Published online Sep 28, 2012. doi: 10.3748/wjg.v18.i36.5051
Revised: May 31, 2012
Accepted: June 8, 2012
Published online: September 28, 2012
AIM: To examine the predictive factors of capsule endoscopy (CE) completion rate (CECR) including the effect of inpatient and outpatient status.
METHODS: We identified 355 consecutive patients who completed CE at Rush University Medical Center between March 2003 and October 2005. Subjects for CE had either nothing by mouth or clear liquids for the afternoon and evening of the day before the procedure. CE exams were reviewed by two physicians who were unaware of the study hypotheses. After retrospective analysis, 21 cases were excluded due to capsule malfunction, prior gastric surgery, endoscopic capsule placement or insufficient data. Of the remaining 334 exams [264 out-patient (OP), 70 in-patient (IP)], CE indications, findings, location of the patients [IP vs OP and intensive care unit (ICU) vs general medical floor (GMF)] and gastrointestinal transit times were analyzed. Statistical analysis was completed using SPSS version 17 (Chicago, IL). Chi-square, t test or fisher exact-tests were used as appropriate. Multivariate logistic regression analysis was used to identify variables associated with incomplete CE exams.
RESULTS: The mean age for the entire study population was 54.7 years. Sixty-one percent of the study population was female, and gender was not different between IPs vs OPs (P = 0.07). The overall incomplete CECR was 14% in our study. Overt obscure gastrointestinal bleeding (OGB) was a significantly more common indication for the IP CE (P = 0.0001), while abdominal pain and assessment of IBD were more frequent indications for the OP CE exams (P = 0.002 and P = 0.01, respectively). Occult OGB was the most common indication and arteriovenous malformations were the most common finding both in the IPs and OPs. The capsule did not enter the small bowel (SB) in 6/70 IPs and 8/264 OPs (P = 0.04). The capsule never reached the cecum in 31.4% (22/70) of IP vs 9.5% (25/ 264) of OP examinations (P < 0.001). The mean gastric transit time (GTT) was delayed in IPs compared to OPs, 98.5 ± 139.5 min vs 60.4 ± 92.6 min (P = 0.008). Minimal SB transit time was significantly prolonged in the IP compared to the OP setting [IP = 275.1 ± 111.6 min vs OP = 244.0 ± 104.3 min (P = 0.037)]. CECR was also significantly higher in the subgroup of patients with OGB who had OP vs IP exams (95% vs 80% respectively, P = 0.001). The proportion of patients with incomplete exams was higher in the ICU (n = 7/13, 54%) as compared to the GMF (n = 15/57, 26%) (P = 0.05). There was only a single permanent SB retention case which was secondary to a previously unknown SB stricture, and the remaining incomplete SB exams were due to slow transit. Medications which affect gastrointestinal system motility were tested both individually and also in aggregate in univariate analysis in hospitalized patients (ICU and GMF) and were not predictive of incomplete capsule passage (P > 0.05). Patient location (IP vs OP) and GTT were independent predictors of incomplete CE exams (P < 0.001 and P = 0.008, respectively).
CONCLUSION: Incomplete CE is a multifactorial problem. Patient location and related factors such as severity of illness and sedentary status may contribute to incomplete exams.
- Citation: Yazici C, Losurdo J, Brown MD, Oosterveen S, Rahimi R, Keshavarzian A, Bozorgnia L, Mutlu E. Inpatient capsule endoscopy leads to frequent incomplete small bowel examinations. World J Gastroenterol 2012; 18(36): 5051-5057
- URL: https://www.wjgnet.com/1007-9327/full/v18/i36/5051.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i36.5051
In recent years, capsule endoscopy (CE) has emerged as a sophisticated and widely used tool to evaluate small bowel pathology. It has applications in Crohn’s disease, celiac disease, Peutz-Jeghers syndrome, familial adenomatous polyposis, and hereditary polyposis syndromes[1-4]. CE is also particularly useful for the investigation of obscure gastrointestinal bleeding (OGB)[5,6], especially if upper and lower endoscopies fail to reveal the underlying pathology and a small bowel source is suspected. Locating the source and treating OGB has long been a challenge for physicians. Identification of the bleeding site is the key step not only to diagnose but also to provide effective treatment. During a brisk overt hemorrhage, the rapidity at which the bleeding site is found is critical. CE is therefore utilized often in patients hospitalized for bleeding and anemia. The hospital setting, however, may not be ideal in obtaining optimal results with CE. In-patients tend to be sedentary and are more prone to delayed transit or ileus, which may prolong gastric and small bowel transit times. Therefore, we hypothesized that in-patient (IP) CE is more often incomplete as compared to out-patient (OP) CE. The two previously published European studies indirectly examining this question have compared IP vs OP CE completion rates (CECR)[7,8], but both studies have limitations: both used a prokinetic agent and/or bowel preparation to increase CECR and neither of these interventions is part of the standard CE protocol at United States institutions. Furthermore, the patient numbers were small and the studies did not examine the effects of inpatient location (i.e., an intensive care unit (ICU) vs a general medical floor (GMF) setting). In this study, we aimed at overcoming these limitations by analyzing all consecutive IPs and OPs, studying a larger IP group, doing a sub-group analysis to compare the ICU and GMF settings, without the use of prokinetic and bowel preparation agents.
This study was conducted in compliance with ethical guidelines and was approved by Rush University Institutional Review Board. All CE examinations done at Rush University Medical Center between March, 2003 and October, 2005 were identified retrospectively. A total of 355 CE exams, which were performed using a Given PillCamTM small bowel (SB) capsule, were reviewed using the Given Rapid® Access software. Patients with capsule malfunction (n = 6), prior gastric surgery (n = 3), endoscopic capsule placement (n = 3) and studies lacking sufficient data for analysis were excluded (n = 9; 8 OP, 1 IP). In the remaining 334 exams (264 OP, 70 IP), patient characteristics such as age, gender, gastric retention of the capsule, completeness of the examination (whether or not the capsule reached the cecum), gastric and small bowel transit times, indications and findings were recorded.
Subjects for capsule endoscopy had either nothing by mouth (except their medications) or clear liquids (excluding any red colored foods) for the afternoon and evening of the day before the procedure. The CE exam was delayed if subjects used iron supplementation within the past 5 d prior to the procedure.
CE exams were reviewed by two physicians. For the majority of cases, gastroenterologists who read the CE exams did not have any information regarding patient location. The hypotheses of the study were conceived after the exams were read and gastroenterologists were unaware of the study hypotheses.
Overt gastrointestinal bleed (GIB) was defined as having melena and/or hematochezia with a hemoglobin < 10 g/dL. Occult obscure GIB was defined as subjects who had a positive fecal occult blood test or complaints of intermittent black bowel movements, and who had both colonoscopy and upper endoscopy without any source of bleeding identified. Accurate SB transit time (SBTT) were obviously not available in those subjects in whom the capsule stopped recording before reaching the colon. In this circumstance the minimum SBTT, which reflects the minimum time the capsule spent in the small bowel, was calculated using the formula, 480 min - the gastric transit time (GTT) (min) = minimum SBTT. Four hundred and eighty minutes was used as this has the generally accepted battery life of the Given PillCam™ SB capsule at the time of the CE exams.
Statistical analysis was completed using SPSS version 17 (Chicago, IL). Chi-square, t test or fisher exact-test was used as appropriate. Multivariate logistic regression analysis was used to identify variables associated with incomplete CE exams. A multivariate logistic regression model was constructed using forward stepwise method with capsule reaching the cecum or not as the dependent variable using the univariate variables that were significantly predictive of incomplete capsule passage. In addition, differences in bowel transit time between the groups were compared by a survival model (Kaplan-Meier) and (Log-Rank test).
The baseline characteristics of the IPs vs OPs that underwent CE are shown in Table 1. The mean age for the entire study population was 54.7 (17-92) years. The mean age for the IPs was greater than the OPs (60.9 ± 18.4 vs 53 ± 17.7, P = 0.001). Sixty-one percents of the study population was female, and gender was not different between IPs vs OPs (P = 0.07). The indications for CE were occult OGB, overt OGB, abdominal pain, and assessment for inflammatory bowel disease (IBD), SB masses, celiac disease and other pathologies. Several patients had more than one indication for the CE examination. Occult OGB was the most common indication for both the IPs and OPs. While evaluation of overt OGB was significantly more common for the IP CE (P = 0.0001), abdominal pain and assessment of IBD were more frequently the indications for the OP CE exams (P = 0.002 and P = 0.01, respectively). Arteriovenous malformations (AVM) were the most common finding both in the IPs and OPs. Active bleeding was seen more often in the IPs (P = 0.001); while SB ulcerations and erosions were more frequently observed in the OP CE exams (P = 0.011).
| Patient characteristics | IP (n = 70) | OP (n = 264) | P value |
| Age (yr) | 60.9 ± 18.4 | 53.0 ± 17.7 | 0.0011 |
| Gender (male/female) | 34/36 | 96/168 | 0.07 |
| Indications | |||
| Occult obscure GIB | 37 (52.9) | 124 (46.9) | 0.381 |
| Overt GIB | 24 (34.3) | 23 (8.7) | 0.0001 |
| Abdominal pain | 11 (15.7) | 92 (36.7) | 0.002 |
| Assess IBD | 8 (11.4) | 83 (31.4) | 0.001 |
| Assess small bowel masses | 1 (1.4) | 17 (6.4) | 0.137 |
| Assess celiac disease | 1 (1.4) | 3 (1.1) | 1.000 |
| Others | 0 (0) | 9 (3.4) | 0.213 |
| Findings | |||
| AVM | 33 (47.1) | 116 (43.9) | 0.632 |
| SB ulceration/erosion | 19 (27.1) | 116 (43.9) | 0.011 |
| Active bleed | 11 (15.7) | 12 (4.6) | 0.001 |
| Esophagitis/duodenitis/gastritis | 7 (10.0) | 28 (10.6) | 1.000 |
| Mass/polyp | 13 (18.6) | 43 (16.3) | 0.649 |
| Excess retained food | 7 (10.0) | 22 (8.3) | 0.660 |
| Others | 6 (8.6) | 19 (7.2) | 0.698 |
The overall incomplete CECR was 14% in our study. A higher number of patients in the OP group had complete CE with visualization of the entire SB, compared to the IP group (90.5% vs 68.6%, P < 0.001). The capsule remained within the stomach for the duration of the study in 8.6% (6/70) of the IPs and 3% (8/264) of the OPs (P = 0.04). When these cases of gastric capsule retention were excluded, 25% (16/64) of the IPs and 6.6% (17/256) of the OPs had exams that were not complete, as the capsule did not reach the cecum (P = 0.001). Therefore, both gastric capsule retention with reduced entry into the SB as well as delayed transit in the SB played a role in the incomplete CE exams in the IPs. Obstructing lesions in the SB were uncommon. There was only a single permanent small bowel retention case in the dataset, who was an OP with a previously unrecognized stricture. The remainder of the incomplete SB exams appeared to be due purely to slow transit. To exclude a diagnosis related bias in the analysis, we also looked at the OGB subset of our data. CECR was also significantly higher in OGB patients who had OP vs IP exams (95% vs 80% respectively, P = 0.001). Therefore, the result obtained can not be solely explained by differences in the OP vs IP diagnoses.
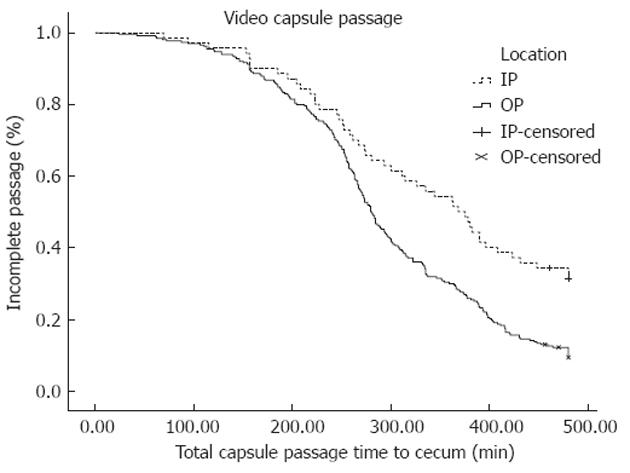
A Kaplan-Meier plot of total bowel transit time for the IPs vs the OPs is shown in Figure 1. As time passes more studies get completed in the OP group compared to the IP group. The median total capsule transit time to the cecum was longer in IP vs OP (368 min vs 279 min) (P < 0.0001, log-rank for median total capsule transit time).
In 10 patients (3 IPs and 7 OPs), GTT were not recorded into the CE report. In the remaining 324 patients, the mean GTT was prolonged in the IP setting. GTT was 98.5 ± 139.5 min in IP vs 60.4 ± 92.6 min in OP setting (P = 0.008). After removing those patients with gastric capsule retention and lack of GTT measurements from the dataset, minimum SBTT were significantly prolonged in the IP setting (275.1 ± 111.6 min) vs the OP setting (244.0 ± 104.3 min) (P = 0.037).
Among the 70 IPs studied, 13 were in the ICU and 57 were on a GMF. There was no statistical difference in mean age and gender in the ICU vs GMF groups, although the ICU patients were relatively older (68.8 ± 13.6 years vs 59.1 ± 19.0 years, P = 0.084).
The proportion of patients with incomplete exams was higher in the ICU (n = 7/13, 54%) as compared to the GMF (n = 15/57, 26%) (P = 0.05). Gastric capsule retention also was more common among ICU patients compared to the GMF [2/13 (15.3%) vs 4/57 (7%), P = 0.331]. When subjects with gastric retention were excluded, the proportion of subjects with incomplete small bowel exams were numerically higher 5/11 (45%) in the ICU setting vs 11/53 (32%) in the GMF setting, but the difference was not statistically significant (P = 0.085). The GTTs were not significantly different between the ICU and GMF patients (154.4 ± 181.4 min vs 85.0 ± 125.8 min, P = 0.108) although a trend toward longer GTT in ICU patients was noted. Minimum SBTT were also not significantly different between the ICU (272.1 ± 131.4 min) and GMF patients (275.8 ± 108.4 min) (P = 0.922).
We looked at the effects of potential risk factors for an incomplete exam in univariate analyses in all subjects (Table 2). Among pre-procedure risk factors, location (IP vs OP setting) and having an indication for gastrointestinal (GI) bleed (either occult or overt) were predictive of incomplete capsule passage. Among post-procedure risk factors, the following findings were predictive of having an incomplete exam: having an AVM, blood, visible vessel, lymphoid hyperplasia, gastric vascular ectesia, findings suggesting Crohn’s disease (CD), excess food particles in the GI tract, phlebectasia, having large gastric folds, white appearing villi, GTT, and SBTT. We could not evaluate the effect of medication use in the entire dataset due to lack of accurate medication data in outpatients.
| Pre-procedure factors | P value | Post-procedure factors | P value |
| Location | 0.0001 | AVM | 0.001 |
| Occult GIB | 0.042 | Blood | 0.016 |
| Overt GIB | 0.054 | Visible vessel | 0.013 |
| Abdominal pain | 0.608 | Lymphoid hyperplasia | 0.014 |
| Assessment of IBD | 0.142 | GAVE | 0.051 |
| History of FAP | 0.999 | Findings suggestive of CD | 0.007 |
| History of DA | 0.199 | Excess food | 0.006 |
| Nausea/vomiting | 0.879 | Phlebectasia | 0.011 |
| Diarrhea | 0.929 | Large gastric folds | 0.010 |
| Colon polyps | 0.999 | White villi | 0.01 |
| History of OVR | 1 | Edema | 0.415 |
| History of carcinoid tumors | 0.999 | Submucosal mass | 0.372 |
| History of PJS | 1 | Mucosal mass | 0.119 |
| History of VE | 1 | Erosions | 0.350 |
| Age | 0.113 | Ulcer | 0.308 |
| Gender | 0.232 | Duodenitis | 0.597 |
| Diverticulitis | 0.461 | ||
| Mucosal break | 0.078 | ||
| Esophagitis | 0.062 | ||
| Stricture | 0.399 | ||
| Reader | 0.982 | ||
| GTT | 0.0001 | ||
| SBTT | 0.001 |
Medications which affect GI system motility were tested both individually and also in aggregate in univariate analysis in hospitalized patients (Table 3). These medications included narcotics, anticholinergics, beta-blockers, calcium channel blockers, inotropic agents, and motility enhancers. None of the medications individually or in aggregate were predictive of capsule passage or incomplete capsule exam (P > 0.05, Table 3). Other factors such as diabetes, neuropathy, hypotension, and immobility were not predictive of capsule passage or incomplete capsule exam either (P > 0.05, Table 3).
| Risk factors | P values |
| Diabetes | 0.416 |
| Neuropathy | 0.382 |
| Hypotension | 0.947 |
| Immobility | 0.381 |
| Medications in aggregate | 0.271 |
| Narcotics | 0.311 |
| Anticholinergics | 0.815 |
| Beta-blockers | 0.711 |
| Calcium channel blockers | 0.931 |
| Inotropes | 0.197 |
| Motility enhancers | 0.337 |
In the multivariate logistic regression model that utilized all patients, except those who did not have gastric transit times recorded, patient location (IP vs OP) and GTT were independent predictors of incomplete CE exams (P < 0.001 and P = 0.008, respectively). Further details of the model are given in Table 4.
| Factors | Beta value | SE | Wald ×2 | P value | Odds ratio | 95% CI |
| Intercept | -3.369 | 0.349 | 93.344 | 0.000 | ||
| Location | 1.457 | 0.415 | 12.328 | 0.000 | 4.294 | 1.904-9.686 |
| GTT | 0.012 | 0.003 | 18.177 | 0.000 | 1.012 | 1.006-1.017 |
Our study represents a first look at the CECR in IPs in the United States, and demonstrates that both GTT and SBTT are significantly prolonged in IP CE exams compared to OP exams. In a multivariate regression model, we show that patient location (IP vs OP) and GTT are independent predictors of incomplete CE, suggesting factors other than GTT must be contributing to the lower CECR in inpatients. Here we demonstrate that increased SBTT is another important contributor for low CECR in addition to GTT. We further show that the CECR is lowest in the ICU setting.
CECR in patients with OGB has been investigated previously. In these studies, CECR are variable and lower than what we note in our study. In a prospective European study of only mild to moderate obscure overt GI bleeders, incomplete IP CEs were 21.6%, but in 75% of the incomplete exams there was some localization of the bleed site[9]. However, this study excluded all severe bleeders and inpatients with other indications. In another study of 48 inpatient obscure overt GI bleeders from Europe, incomplete CE rates were 27%[10]. In a recent Canadian study of 535 patients, overt GI bleed was found to be a major risk factor for incomplete CE (P = 0.002, OR = 2.7) and the overall incomplete CE rate was 29.5%[11]. However, in this study, only 31 subjects were inpatients. Therefore, hospitalization status did not reach statistical significance despite a large trend (P = 0.054). In studies that analyzed CECR in patients with GIB, CECR in relation to patient location was not examined and direct comparisons to outpatients were not complete. Using subgroup analysis of our patients with GIB, we found a lower IP CECR compared to these prior studies (95% in OP vs 80% in IP). More interestingly, regardless of the GIB diagnosis, incomplete CE rate was also significantly lower in the OP setting compared to the IP setting (P = 0.001).
The relationship between CECR and hospitalization was addressed more directly in two other previous European studies. When all indications were taken into account, incomplete CE rate was higher in hospitalized patients, 34%-35%[7,8]. Our results in the United States confirm these findings in a larger dataset. GTT was elevated in the IP setting in both studies, similar to our findings. In the former studies, there were additional interventions to improve CE quality: per protocol, some patients received bowel preparation[8], while others received both bowel preparation and prokinetic agents[7]. Bowel preparation and the use of prokinetic agents are not routine in the United States. None of these prior studies specifically investigated the effect of IP location in CECR, and in one of these studies, patients underwent a 23 h hospitalization solely to perform the CE, which is not done in the United States. In addition lower IP CECR shown in these studies were attributed to decreased gastric motility; whereas we now show that the minimum SBTTs are also significantly prolonged in patients who had IP CE, suggesting that factors such as generalized dysmotility of gastrointestinal tract may be at play.
The overall CECR has been reported to be 81.1% and 83.5% in two recent meta-analyses[12,13]. The first meta-analysis compared CECR in patients who were prepped with purgatives vs clear liquid diet prior to the CE and showed that there was no significant difference in CECR, although the purgative use resulted in better small bowel visualization quality and increased diagnostic yield[13]. The second study reported an overall CECR of 83.5% in patients with OGIB, CD, and neoplastic lesions[12]. The overall CECR in our study at 86% reflects a slightly higher value than reported in these two meta-analyses. Nevertheless, even if we were to assume that the CECR in our OP group was as low as 81%-83% (similar to the two meta-analyses), this would still be markedly numerically higher than our observed CECR of 68% in the IPs, and should not alter our conclusions. Our CECR may perhaps be reflective of the careful case selection at a tertiary urban medical center, in order to avoid retention. Alternative explanations could include a larger number of OPs compared to IPs in our study. Notably, the percentage of OPs and IPs were not delineated in the meta-analyses. While the latter studies included in the meta-analyses all come from the English literature, they could be conducted in various countries, where CE may not be routinely done as an outpatient. Additionally even within the United States, the capture area of each medical center may be different and may require some subjects to be IPs especially if the patient catchment area is wide and mostly rural. Our findings of lower CECR rates in the ICU setting might suggest that the severity of the acute illness may also play important roles in CECR. However, possible confounding factors such as medication use and certain comorbidities such as diabetes, neuropathy, and hypotension were not predictive of incomplete CE in hospitalized patients. This suggests that multiple factors may have a combined effect in the outcomes observed in hospitalized patients. While our hospitalized patients were older than our outpatients and while our ICU patients were older than our GMF patients, age was not an independent factor that explained CECR differences, which have previously been demonstrated[10,14].
Our study has several strengths including a larger IP sample size and a sub-group analysis on patient location and GIB. In our study, no confounding prokinetic agents or bowel preparations were used, and the hospitalization status was exclusively based on the severity of patients’ clinical condition reflective of the current practice patterns in the United States. The major limitation of our study is its retrospective design; therefore, further prospective studies in inpatients are necessary to confirm our observations. Additionally, we took consecutive patients who had CE, and did not do a matched case-control design. This resulted in more subjects in our OP group compared to IP group - due to increasing use of CE in the OP setting. Therefore, it is possible that having less number of IPs may have resulted in potential unidentified confounders. As such, larger studies on IPs are needed to confirm our findings. Another potential limitation of our study is that, the readers of CE examinations were not blinded to the patient’s clinical data. However, the readers were blinded to the hypotheses, making an outcome bias less likely to impact the study results. The lack of data regarding medication use, diabetes, and neuropathy in the outpatient group, lack of a generalized quality measure of the views obtained during CE exams, and the single center design are other limitations of our study.
Several interventions have been undertaken to improve CECR. The results of the studies which investigated the effect of bowel preparation on CECR were not consistent with each other[15-22], but improved image quality has been reported. Previous studies also investigated the role of endoscopic placement of capsule and/or real-time viewing and showed that these approaches can improve CECR[23-26]. Use of 12 h CEs could also be used to improve CECR[27]. Future studies are needed to examine the effects of such interventions to improve CECR among inpatients.
In conclusion, inpatients have a lower than usual CECR, which is more pronounced if admitted to the ICU. While gastric retention is a reason in some patients for the lower CECR, there seems to be generalized dysmotility in inpatients with incomplete CE exams. Other factors embodied in the IP setting such as sedentary status and acuity of the illness could be partially contributing. A large prospective study is needed to further delineate the most important factors that contribute to incomplete exams in the inpatient setting and determine the impact of incomplete CE on patient outcomes.
Capsule endoscopy (CE) is being used widely to evaluate small bowel pathologies. It has applications in Crohn’s disease, celiac disease, Peutz-Jeghers syndrome, familial adenomatous polyposis, hereditary polyposis syndromes, and particularly in the investigation of obscure gastrointestinal bleeding. However, the hospital setting may not be ideal in obtaining optimal results with CE as hospitalized patients tend to be more sedentary and have prolonged gastric and small bowel transit times.
Two previously published studies indirectly examined the effect of patient location on CE completion rates (CECR), but both studies had limitations. Earlier studies also investigated the effect of bowel preparation, endoscopic placement of capsule and/or real-time viewing, and the use of 12 h CEs on CECR.
This is the first study to investigate the effect of patient location on CECR in United States. The authors demonstrated that both gastric transit time (GTT) and small bowel transit time (SBTT) are significantly prolonged in in-patient (IP) CE exams compared to out-patient (OP) exams. The authors also showed that patient location (IP vs OP) and GTT are independent predictors of incomplete CE. The authors further show that the CECR is lowest in the intensive care unit setting. While gastric retention is a reason in some patients for the lower CECR, there seems to be generalized dysmotility in IPs with incomplete CE exams. Other factors such as sedentary status and acuity of the illness could be partially contributing to lower CECR in hospitalized patients.
In addition to patient location other factors such as severity of illness and sedentary status may contribute to incomplete exams in hospitalized patients. CECR can be improved by doing CE studies as outpatient, placing the capsule endoscopically, using real-time viewing or 12 h CEs, and limiting the use of medications which decrease gastrointestinal transit time.
CE is a procedure in which a small camera (capsule) swallowed to investigate the small bowel pathologies; CECR shows the percentage of CE exams that are completed; GTT is the time capsule spends in the stomach during the study; SBTT is the time capsule spends in the small bowel during the study.
In this study, the authors investigated the effect of IP and OP status on SBTT and CECR. This is an interesting and well written paper.
Peer reviewers: Pietro Valdastri, MScEE, PhD, Assistant Professor of Industrial BioEngineering, The BioRobotics Institute, Scuola Superiore Sant’Anna, Polo Sant’Anna Valdera, Viale Rinaldo Piaggio, 34, 56025 Pontedera, Italy; Spiros Ladas, Professor, 1st Department of Internal Medicine-Propaedeutic, Medical School, Athens University, “Laiko” General Hospital, Agiou Thoma 17, 11527 Athens, Greece; Suryakanth Gurudu, MD, Mayo Clinic Arizona, 13400 East shea blvd, Scottsdale, AZ 85259, United States
S- Editor Lv S L- Editor A E- Editor Li JY
| 1. | Westerhof J, Koornstra JJ, Hoedemaker RA, Sluiter WJ, Kleibeuker JH, Weersma RK. Diagnostic yield of small bowel capsule endoscopy depends on the small bowel transit time. World J Gastroenterol. 2012;18:1502-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Herrerías JM, Caunedo A, Rodríguez-Téllez M, Pellicer F, Herrerías JM. Capsule endoscopy in patients with suspected Crohn's disease and negative endoscopy. Endoscopy. 2003;35:564-568. [PubMed] |
| 3. | Soares J, Lopes L, Vilas Boas G, Pinho C. Wireless capsule endoscopy for evaluation of phenotypic expression of small-bowel polyps in patients with Peutz-Jeghers syndrome and in symptomatic first-degree relatives. Endoscopy. 2004;36:1060-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Caspari R, von Falkenhausen M, Krautmacher C, Schild H, Heller J, Sauerbruch T. Comparison of capsule endoscopy and magnetic resonance imaging for the detection of polyps of the small intestine in patients with familial adenomatous polyposis or with Peutz-Jeghers' syndrome. Endoscopy. 2004;36:1054-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Lewis BS, Swain P. Capsule endoscopy in the evaluation of patients with suspected small intestinal bleeding: Results of a pilot study. Gastrointest Endosc. 2002;56:349-353. [PubMed] [DOI] [Full Text] |
| 6. | Triester SL, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:2407-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 419] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 7. | Westerhof J, Weersma RK, Koornstra JJ. Risk factors for incomplete small-bowel capsule endoscopy. Gastrointest Endosc. 2009;69:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Ben-Soussan E, Savoye G, Antonietti M, Ramirez S, Lerebours E, Ducrotté P. Factors that affect gastric passage of video capsule. Gastrointest Endosc. 2005;62:785-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Apostolopoulos P, Liatsos C, Gralnek IM, Kalantzis C, Giannakoulopoulou E, Alexandrakis G, Tsibouris P, Kalafatis E, Kalantzis N. Evaluation of capsule endoscopy in active, mild-to-moderate, overt, obscure GI bleeding. Gastrointest Endosc. 2007;66:1174-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Scaglione G, Russo F, Franco MR, Sarracco P, Pietrini L, Sorrentini I. Age and video capsule endoscopy in obscure gastrointestinal bleeding: a prospective study on hospitalized patients. Dig Dis Sci. 2011;56:1188-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Lee MM, Jacques A, Lam E, Kwok R, Lakzadeh P, Sandhar A, Segal B, Svarta S, Law J, Enns R. Factors associated with incomplete small bowel capsule endoscopy studies. World J Gastroenterol. 2010;16:5329-5333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71:280-286. [PubMed] |
| 13. | Rokkas T, Papaxoinis K, Triantafyllou K, Pistiolas D, Ladas SD. Does purgative preparation influence the diagnostic yield of small bowel video capsule endoscopy?: A meta-analysis. Am J Gastroenterol. 2009;104:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Papadopoulos AA, Triantafyllou K, Kalantzis C, Adamopoulos A, Ladas D, Kalli T, Apostolopoulos P, Kalantzis N, Ladas SD. Effects of ageing on small bowel video-capsule endoscopy examination. Am J Gastroenterol. 2008;103:2474-2480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Endo H, Kondo Y, Inamori M, Ohya TR, Yanagawa T, Asayama M, Hisatomi K, Teratani T, Yoneda M, Nakajima A. Ingesting 500 ml of polyethylene glycol solution during capsule endoscopy improves the image quality and completion rate to the cecum. Dig Dis Sci. 2008;53:3201-3205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Wei W, Ge ZZ, Lu H, Gao YJ, Hu YB, Xiao SD. Purgative bowel cleansing combined with simethicone improves capsule endoscopy imaging. Am J Gastroenterol. 2008;103:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Dai N, Gubler C, Hengstler P, Meyenberger C, Bauerfeind P. Improved capsule endoscopy after bowel preparation. Gastrointest Endosc. 2005;61:28-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Kantianis A, Karagiannis S, Liatsos C, Galanis P, Psilopoulos D, Tenta R, Kalantzis N, Mavrogiannis C. Comparison of two schemes of small bowel preparation for capsule endoscopy with polyethylene glycol: a prospective, randomized single-blind study. Eur J Gastroenterol Hepatol. 2009;21:1140-1144. [PubMed] |
| 19. | Lapalus MG, Ben Soussan E, Saurin JC, Favre O, D'Halluin PN, Coumaros D, Gaudric M, Fumex F, Antonietti M, Gaudin JL. Capsule endoscopy and bowel preparation with oral sodium phosphate: a prospective randomized controlled trial. Gastrointest Endosc. 2008;67:1091-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Kalantzis C, Triantafyllou K, Papadopoulos AA, Alexandrakis G, Rokkas T, Kalantzis N, Ladas SD. Effect of three bowel preparations on video-capsule endoscopy gastric and small-bowel transit time and completeness of the examination. Scand J Gastroenterol. 2007;42:1120-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Niv Y, Niv G, Wiser K, Demarco DC. Capsule endoscopy - comparison of two strategies of bowel preparation. Aliment Pharmacol Ther. 2005;22:957-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Postgate A, Tekkis P, Patterson N, Fitzpatrick A, Bassett P, Fraser C. Are bowel purgatives and prokinetics useful for small-bowel capsule endoscopy? A prospective randomized controlled study. Gastrointest Endosc. 2009;69:1120-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Lai LH, Wong GL, Lau JY, Sung JJ, Leung WK. Initial experience of real-time capsule endoscopy in monitoring progress of the videocapsule through the upper GI tract. Gastrointest Endosc. 2007;66:1211-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Gao YJ, Ge ZZ, Chen HY, Li XB, Dai J, Ye CA, Xiao SD. Endoscopic capsule placement improves the completion rate of small-bowel capsule endoscopy and increases diagnostic yield. Gastrointest Endosc. 2010;72:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Shiotani A, Honda K, Kawakami M, Nishi R, Murao T, Ishii M, Matsumoto H, Kusunoki H, Hata J, Haruma K. Use of an external real-time image viewer coupled with prespecified actions enhanced the complete examinations for capsule endoscopy. J Gastroenterol Hepatol. 2011;26:1270-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Almeida N, Figueiredo P, Lopes S, Freire P, Lérias C, Gouveia H, Correia Leitão M. Capsule endoscopy assisted by traditional upper endoscopy. Rev Esp Enferm Dig. 2008;100:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Pioche M, Gaudin JL, Filoche B, Jacob P, Lamouliatte H, Lapalus MG, Duburque C, Chaput U, Ben Soussan E, Daudet J. Prospective, randomized comparison of two small-bowel capsule endoscopy systems in patients with obscure GI bleeding. Gastrointest Endosc. 2011;73:1181-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |