Published online Sep 28, 2012. doi: 10.3748/wjg.v18.i36.5096
Revised: May 14, 2012
Accepted: May 26, 2012
Published online: September 28, 2012
AIM: To compare ghrelin levels in plasma and gastric mucosa before and after Helicobacter pylori (H. pylori) treatment in children with H. pylori-associated functional dyspepsia.
METHODS: Children with H. pylori-associated functional dyspepsia were enrolled in this study. H. pylori infection was confirmed by positive bacterial culture results. All of the children received triple H. pylori eradication therapy (a 2 wk course of omeprazole, amoxicillin, and clarithromycin). The children were divided into two groups based on the success of the H. pylori treatment: group 1 (eradicated) - patients who had a negative 13C-urea breath test 2 mo after the end of therapy; and group 2 (non-eradicated) - patients who had a positive 13C-urea breath test. Plasma ghrelin, gastric ghrelin mRNA, and the body mass index were evaluated in both groups before and after the H. pylori treatment. The plasma ghrelin levels were measured by a radioimmunoassay. The expression of gastric ghrelin mRNA was determined by real-time reverse transcription polymerase chain reaction.
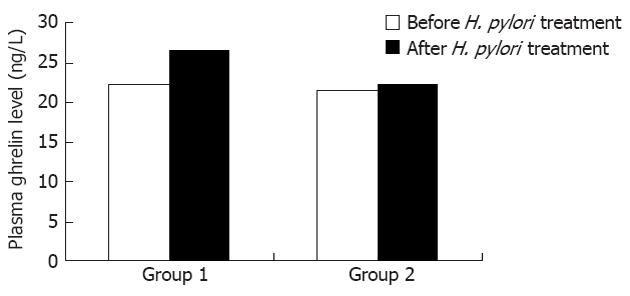
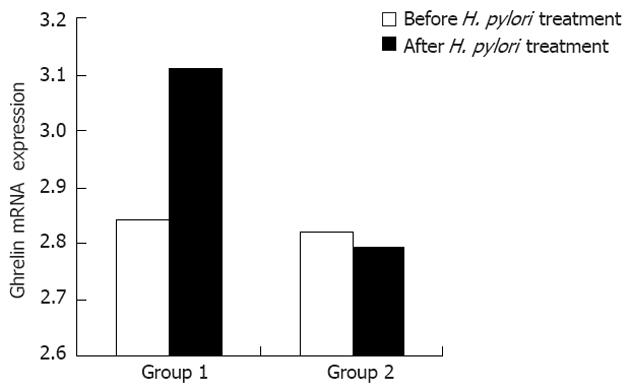
RESULTS: A total of 50 children with H. pylori-associated functional dyspepsia were treated with triple H. pylori eradication therapy. The mean age of the children was 5.52 ± 0.83 years, and there were 28 males and 22 females. Among the 50 H. pylori-positive children, 30 successfully achieved eradication, and 20 did not. The mean plasma ghrelin levels of group 1 were 22.17 ± 1.73 ng/L and 26.59 ± 2.05 ng/L before and after the treatment, respectively, which was a significant increase (P = 0.001). However, the mean plasma ghrelin level of group 2 before and after the H. pylori treatment was 21.34 ± 2.40 ng/L and 22.24 ± 2.10 ng/L (P = 0.785). The plasma ghrelin levels increased substantially after treatment in group 1 but showed only minor changes in group 2. Similarly, the gastric ghrelin mRNA expression in group 1 before treatment was 2.84 ± 0.08. After treatment, the level was 3.11 ± 0.65, which was significantly different (P = 0.023). The gastric ghrelin mRNA expression in group 2 did not change significantly during the treatment (2.82 ± 0.44 vs 2.79 ± 0.31, P = 0.875). The plasma ghrelin and gastric ghrelin mRNA levels in group 1 increased substantially after the treatment but did not do so in group 2. In addition, the body mass index the two groups did not differ significantly 2 mo before and after the H. pylori treatment.
CONCLUSION: H. pylori eradication increases the plasma and tissue ghrelin levels in children with H. pylori-associated functional dyspepsia.
-
Citation: Deng ZH, Chu B, Xu YZ, Zhang B, Jiang LR. Influence of
Helicobacter pylori infection on ghrelin levels in children. World J Gastroenterol 2012; 18(36): 5096-5100 - URL: https://www.wjgnet.com/1007-9327/full/v18/i36/5096.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i36.5096
Helicobacter pylori (H. pylori) infection is an emerging global health problem. It is regarded as a major cause of chronic gastritis, duodenitis and peptic ulcers. H. pylori is generally acquired early in life[1,2]. The prevalence of H. pylori infection is higher in children from developing countries than in those from developed countries. H. pylori is the major pathogen of the gastrointestinal tract and may also be responsible for dyspeptic symptoms and reduced appetite that can lead to malnutrition and impaired growth[3,4].
The gastrointestinal hormone ghrelin is a recently discovered gut-brain peptide that regulates food intake in humans and has strong growth hormone-releasing activity[5,6]. Ghrelin is composed of 28 amino acids and has been confirmed to both stimulate the hypothalamic appetite center through neuropeptide YNPY/AGRP and to directly regulate gastrointestinal function and appetite[7,8]. In addition, ghrelin enhances gastrointestinal tract motility and accelerates gastric emptying, which explains its appetite-promoting effects[9,10]. Ghrelin is mainly synthesized and secreted by gastric endocrine cells. Thus, chronic persistent damage to the gastric mucosa, which occurs in H. pylori infection, may affect ghrelin production and lead to changes in food intake and body weight.
In adults, there are contradictory reports on the relationship between H. pylori infection and ghrelin. Some studies have demonstrated that H. pylori infection decreased ghrelin secretion[11,12], whereas other studies have reported that H. pylori infection has no effect on plasma ghrelin levels[13,14]. There have been only a few small studies evaluating the relationship between ghrelin and H. pylori infection in children. A direct relationship between H. pylori infection and gastric ghrelin production in children remains to be demonstrated.
Thus, we studied children with H. pylori-associated functional dyspepsia (FD) and evaluated their plasma and gastric ghrelin production before and after treating their H. pylori infection. FD is a syndrome characterized by chronic and recurrent gastroduodenal symptoms in the absence of any organic or metabolic disease. H. pylori infection has been suggested to play a role in the development of FD. FD in patients with H. pylori infection is defined as a different disease entity. We recruited FD subjects to minimize any confounding factors that might have affected the study.
This study examined children with FD and H. pylori infection that had been confirmed by positive bacterial culture results. The FD diagnosis was based on the Rome III criteria[15]. Patients with histopathological examination showed normal mucosa. The children were divided into two groups, depending on the H. pylori treatment results: the H. pylori eradication group (group 1) and the H. pylori failed eradication group (group 2). The patients who had the following symptoms or diseases were excluded from the study: serious infections, heart, lung, kidney or other major organ disorders, use of drugs that may affect the H. pylori test results (antibiotics, H2 blockers, proton pump inhibitors and bismuth preparations) and use of corticosteroids. The patients who showed severe gastritis, ulcers or erosion by endoscopy were also excluded.
All of the participants underwent an initial upper gastrointestinal endoscopy. Six biopsy specimens were taken from the intact mucosa in the gastric antrum. Two of these specimens were used for bacterial cultures. Another two specimens were used for histological assessment. The remaining specimens were immediately snap frozen and stored in liquid nitrogen for ghrelin mRNA detection.
On the day of the endoscopy, blood samples were taken between 8:00 am and 10:00 am after an overnight fast, transferred into tubes containing EDTA and centrifuged. The plasma was then separated and stored at -80 °C until needed. The blood samples were taken before and after the H. pylori treatment. The plasma ghrelin concentrations were measured by radioimmunoassay.
Quantitative reverse transcription polymerase chain reaction was used to detect ghrelin mRNA. cDNA was generated using M-MLV reverse transcriptase (Sangon Biological Engineering Technology And Service Co., Shanghai, China), 1 μg of total RNA as a template and random hexamer priming. The resulting cDNA was amplified using an Exicycler. The real-time PCR analysis was conducted using SYBR® Premix Ex Taq™ and specific primers. The forward ghrelin primer was TGGAGGTCAAGCAGAAGGG, and the reverse primer was GCAGAAGCAAGCGAAAAGC. The mRNA levels were normalized using β-actin.
Selective Columbia blood agar (containing 5 mg/L TMP, 10 mg/L vancomycin, 2500 U/L polymyxin and 2 mg/L amphotericin B) was used for the culture. The H. pylori identification was based on colony morphology, the catalase and urease tests, and Gram staining to determine the morphological and staining properties.
The H. pylori-infected children were treated with omeprazole (20 mg/d), amoxicillin (50 mg/kg per day), and clarithromycin (15 mg/kg per day) for two weeks. The children were not given any other medication after the triple therapy. The H. pylori infection status was evaluated using the 13C-urea breath test 2 mo after the end of the therapy.
The patients were followed up 2 mo after the end of therapy. Endoscopy and biopsy sampling were conducted, and plasma was collected again. We monitored serum ghrelin and gastric ghrelin mRNA and assessed the changes in their body mass indexs (BMIs).
All of the data were expressed as mean ± SE. The Student’s t test was used to compare mean. P < 0.05 was considered to be significantly different.
From January 2009 to April 2011, we studied 50 children with H. pylori-associated FD. The characteristics of the children, including their mean age, gender and BMI, are shown in Table 1. The H. pylori therapy was effective in 30 children and failed in 20 children.
| Group 1 | Group 2 | |
| Number of children (male/female) | 17/12 | 11/10 |
| Age (yr) | 6.15 ± 0.74 | 5.49 ± 0.23 |
| BMI (kg/m2) | 14.7 ± 1.5 | 15.9 ± 1.7 |
In group 1, the plasma ghrelin levels before and after the treatment were significantly different (22.17 ± 1.73 ng/L and 26.59 ± 2.05 ng/L, respectively, P = 0.001). However, the plasma ghrelin levels in group 2 before and after the treatment were similar (21.34 ± 2.40 ng/L and 22.24 ± 2.10 ng/L, respectively, P = 0.785). The plasma ghrelin levels in group 1 increased substantially after the therapy, but those in group 2 showed only minor changes.
We also compared gastric ghrelin mRNA expression in the two groups before and after the H. pylori treatment. Similar to the changes in the plasma ghrelin levels, the gastric ghrelin mRNA expression in group 1 was 2.84 ± 0.08 and 3.11 ± 0.65 (P = 0.023) before and after the treatment, respectively, which was significantly different. These values were not significantly different in group 2 (2.82 ± 0.44 and 2.79 ± 0.31, P = 0.875).
The plasma ghrelin levels and gastric ghrelin mRNA increased significantly in group 1 but showed inconsistent changes in group 2 (Figures 1 and 2). The plasma ghrelin levels paralleled the gastric ghrelin mRNA expression levels in the H. pylori eradication group. These results suggest that attenuating ghrelin production in the gastric mucosa accounts for the decrease in the plasma ghrelin concentrations.
The mean BMI in the H. pylori eradication group before and after the treatment was 14.7 ± 1.5 kg/m2 and 15.6 ± 1.2 kg/m2 (P = 0.765), respectively. The mean BMI in the H. pylori failed eradication group before and after the treatment was 15.9 ± 1.7 kg/m2 and 15.5 ± 1.6 kg/m2 (P = 0.825), respectively. Our study found that BMI did not change significantly before and after the H. pylori treatment in either group.
Our study found that the plasma ghrelin and gastric ghrelin mRNA levels increased significantly in those patients for whom the H. pylori treatment was successful and were not significantly different in those patients for whom the H. pylori treatment failed. These findings are consistent with those of other studies in which subjects underwent H. pylori treatment and had increased plasma ghrelin[16,17] and gastric ghrelin mRNA levels[18].
Inflammation of the gastric mucosa is the one of the important mechanisms of H. pylori-induced changes in ghrelin production. However, severe inflammation of the mucosal layers by other factors, such as ulcers and atrophic gastritis, can also influence ghrelin production. Cummings et al[19] found that plasma ghrelin levels were low in a young woman with an evolving autoimmune gastric process. Thus, the change in ghrelin production caused by H. pylori infection might be confused with the severe inflammation caused by diseases such as ulcers and atrophic gastritis. Therefore, we recruited FD subjects without severe histopathological changes to minimize the confounding factors and found that ghrelin production increased after H. pylori eradication independent of inflammation. Furthermore, diseases such as ulcers, atrophic gastritis and cancer are seen much less frequently in children than in adults, and the host genetic and environmental factors (such as diet, alcohol, and smoking) that play an important role in ghrelin production are more uniform in children. Studies of children can clarify the relationship between H. pylori and ghrelin more directly than can studies of adults.
The reports on the influence of H. pylori eradication on ghrelin concentrations in children are contradictory. Pacifico et al[20] have reported that successful H. pylori treatment in children was associated with increased BMI and lean and fat mass but not with increased ghrelin levels, which were actually decreased. These results may suggest that changes in ghrelin levels after the H. pylori infection has been cured are epiphenomenal. By contrast, Konturek et al[21] found that eliminating H. pylori from the stomach using triple eradication therapy resulted in enhanced circulating plasma ghrelin in H. pylori-positive shepherds and their children. The discrepancies in the response to H. pylori treatment may be related to the strain of H. pylori. Patients with the type I strain, which expresses the cytotoxin-associated gene product and vacuolating cytotoxin A virulence factors, have lower circulating ghrelin levels than those with the less virulent type II strain, which does not express these virulence factors[22]. The features of H. pylori strains are known to differ between developed and developing countries, and H. pylori infection is also known to be associated with socio-economic conditions. The patients in the study by Konturek et al[21] were shepherds who may have the same H. pylori strain as our patients, and our results are similar to those reported by Konturek et al[21].
Ghrelin-producing cells have been found mainly in the oxyntic mucosa of the stomach. Decades of acquired H. pylori infection can lead to chronic gastritis and atrophy. Thus, some authors have demonstrated that ghrelin production was influenced by reductions in the number of oxyntic cells caused by atrophy. Cindoruk et al[14] have shown that eradicating H. pylori has no affect on the plasma ghrelin concentration in patients without atrophy. Osawa et al[11] have also suggested that impaired gastric ghrelin production in association with the atrophic gastritis caused by H. pylori infection accounted for the decreased plasma ghrelin concentration. There was no evidence of gastric atrophy or intestinal metaplasia in our study. The functional impairment of ghrelin-producing cells due to inflammation, perhaps mediated by cytokines, may account for reduced ghrelin production[23,24]. Recent studies have shown that H. pylori infection causes the release of many pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6 and IL-8, and influences decreases in ghrelin production[25]. Abiko et al[26] further confirmed the relationships among H. pylori infection, ghrelin secretion and cytokines. A comparison of the ghrelin level, appetite and body weight of H. pylori-infected IL-1 gene knockout and wild-type mice showed no obvious changes in the knockout mice but significant reductions in the ghrelin level, appetite and body weight of the infected wild-type mice. These results indicated that H. pylori infection can affect ghrelin secretion via IL-1. Therefore, further investigations are required to understand the relationships among ghrelin, H. pylori infection and inflammatory cytokines in children.
Compared with previous studies of children, our study not only demonstrated that plasma ghrelin concentrations were influenced by H. pylori eradication but also focused on the gastric mucosa to better understand the effects of H. pylori infection on ghrelin expression changes. Increases in gastric ghrelin production may account for the higher concentrations of plasma ghrelin seen after H. pylori eradication.
A previous study has reported that BMI increased significantly 12 mo after H. pylori eradication[27]. Our study was designed to evaluate ghrelin changes at 2 mo after H. pylori treatment and was therefore too short to detect changes in BMI.
The changes in ghrelin production can be related to successfully eradicating the H. pylori infection. Ghrelin has also been suggested as a potential marker for H. pylori treatment success and may be an indicator of children who should be treated for H. pylori infection.
In conclusion, we demonstrated that plasma and gastric tissue ghrelin levels in children are increased after H. pylori eradication. H. pylori may influence ghrelin production. We also hypothesize that ghrelin may play an important role in the mechanism of H. pylori-associated FD in children. Future studies are needed to investigate this hypothesis.
Helicobacter pylori (H. pylori) infection in children is higher in developing country. It not only is the major pathogen of the gastrointestinal tract but also may be responsible for dyspeptic symptoms and reduces appetite that might lead to malnutrition to impair growth. Ghrelin is primarily secreted from the stomach and has been implicated in the coordination of eating behavior and weight regulation. Thus, there exists the possibility that chronic persistent damage of the gastric mucosa, such as H. pylori infection, might affect ghrelin production and lead to changes in food intake and body weight. The direct relationship between H. pylori infection and gastric ghrelin production is needed to be demonstrated.
Many studies assessing the relationship between H. pylori and Ghrelin in adults have been published but a comparable study in children is lacking. The important area in the study is selected children with H. pylori-associated dyspepsia as subjects and evaluates plasma and gastric ghrelin production before and after treating H. pylori.
Compared with previous studies in children, the authors’ study not only demonstrates that plasma ghrelin concentrations are influenced after H. pylori eradication but also focused on the gastric musca to better understand the effects of H. pylori infection on the alteration of ghrelin expression. Increases in gastric ghrelin production may account for higher concentration of plasma ghrelin after H. pylori eradication. The authors’ study also suggested that level of ghrelin can be considered as one of the maker of H. pylori successfully or not and can be one of the indication for children H. pylori infection therapy. Furthermore, this report provide novel insights for understanding the physiological function of ghrelin and its relation to associated disease.
The study results suggest that gherlin levels in plasma and gastric tissue in children are increased after H. pylori eradication and ghrelin may play an important role in the mechanism of H. pylori-associated dyspepsia in children.
Ghrelin: Ghrelin is a gastrointestinal hormone and possesses strong growth hormone-releasing activity and is recently discovered gut-brain peptide that regulates food intake of human; Functional dyspepsia: Functional dyspepsia is a syndrome characterized by chronic and recurrent gastroduodenal symptoms in absence of any organic or metabolic disease.
This is a novel study in which authors evaluate plasma and gastric ghrelin production before and after treating H. pylori in children with H. pylori-associated dyspepsia. The results suggest that ghrelin may play an important role in the mechanism of H. pylori-associated dyspepsia in children.
Peer reviewers: Tamara Vorobjova, Senior Researcher in Immunology, Department of Immunology, Institute of General and Molecular Pathology, University of Tartu, Ravila, 19, 51014 Tartu, Estonia; Andrew S Day, MB, ChB, MD, FRACP, AGAF, Associate Professor, Department of Paediatrics, University of Otago, Christchurch, PO Box 4345, Christchurch 8140, New Zealand
S- Editor Lv S L- Editor A E- Editor Li JY
| 1. | Sherman PM. Appropriate strategies for testing and treating Helicobacter pylori in children: when and how? Am J Med. 2004;117 Suppl 5A:30S-35S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1907] [Article Influence: 82.9] [Reference Citation Analysis (3)] |
| 3. | Perri F, Pastore M, Leandro G, Clemente R, Ghoos Y, Peeters M, Annese V, Quitadamo M, Latiano A, Rutgeerts P. Helicobacter pylori infection and growth delay in older children. Arch Dis Child. 1997;77:46-49. [PubMed] |
| 4. | Ertem D, Pehlivanoglu E. Helicobacter pylori may influence height in children independent of socioeconomic factors. J Pediatr Gastroenterol Nutr. 2002;35:232-233. [PubMed] |
| 5. | Akamizu T, Kangawa K. Translational research on the clinical applications of ghrelin. Endocr J. 2006;53:585-591. [PubMed] |
| 6. | Williams DL, Cummings DE. Regulation of ghrelin in physiologic and pathophysiologic states. J Nutr. 2005;135:1320-1325. [PubMed] |
| 7. | Nogueiras R, Williams LM, Dieguez C. Ghrelin: new molecular pathways modulating appetite and adiposity. Obes Facts. 2010;3:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Crespo MA, González Matías LC, Lozano MG, Paz SF, Pérez MR, Gago EV, Ferrer FM. [Gastrointestinal hormones in food intake control]. Endocrinol Nutr. 2009;56:317-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Chen CY, Asakawa A, Fujimiya M, Lee SD, Inui A. Ghrelin gene products and the regulation of food intake and gut motility. Pharmacol Rev. 2009;61:430-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 10. | Takeshita E, Matsuura B, Dong M, Miller LJ, Matsui H, Onji M. Molecular characterization and distribution of motilin family receptors in the human gastrointestinal tract. J Gastroenterol. 2006;41:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Osawa H, Nakazato M, Date Y, Kita H, Ohnishi H, Ueno H, Shiiya T, Satoh K, Ishino Y, Sugano K. Impaired production of gastric ghrelin in chronic gastritis associated with Helicobacter pylori. J Clin Endocrinol Metab. 2005;90:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Tatsuguchi A, Miyake K, Gudis K, Futagami S, Tsukui T, Wada K, Kishida T, Fukuda Y, Sugisaki Y, Sakamoto C. Effect of Helicobacter pylori infection on ghrelin expression in human gastric mucosa. Am J Gastroenterol. 2004;99:2121-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Isomoto H, Ueno H, Nishi Y, Wen CY, Nakazato M, Kohno S. Impact of Helicobacter pylori infection on ghrelin and various neuroendocrine hormones in plasma. World J Gastroenterol. 2005;11:1644-1648. [PubMed] |
| 14. | Cindoruk M, Yetkin I, Deger SM, Karakan T, Kan E, Unal S. Influence of H pylori on plasma ghrelin in patients without atrophic gastritis. World J Gastroenterol. 2007;13:1595-1598. [PubMed] |
| 15. | Drossman DA. Rome III: the new criteria. Chin J Dig Dis. 2006;7:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1467] [Cited by in RCA: 1476] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 16. | Kawashima J, Ohno S, Sakurada T, Takabayashi H, Kudo M, Ro S, Kato S, Yakabi K. Circulating acylated ghrelin level decreases in accordance with the extent of atrophic gastritis. J Gastroenterol. 2009;44:1046-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N, Urabe A. Helicobacter pylori infection is significantly associated with metabolic syndrome in the Japanese population. Am J Gastroenterol. 2008;103:3005-3010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Osawa H, Kita H, Ohnishi H, Nakazato M, Date Y, Bowlus CL, Ishino Y, Watanabe E, Shiiya T, Ueno H. Changes in plasma ghrelin levels, gastric ghrelin production, and body weight after Helicobacter pylori cure. J Gastroenterol. 2006;41:954-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2010] [Cited by in RCA: 1933] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 20. | Pacifico L, Anania C, Osborn JF, Ferrara E, Schiavo E, Bonamico M, Chiesa C. Long-term effects of Helicobacter pylori eradication on circulating ghrelin and leptin concentrations and body composition in prepubertal children. Eur J Endocrinol. 2008;158:323-332. [PubMed] |
| 21. | Konturek PC, Cześnikiewicz-Guzik M, Bielanski W, Konturek SJ. Involvement of Helicobacter pylori infection in neuro-hormonal control of food intake. J Physiol Pharmacol. 2006;57 Suppl 5:67-81. [PubMed] |
| 22. | Isomoto H, Nishi Y, Ohnita K, Mizuta Y, Kohno S, Ueno H, Nakazato M. The Relationship between Plasma and Gastric Ghrelin Levels and Strain Diversity in Helicobacter pylori Virulence. Am J Gastroenterol. 2005;100:1425-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Isomoto H, Nakazato M, Ueno H, Date Y, Nishi Y, Mukae H, Mizuta Y, Ohtsuru A, Yamashita S, Kohno S. Low plasma ghrelin levels in patients with Helicobacter pylori-associated gastritis. Am J Med. 2004;117:429-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Cummings DE. Helicobacter pylori and ghrelin: Interrelated players in body-weight regulation? Am J Med. 2004;117:436-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Chuang CH, Sheu BS, Yang HB, Lee SC, Kao AW, Cheng HC, Chang WL, Yao WJ. Gender difference of circulating ghrelin and leptin concentrations in chronic Helicobacter pylori infection. Helicobacter. 2009;14:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Abiko Y, Suzuki H, Masaoka T, Nomura S, Kurabayashi K, Hosoda H, Kangawa K, Hibi T. Enhanced plasma ghrelin levels in Helicobacter pylori-colonized, interleukin-1-receptor type 1-homozygous knockout (IL-1R1-/-) mice. World J Gastroenterol. 2005;11:4148-4153. [PubMed] |
| 27. | Azuma T, Suto H, Ito Y, Muramatsu A, Ohtani M, Dojo M, Yamazaki Y, Kuriyama M, Kato T. Eradication of Helicobacter pylori infection induces an increase in body mass index. Aliment Pharmacol Ther. 2002;16 Suppl 2:240-244. [PubMed] |