hveem K, Lagergren J. Salt and gastric adenocarcinoma: a population-based cohort study in Norway. Cancer Epidemiol Biomarkers Prev. 2008;17:1997-2001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Hamilton SR, Aaltonen LA. World Health Organization classification of tumours. Pathology and genetics of tumours of the digestive system. Lyon: IARC Press 2000; Available from: http://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb2/bb2-cover.pdf. |
| 8. | Jenab M, Riboli E, Ferrari P, Sabate J, Slimani N, Norat T, Friesen M, Tjønneland A, Olsen A, Overvad K. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Carcinogenesis. 2006;27:2250-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 10. | Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006;12:2979-2990. [PubMed] |
| 11. | Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135:41-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 490] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 12. | Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gann. 1968;59:251-258. [PubMed] |
| 13. | Namikawa T, Hanazaki K. Mucin phenotype of gastric cancer and clinicopathology of gastric-type differentiated adenocarcinoma. World J Gastroenterol. 2010;16:4634-4639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Qiao XT, Gumucio DL. Current molecular markers for gastric progenitor cells and gastric cancer stem cells. J Gastroenterol. 2011;46:855-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Suzuki S, Tsuyama S, Murata F. Cells intermediate between mucous neck cells and chief cells in rat stomach. Cell Tissue Res. 1983;233:475-484. [PubMed] |
| 16. | Thompson M, Fleming KA, Evans DJ, Fundele R, Surani MA, Wright NA. Gastric endocrine cells share a clonal origin with other gut cell lineages. Development. 1990;110:477-481. [PubMed] |
| 17. | Hanby AM, Poulsom R, Playford RJ, Wright NA. The mucous neck cell in the human gastric corpus: a distinctive, functional cell lineage. J Pathol. 1999;187:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Wright NA. Epithelial stem cell repertoire in the gut: clues to the origin of cell lineages, proliferative units and cancer. Int J Exp Pathol. 2000;81:117-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Brittan M, Wright NA. The gastrointestinal stem cell. Cell Prolif. 2004;37:35-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Teir H, Rasanen T. A study of mitotic rate in renewal zones of nondiseased portions of gastric mucosa in cases of peptic ulcer and gastric cancer, with observations on differentiation and so-called "intestinalization" of gastric mucosa. J Natl Cancer Inst. 1961;27:949-971. [PubMed] |
| 21. | Hansen OH, Pedersen T, Larsen JK. Cell proliferation kinetics in normal human gastric mucosa. Studies on diurnal fluctuations and effect of food ingestion. Gastroenterology. 1976;70:1051-1054. [PubMed] |
| 22. | Patel S, Rew DA, Taylor I, Potten CS, Owen C, Roberts SA. Study of the proliferation in human gastric mucosa after in vivo bromodeoxyuridine labelling. Gut. 1993;34:893-896. [PubMed] |
| 23. | Kedinger M, Duluc I, Fritsch C, Lorentz O, Plateroti M, Freund JN. Intestinal epithelial-mesenchymal cell interactions. Ann N Y Acad Sci. 1998;859:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999;277:C183-C201. [PubMed] |
| 25. | Tahara E. Molecular mechanism of stomach carcinogenesis. J Cancer Res Clin Oncol. 1993;119:265-272. [PubMed] |
| 26. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5760] [Article Influence: 240.0] [Reference Citation Analysis (0)] |
| 27. | Yokozaki H, Kuniyasu H, Semba S, Yasui W, Tahara E. Molecular bases of human stomach carcinogenesis. Molecular pathology of gastroenterological cancer. New York/Tokyo: Springer, Berlin Heidelberg 1997; 55-70. |
| 28. | Gutiérrez-González L, Wright NA. Biology of intestinal metaplasia in 2008: more than a simple phenotypic alteration. Dig Liver Dis. 2008;40:510-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 611] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 30. | Hoffmann W. Regeneration of the gastric mucosa and its glands from stem cells. Curr Med Chem. 2008;15:3133-3144. [PubMed] |
| 31. | Slack JM. Epithelial metaplasia and the second anatomy. Lancet. 1986;2:268-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 732] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 33. | Goldstone AR, Quirke P, Dixon MF. Helicobacter pylori infection and gastric cancer. J Pathol. 1996;179:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Ruddell WS, Bone ES, Hill MJ, Walters CL. Pathogenesis of gastric cancer in pernicious anaemia. Lancet. 1978;1:521-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 72] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Gonda TA, Tu S, Wang TC. Chronic inflammation, the tumor microenvironment and carcinogenesis. Cell Cycle. 2009;8:2005-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 36. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [PubMed] |
| 37. | Takada K. Epstein-Barr virus and gastric carcinoma. Mol Pathol. 2000;53:255-261. [PubMed] |
| 38. | Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904-5912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1334] [Cited by in RCA: 1727] [Article Influence: 101.6] [Reference Citation Analysis (0)] |
| 39. | Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 776] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 40. | Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol. 2004;5:996-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 303] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 41. | Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13:193-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 359] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 42. | Leach SA, Thompson M, Hill M. Bacterially catalysed N-nitrosation reactions and their relative importance in the human stomach. Carcinogenesis. 1987;8:1907-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | McColl KE, el-Omar E, Gillen D, Banerjee S. The role of Helicobacter pylori in the pathophysiology of duodenal ulcer disease and gastric cancer. Semin Gastrointest Dis. 1997;8:142-155. [PubMed] |
| 44. | Crabtree JE, Shallcross TM, Heatley RV, Wyatt JI. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991;32:1473-1477. [PubMed] |
| 45. | Peek RM, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208:233-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 446] [Article Influence: 22.3] [Reference Citation Analysis (2)] |
| 46. | Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 679] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 47. | El-Omar EM. The importance of interleukin 1beta in Helicobacter pylori associated disease. Gut. 2001;48:743-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 234] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 48. | El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N. The role of interleukin-1 polymorphisms in the pathogenesis of gastric cancer. Nature. 2001;412:99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 160] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 49. | Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2416] [Cited by in RCA: 2564] [Article Influence: 122.1] [Reference Citation Analysis (0)] |
| 50. | Kamangar F, Dawsey SM, Blaser MJ, Perez-Perez GI, Pietinen P, Newschaffer CJ, Abnet CC, Albanes D, Virtamo J, Taylor PR. Opposing risks of gastric cardia and noncardia gastric adenocarcinomas associated with Helicobacter pylori seropositivity. J Natl Cancer Inst. 2006;98:1445-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 232] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 51. | Duncan LM, Richards LA, Mihm MC. Increased mast cell density in invasive melanoma. J Cutan Pathol. 1998;25:11-15. [PubMed] |
| 52. | Nowicki A, Szenajch J, Ostrowska G, Wojtowicz A, Wojtowicz K, Kruszewski AA, Maruszynski M, Aukerman SL, Wiktor-Jedrzejczak W. Impaired tumor growth in colony-stimulating factor 1 (CSF-1)-deficient, macrophage-deficient op/op mouse: evidence for a role of CSF-1-dependent macrophages in formation of tumor stroma. Int J Cancer. 1996;65:112-119. [PubMed] |
| 53. | Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 444] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 54. | Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 542] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 55. | Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, Blay JY. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778-4791. [PubMed] |
| 56. | Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2227] [Cited by in RCA: 2412] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 57. | Yang XF. Immunology of stem cells and cancer stem cells. Cell Mol Immunol. 2007;4:161-171. [PubMed] |
| 58. | Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2609] [Cited by in RCA: 2787] [Article Influence: 146.7] [Reference Citation Analysis (0)] |
| 59. | Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807-811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1279] [Cited by in RCA: 1214] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 60. | Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1135] [Cited by in RCA: 1139] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 61. | Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19 Suppl 1:S37-S43. [PubMed] |
| 62. | Nardone G, Rocco A, Malfertheiner P. Review article: helicobacter pylori and molecular events in precancerous gastric lesions. Aliment Pharmacol Ther. 2004;20:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 63. | Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3911] [Cited by in RCA: 3963] [Article Influence: 165.1] [Reference Citation Analysis (0)] |
| 64. | Kulbe H, Thompson R, Wilson JL, Robinson S, Hagemann T, Fatah R, Gould D, Ayhan A, Balkwill F. The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Res. 2007;67:585-592. [PubMed] |
| 65. | Marx J. Cancer research. Inflammation and cancer: the link grows stronger. Science. 2004;306:966-968. [PubMed] |
| 66. | Weinberg RA. Mechanisms of malignant progression. Carcinogenesis. 2008;29:1092-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 67. | Matkar SS, Durham A, Brice A, Wang TC, Rustgi AK, Hua X. Systemic activation of K-ras rapidly induces gastric hyperplasia and metaplasia in mice. Am J Cancer Res. 2011;1:432-445. [PubMed] |
| 68. | Konturek PC, Kania J, Konturek JW, Nikiforuk A, Konturek SJ, Hahn EG. H. pylori infection, atrophic gastritis, cytokines, gastrin, COX-2, PPAR gamma and impaired apoptosis in gastric carcinogenesis. Med Sci Monit. 2003;9:SR53-SR66. [PubMed] |
| 69. | Naghshvar F, Torabizadeh Zh, Emadian O, Enami K, Ghahremani M. Correlation of cyclooxygenase 2 expression and inflammatory cells infiltration in colorectal cancer. Pak J Biol Sci. 2009;12:98-100. [PubMed] |
| 70. | Lee CW, Rickman B, Rogers AB, Muthupalani S, Takaishi S, Yang P, Wang TC, Fox JG. Combination of sulindac and antimicrobial eradication of Helicobacter pylori prevents progression of gastric cancer in hypergastrinemic INS-GAS mice. Cancer Res. 2009;69:8166-8174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 71. | Schmidt PH, Lee JR, Joshi V, Playford RJ, Poulsom R, Wright NA, Goldenring JR. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639-646. [PubMed] |
| 72. | Goldenring JR, Nam KT, Wang TC, Mills JC, Wright NA. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology. 2010;138:2207-210, 2210.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 73. | Lennerz JK, Kim SH, Oates EL, Huh WJ, Doherty JM, Tian X, Bredemeyer AJ, Goldenring JR, Lauwers GY, Shin YK. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am J Pathol. 2010;177:1514-1533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 74. | Lee HJ, Nam KT, Park HS, Kim MA, Lafleur BJ, Aburatani H, Yang HK, Kim WH, Goldenring JR. Gene expression profiling of metaplastic lineages identifies CDH17 as a prognostic marker in early stage gastric cancer. Gastroenterology. 2010;139:213-25.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 75. | Nozaki K, Ogawa M, Williams JA, Lafleur BJ, Ng V, Drapkin RI, Mills JC, Konieczny SF, Nomura S, Goldenring JR. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008;134:511-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 76. | Nam KT, Varro A, Coffey RJ, Goldenring JR. Potentiation of oxyntic atrophy-induced gastric metaplasia in amphiregulin-deficient mice. Gastroenterology. 2007;132:1804-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 77. | Qiao XT, Ziel JW, McKimpson W, Madison BB, Todisco A, Merchant JL, Samuelson LC, Gumucio DL. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology. 2007;133:1989-1998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 78. | Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1527] [Cited by in RCA: 1526] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 79. | Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3347] [Cited by in RCA: 3527] [Article Influence: 185.6] [Reference Citation Analysis (1)] |
| 80. | Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9:1392-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1053] [Cited by in RCA: 1207] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 82. | Kawachi H, Takizawa T, Eishi Y, Shimizu S, Kumagai J, Funata N, Koike M. Absence of either gastric or intestinal phenotype in microscopic differentiated gastric carcinomas. J Pathol. 2003;199:436-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Park do Y, Srivastava A, Kim GH, Mino-Kenudson M, Deshpande V, Zukerberg LR, Song GA, Lauwers GY. Adenomatous and foveolar gastric dysplasia: distinct patterns of mucin expression and background intestinal metaplasia. Am J Surg Pathol. 2008;32:524-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Vescovi AL, Galli R, Reynolds BA. Brain tumour stem cells. Nat Rev Cancer. 2006;6:425-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 724] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 85. | Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nat Rev Cancer. 2007;7:659-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 450] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 86. | Tatematsu M, Tsukamoto T, Inada K. Stem cells and gastric cancer: role of gastric and intestinal mixed intestinal metaplasia. Cancer Sci. 2003;94:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 87. | Saikawa Y, Fukuda K, Takahashi T, Nakamura R, Takeuchi H, Kitagawa Y. Gastric carcinogenesis and the cancer stem cell hypothesis. Gastric Cancer. 2010;13:11-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |