Published online Sep 21, 2012. doi: 10.3748/wjg.v18.i35.4875
Revised: April 23, 2012
Accepted: April 27, 2012
Published online: September 21, 2012
AIM: To investigate the effect of Tangweian Jianji (TWAJJ) on the biomechanical and morphometrical remodeling of the upper gastrointestinal tract in diabetic rats.
METHODS: Diabetes was induced in 27 rats by injecting streptozotocin (40 mg/kg body weight), the animals were then divided into three groups (n = 9 in each group), i.e., diabetic control (DM); high dose (10 g/kg, T1) and low dose (5 g/kg, T2). Another 10 rats acted as normal controls (Control). TWAJJ was administered by gavage once daily. Blood glucose and serum insulin levels were measured. Circumferential length, wall thickness and opening angle were measured from esophageal, duodenal, jejunal and ileal ring segments. The residual strain was calculated from the morphometric data. Step-wise distension was carried out on esophageal and jejunal segments. The obtained data on the length, diameter and pressure changes were then used to calculate the circumferential and longitudinal stresses and strains. Real-time reverse transcription polymerase chain reaction was used to detect the receptor of advanced glycation end-products (RAGE) mRNA level in jejunal tissues.
RESULTS: At the end of the experiment, the blood glucose level was significantly higher and the serum insulin level was significantly lower in DM, T1 and T2 groups than in the control group (Glucose: 30.23 ± 0.41 mmol/L, 27.48 ± 0.27 mmol/L and 27.84 ± 0.29 mmol/L vs 5.05 ± 0.04 mmol/L, P = 1.65 × 10-16, P = 5.89 × 10-19 and P = 1.63 × 10-18, respectively; Insulin: 1.47 ± 0.32 μg/L, 2.66 ± 0.44 μg/L, 2.03 ± 0.29 μg/L and 4.17 ± 0.54 μg/L, P = 0.0001, P = 0.029 and P = 0.025, respectively). However, these levels did not differ among the DM, T1 and T2 groups. The wet weight per unit length, wall thickness and opening angle of esophageal and intestinal segments in the DM group were significantly higher than those in the control group (from P = 0.009 to P = 0.004). These parameters in the T1 group were significantly lower than those in the DM group (wet weight, duodenum: 0.147 ± 0.003 g/cm vs 0.158 ± 0.001 g/cm, P = 0.047; jejunum, 0.127 ± 0.003 g/cm vs 0.151 ± 0.002 g/cm, P = 0.017; ileum, 0.127 ± 0.004 g/cm vs 0.139 ± 0.003 g/cm, P = 0.046; wall thickness, esophagus: 0.84 ± 0.03 mm vs 0.94 ± 0.02 mm, P = 0.014; duodenum: 1.27 ± 0.06 mm vs 1.39 ± 0.05 mm, P = 0.031; jejunum: 1.19 ± 0.07 mm vs 1.34 ± 0.04 mm, P = 0.047; ileum: 1.09 ± 0.04 mm vs 1.15 ± 0.03 mm, P = 0.049; opening angle, esophagus: 112.2 ± 13.2˚ vs 134.7 ± 14.7˚, P = 0.027; duodenum: 105.9 ± 12.3˚ vs 123.1 ± 13.1˚, P = 0.046; jejunum: 90.1 ± 15.4˚ vs 115.5 ± 13.3˚, P = 0.044; ileum: 112.9 ± 13.4˚ vs 136.1 ± 17.1˚, P = 0.035). In the esophageal and jejunal segments, the inner residual stain was significantly smaller and the outer residual strain was larger in the DM group than in the control group (P = 0.022 and P = 0.035). T1 treatment significantly restored this biomechanical alteration (P = 0.011 and P = 0.019), but T2 treatment did not. Furthermore, the circumferential and longitudinal stiffness of the esophageal and jejunal wall increased in the DM group compared with those in the control group. T1, but not T2 treatment, significantly decreased the circumferential wall stiffness in the jejunal segment (P = 0.012) and longitudinal wall stiffness in the esophageal segment (P = 0.023). The mRNA level of RAGE was significantly decreased in the T1 group compared to that in the DM group (P = 0.0069).
CONCLUSION: TWAJJ (high dose) treatment partly restored the morphometric and biomechanical remodeling of the upper gastrointestinal tract in diabetic rats.
- Citation: Liu GF, Zhao JB, Zhen Z, Sha H, Chen PM, Li M, Zhang JC, Yuan MZ, Gao W, Gregersen H, Tong XL. Effect of Tangweian Jianji on upper gastrointestinal remodeling in streptozotocin-induced diabetic rats. World J Gastroenterol 2012; 18(35): 4875-4884
- URL: https://www.wjgnet.com/1007-9327/full/v18/i35/4875.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i35.4875
Diabetic gastrointestinal disorder (DGID) is a common complication of diabetes. Up to 76% of diabetic patients express gastrointestinal symptoms including dysphagia, early satiety, reflux, constipation, abdominal pain, nausea, vomiting and diarrhea[1,2]. Duration of the disease and poor glycemic control seem to be associated with the severity of gastrointestinal (GI) problems. In general, the pathogenesis of DGID includes high blood glucose level, smooth muscle degeneration, abnormal autonomic neuropathy, gastrointestinal hormone secretion disorders and oxidative stress[3,4].
The GI tract is functionally subjected to dimensional changes. Hence, biomechanical properties such as the stress-strain relationships are of particular importance[5]. These properties are remodeled in response to growth[6], fasting[7] and disease[8]. The biomechanical properties are crucial for GI motor function because peristaltic motion which propels the food through the GI tract is a result of an interaction between the passive and active tissue forces and the hydrodynamic forces in the food bolus. Remodeling of the mechanical properties reflects the changes in the tissue structure that determine a specific motor dysfunction. During the past few years, several studies have demonstrated that experimental diabetes induces GI morphological and biomechanical remodeling[9-13]. Following the development of diabetes, the GI wall becomes thicker and stiffer in a time-dependent manner. Therefore, diabetic gastrointestinal morphological and biomechanical remodeling play an important role in DGID and has become the new perspective of diabetic gastrointestinal pathogenesis[11].
Some studies on diabetic arteries have demonstrated that non-enzymatic glycation of arterial wall tissues is associated with remodeling of the wall[14,15]. We believe that the same applies to the diabetic GI wall. Advanced glycation end-products (AGEs) may contribute to diabetic GI morphological and biomechanical remodeling by two major mechanisms. The first is receptor-independent alteration of the extracellular matrix architecture by non-enzymatic glycation and the formation of protein cross-links. The second mechanism is receptor-dependent and consists of modulation of cellular functions through ligation of specific cell surface receptors[16-18].
Western medical treatment of DGID is mainly focused on symptomatic control with improvement of gastric motility, using promoting agents and supportive measures based on blood glucose control[2]. Although these therapies can partially improve the clinical symptoms, they do not fundamentally reverse the diabetes-induced changes and result in a very high relapse rate. In clinics it was shown that Tangweian Jianji (TWAJJ) significantly improved DGID with a low relapse rate, however, the mechanism involved in this improvement is not fully understood. Therefore, the aim of the present study was to investigate whether TWAJJ treatment can improve the morphometric and biomechanical remodeling of the GI tract in streptozotocin (STZ)-induced diabetic rats. Furthermore, the receptor of advanced glycation end-products (RAGE) mRNA level in the jejunal tissues was also investigated to explore the possible mechanism of TWAJJ in the treatment of DGID.
Forty male SD rats weighing 220-250 g were included in this study. Diabetes was induced in 30 rats by a single tail vein injection of 40 mg/kg body weight of streptozotocin (STZ, Sigma-Aldrich, China). This dose of STZ resulted in a random blood glucose level ≥ 16.7 mmoL/L in 90% of rats 7 d after the injection. The remaining 10% of rats were excluded from this study. Twenty-seven STZ-induced diabetic rats were subdivided into three groups (n = 9 in each group), i.e., diabetic control group (DM); high dose of TWAJJ group (T1) and low dose of TWAJJ group (T2). Another 10 rats of similar age and body weight from the same vendor were used as a non-diabetic control group (Control).
TWAJJ is composed of Citrus aurantium, Codonopsis, fried Atractylodes and wine Rhubarb and was provided by Guang’anmen Hospital, China Academy of Chinese Medical Sciences. The medicine was administered by gavage which passed though the esophagus and reached the stomach lumen. The drug was perfused directly into the stomach once daily from the beginning of the experiment. The dosage was 10 g/kg for T1 and 5 g/kg for T2, respectively. The rats in the DM and control groups were perfused with physiological saline.
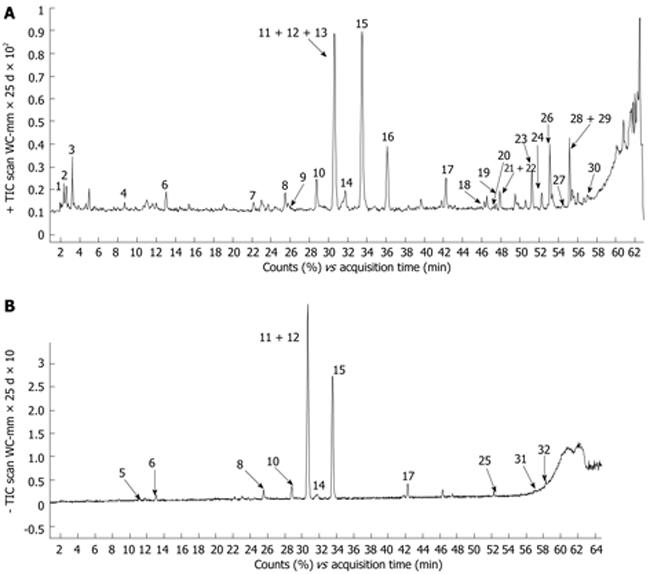
The formulation of TWAJJ was prepared according to the corresponding monograph in the 2005 Edition of Chinese Pharmacopoeia. There were quantitative control limits for the raw herbs (for example, the content of Naringin in Citrus aurantium was not less than 4.0% and Neohesperidin was not less than 3.0%) and for the final drug product. The chemical composition of TWAJJ was determined by using an ultra-fast high performance liquid chromatography-electrospray-ionization-quadrupole time-of-flight mass spectrometry method. An example chromatographic fingerprint is shown in Figure 1. The major compounds in the final dosage form were identified as Naringin, Neohesperidin, Lobetyolin, Atractylenolide and Emodin. These compounds were used as the quality control markers in the final TWAJJ formulation.
Weight and blood glucose levels were measured at 2-wk intervals after initiating the experiment. For blood glucose measurement, one drop of blood was obtained from rat tail vein and blood glucose was measured by a Johnson and Johnson One Touch Ultra Blood Glucose Meter. For insulin measurement, blood was obtained from the abdominal aorta and 0.2 mL serum was separated. The serum insulin was measured by radioimmunoassay (rat insulin radioimmunoassay kit, Linco Company, United States) at the end of the experiment.
The experimental period was 60 d. At the end of the experiment, the rats were fasted overnight and anesthetized with 4% Chloral hydrate (10 mL/kg, ip). Following laparotomy, the whole esophagus and ten centimeters of duodenum, jejunum and ileum were harvested. The duodenum was taken from the descending section, 1 cm down from the pylorus; the jejunum from 5 cm distal to the ligament of Treitz, and the ileum from 5 cm proximal to the ileo-cecal valve. After gently cleaning the lumen of the segments with saline, the length and wet weight were measured.
The esophageal and jejunal segments were divided into three sections, the proximal 1 cm segment was immediately stored at -70 °C for protein and RAGE mRNA detection. The adjacent 1 cm long segment was used for the zero-stress state experiment. The remaining section was used for the distension test. The duodenal and ileal segments were divided into two and used for protein and mRNA detection and the zero-stress state experiment. In this experiment, RAGE mRNA detection was performed only on the jejunal segments.
To obtain data on the zero-stress state, three 1-2 mm wide esophageal and intestinal rings were cut and placed in Krebs solution at room temperature. The composition of Krebs solution (mmol/L) was: NaCl, 118; KCl, 4.7; NaHCO3, 25; NaH2PO4, 1.0; MgCl2, 1.2; and ascorbic acid, 0.11. A photograph was taken of the cross-section of the rings using a Canon camera (Canon, Japan) and was presented as the no-load state. Each ring-shaped segment was then cut radially from the opposite mesentery site and the photographs were taken about 60 min after the radial cutting to allow viscoelastic creep to take place. This is presented as the zero-stress state.
The distal end of the remaining esophageal and jejunal segments was tied with a suture and the proximal end was cannulated with a tube for the distension experiment. After preconditioning of the segments, they were inflated with Krebs solution using a step-wise distension protocol from 0 cm to 20 cm H2O (0 cm, 1 cm, 2 cm, 3 cm, 5 cm, 10 cm, 15 cm and 20 cm H2O) for the esophageal segment and from 0 cm to 10 cm H2O (0 cm, 0.5 cm, 1 cm, 2 cm, 4 cm, 6 cm, 8 cm and 10 cm H2O) for the jejunal segment. The segments conformed to a cylindrical geometry during the distensions. Each pressure lasted for 2 min and then the outer diameter and length of the segments were photographed (Canon, Japan).
The morphometric data were obtained from digitized images of the segments in the zero-stress, no-load and pressurised states. Measurements were undertaken using image analysis software (Sigmascan ver. 4.0, Sigma Corp., San Rafael, CA, United States). The following data were measured from each specimen: the circumferential length (C), the wall thickness (h), the wall area (A), and the opening angle at zero-stress state (α). The subscripts i, o, n, z and p refer to the inner (mucosal) surface, outer (serosal) surface, no-load state, zero-stress state and pressurised condition. The opening angle α was defined as the angle subtended by two radii drawn from the midpoint of the inner wall to the inner tips of two ends of the specimen. Furthermore, the outer diameter (D) and the length (Lp) were measured from the images of the pressurised segments.
The measured data were used for the calculation of biomechanical parameters defined as:
Residual Green’s strain at the mucosal surface:
(1)
Residual Green's strain at the serosal surface:
(2)
The stress and strain of the esophageal and intestinal segments in the pressurised state were determined under the assumptions that the wall was homogenous and the organ shape was cylindrical. Calculations were performed knowing the no-load state dimensions, the outer diameters and lengths of the specimens at varying pressures, and assuming incompressibility of the wall. The longitudinal stretch ratio, ;
the luminal radius, ;
the wall thickness, hp = ro-p - ri-p; the mucosal circumferential length, Ci-p = 2 ×π× ri-p; the serosal circumferential length, Co-p = 2 ×π× ro-p; the mid-wall circumferential length, ;
the circumferential stretch ratio,
(where the middle-wall circumferential length at
zero-stress state, ) were calculated.
Then the Kirchhoff’s stress and Green’s strain in a wall at a given pressure were calculated according to the following equations:
Circumferential Kirchhoff’s stress:
(3)
Longitudinal Kirchhoff’s stress:
(4)
Circumferential mid-wall Green’s strain:
(5)
Longitudinal Green’s strain:
(6)
∆P is the transmural pressure difference. The longitudinal mid-wall stretch ratio was referenced to the no-load state because tissue strips could not be cut to obtain the zero-stress state in the longitudinal direction. However, the longitudinal mid-wall length in rat intestine does not differ between the no-load and zero-stress states[19].
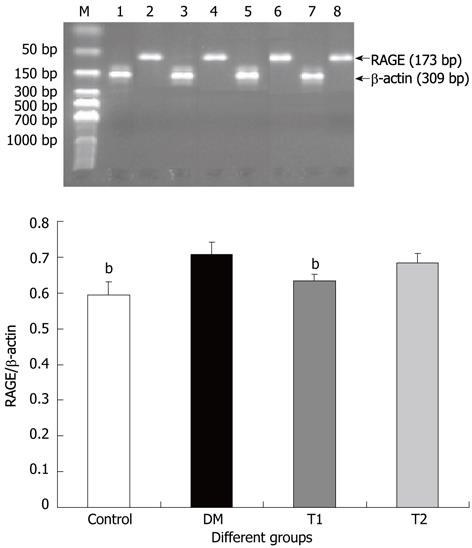
Real-time polymerase chain reaction (RT-PCR) was used to detect RAGE mRNA level in the jejunal tissues. Total RNA was isolated from 100 mg tissue of each specimen using ultra-pure Trizol reagent according to the procedure described by the manufacturer (Invitrogen, United States). RNA concentration was determined when equal amounts of total RNA (3 μg) were used in the reverse transcription with the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, United States). RT-PCR was used to quantify the mRNA expression of RAGE. Amplification was performed using iQ Supermix with the iCycler iQ Real-Time Detection System according to the manufacturer’s instructions (Applied Biosystems). A standard curve was generated for each primer pair and a probe to determine PCR efficiency. Mean threshold cycle (Ct) values for each sample were normalized to β-actin and calibrated to the control group to obtain the threshold cycle difference (ΔΔCt) with 2-ΔΔCt being the fold change relative to the control group. The probes and primer sequences used were as follows: RAGE: Sense: 5’-TCT CAG AAG CCC AAG GAA GAG T-3’; Antisense: 5’-CCT AGG TCT GAA GGC CCT GAG T-3’, amplified cDNA length: 173 bp; β-actin: Sense: 5’-AGA TCC ACA ACG GAT ACA TT-3’; Antisense: 5’-TCC CTC AAG ATT GTC AGC AA-3’, amplified cDNA length: 309 bp.
The data were representative of a normal distribution and accordingly the results were expressed as mean ± SE. The stress-strain curve for each direction was fitted using the exponential function equation S = (S* + β) eα (E-E*) - β.S* and E* are the stress and strain at a physiological reference level[5]. The constants α and β from the above exponential function were used for the statistical evaluation of the stress-strain data. Analysis of variance was used to detect differences in the parameters and groups (Sigmastat 2.0TM). The results were regarded as significant when P < 0.05.
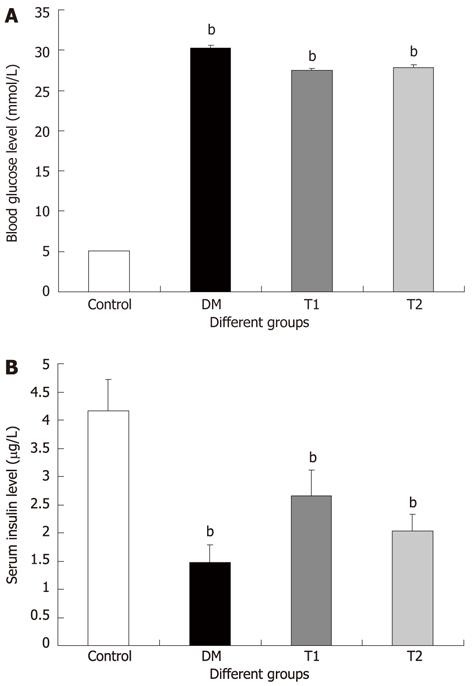
Blood glucose and serum insulin levels at the end of the experiment are shown in Figure 2. The blood glucose level was 4-fold higher in the DM group compared with the control group (Figure 2A, P < 0.01). The serum insulin level was significantly lower in the DM group compared with the control group (Figure 2B, P < 0.01). Compared with the DM group, the blood glucose and insulin levels did not significantly change in the T1 and T2 groups.
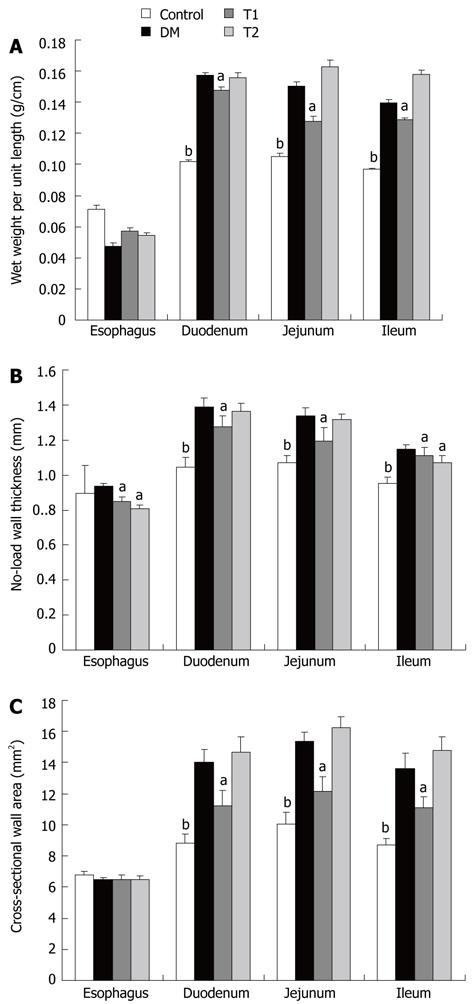
The wet weight per unit length (Figure 3A), no-load wall thickness (Figure 3B) and cross-sectional wall area (Figure 3C) of the esophageal and intestinal segments increased in the DM group compared with the control group (P < 0.01, P < 0.05). After treatment with T1, the wall thickness decreased in all segments (Figure 3B, P < 0.05), whereas the wet weight and wall area decreased in the duodenal, jejunal and ileal segments (Figure 3A, D, P < 0.01, P < 0.05), but not in the esophageal segment (P = 0.12). All the above parameters did not change significantly in the T2 group.
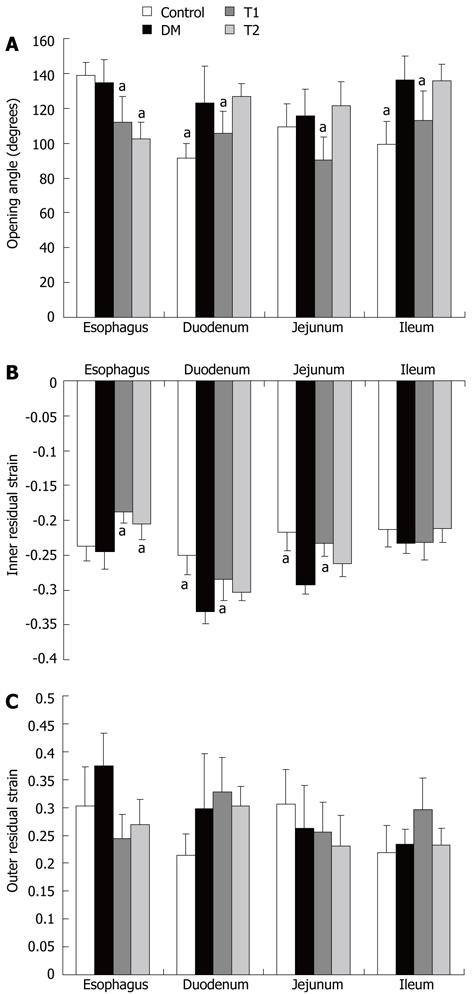
At the end of the experiment, the opening angle of all three intestinal segments was significantly increased in the DM group compared with the control group (Figure 4A, P < 0.05). However, the opening angle in the esophageal segment did not change. Treatment with high dose TWAJJ decreased the opening angle in all segments studied (Figure 4A, P < 0.05). Interestingly, the opening angle of the esophageal segment was also decreased in the T2 group (P < 0.05).
A similar pattern to that found in the opening angle was found in the inner residual strain for all segments and groups, with the exception of the ileal segment (Figure 4B), where no difference was found between the different groups (P > 0.05). The outer residual strain did not differ between the groups and segments (Figure 4C).
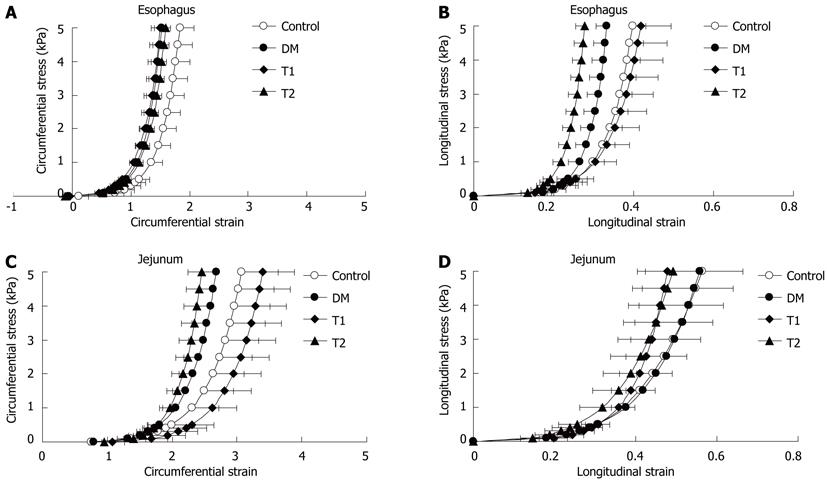
At the end of the experiment, the stress-strain analysis showed that the circumferential stress-strain curves of esophageal and jejunal segments (Figure 5A, C), and the longitudinal stress-strain curve of the esophageal segment (Figure 5B) in the DM group shifted to the left compared with those in the control group, indicating that the diabetic esophageal and intestinal wall became stiffer. Calculation of the mechanical constants demonstrated a difference between the DM group and the control group (P < 0.05, Table 1). However, the longitudinal stress-strain distribution of the jejunal segment did not differ between the DM and control groups (P > 0.05, Figure 5D). High dose TWAJJ (T1) decreased the stiffness of the esophageal wall in the longitudinal direction (Figure 5B and Table 1, P < 0.05) and the intestinal wall in the circumferential direction (Figure 5C and Table 1, P < 0.05). Low dose TWAJJ did not improve the stiffening of the esophageal and jejunal wall caused by diabetes (P > 0.05, Figure 5 and Table 1).
| Circumferential direction | Longitudinal direction | |||
| Esophagus | Jejunum | Esophagus | Jejunum | |
| Control (n = 10) | 3.56 ± 0.35 | 2.65 ± 0.37 | 18.33 ± 2.36 | 13.51 ± 3.66 |
| DM (n = 9) | 4.59 ± 0.79a | 3.81 ± 0.31a | 27.62 ± 3.93a | 14.79 ± 2.26 |
| T1 (n = 9) | 3.77 ± 0.39 | 2.55 ± 0.51c | 19.43 ± 4.13c | 14.67 ± 1.56 |
| T2 (n = 9) | 3.84 ± 0.44 | 4.02 ± 0.71a | 27.82 ± 1.74a | 15.41 ± 4.36 |
RAGE mRNA level of the jejunal segment increased in the DM group compared to the control group (Figure 6, P < 0.01). T1 treatment reduced the RAGE mRNA level (Figure 6, P < 0.01). We did not observe a significant difference between the T2 group and the DM group (Figure 6, P > 0.05).
Diabetes mellitus is a pandemic disease. According to WHO´s statistical report; 370 million diabetic patients are expected by 2030. The serious impact caused by diabetes and its complications does not only affect patients but is also a heavy financial burden to the health care system and on families[20]. DGID is a common complication of diabetes. A previous study showed that prominent proliferations of the GI wall, especially the small intestine and esophagus mucosae, occur in experimental diabetes[21]. Other studies demonstrated morphological and biomechanical remodeling of the upper GI tract in diabetes[9-10,22], which may be caused by over-expression of AGE and RAGE in the diabetic GI tract. Furthermore, a linear association between the glucose level and morphometric and biomechanical remodeling was observed[23].
In the view of traditional Chinese medicine, DGID is in the category of fullness, epigastric pain, vomiting, diarrhea, and constipation concurrent with diabetes. The major syndrome of DGID includes spleen deficiency and qi stagnation. Therefore, the principal treatment is to strengthen the spleen and remove stagnation. TWAJJ is a practical Chinese medicinal compound for DGID[24-26] and is composed of several herbs. Atractylodes macrocephala can strengthen the spleen, harmonize the stomach, regulate GI motility and protect the gastric mucosa[27]. Citrus aurantium and wine rhubarb move qi and accumulation. Pinellia downbears counterflow and inhibits vomiting. Ginseng tones qi and has a beneficial affect on body fluid. Citrus aurantium has a non-competitive antagonist effect on acetylcholine and histamine in intestinal smooth muscle contraction, possibly by influencing Ca2+ channels. Its total alkaloid level can prevent vomiting and can promote GI tract movement in rats[28-30]. The active ingredients of ginseng ginsenosides can reduce blood sugar by stimulating insulin secretion and anti-apoptosis of pancreatic β-cells[31,32]. Furthermore, the results of a retrospective clinical study on TWAJJ treatment in DGID patients have been reported[24,25]. After treatment with TWAJJ, symptoms such as fullness, eructation, nausea (vomiting) and constipation were significantly improved. Although the fasting blood glucose tended to decrease after treatment, this decrease was not statistically significant. However, clarification of the clinical validity of TWAJJ in randomized controlled clinical trials is necessary. In this study, we also found that TWAJJ had a tendency to decrease blood glucose and increase serum insulin, however, statistical significance was not reached. Therefore, we consider that the functional mechanism of TWAJJ in DGID is through other pathways rather than through blood glucose regulation.
The esophagus and small intestine are tubular organs. One important function is transportation of food by wall movement; therefore the biomechanical properties of the wall are important for this function[8]. The biomechanical properties of the esophagus and small intestine depend on their structure and can be evaluated by the opening angle, residual stress and strain, and stress-strain relationship[11]. Previous studies have demonstrated that the esophageal wall[12,33,34] and intestinal wall[9-11] were remodeled during the development of diabetes. Therefore, it is important to determine whether TWAJJ can improve DGID through a pathway involved in changing the biomechanical properties of the GI tract.
The present study confirmed previous findings that morphometric and biomechanical remodeling of the esophageal and intestinal wall occur in STZ-induced diabetic rats. Although the treatment with TWAJJ did not significantly change the blood glucose and serum insulin levels, high dose TWAJJ, to a large extent, improved the morphometric and biomechanical remodeling caused by diabetes. The improvement in morphometric remodeling was expressed as reduced wall thickness and area. Improvements in biomechanical remodeling were expressed as reduced opening angle, reduced absolute value of residual strain and decreased wall stiffness. The present study indicated that the effect of TWAJJ on improvements in DGID in the clinic may be partially associated with improvements in biomechanical remodeling of the GI wall. Due to the complex geometry of the stomach[22,35], we did not focus on the biomechanical parameters of the gastric wall in the present study. Future studies may focus on the effect of TWAJJ on the stomach due to the importance of stomach function in diabetic patients.
The alterations in residual strain and wall stiffness in diabetes will change the tension and stress distribution in the location of mechanosensitive afferents in the GI tract[36,37]. Therefore, biomechanical remodeling of the diabetic GI wall indirectly affects GI motor function. High dose TWAJJ partially restored the changes in biomechanical properties caused by diabetes. This may be associated with improved motor and sensory function of the diabetic GI tract.
AGEs is a heterogeneous group of macromolecules formed by the non-enzymatic glycation of proteins, lipids and nucleic acids[38]. Endogenous AGEs are generated at higher rates in diabetes due to abnormal glucose metabolism. Chronic high blood glucose can cause excessive glycoprotein accumulation in the body including the GI tract causing GI wall stiffening[8]. There is growing evidence to show that AGEs and RAGE are implicated in various disorders. AGE levels in serum and tissues are associated with chronic complications of DM including DGID. Furthermore, RAGE is involved in signal transduction of AGEs in a variety of cells[39]. Our previous studies showed that AGE and RAGE were over-expressed in diabetic GI tissues[40]. In the present study, we found that the RAGE mRNA level in the jejunal segment increased in diabetic rats compared with normal rats. Following high dose TWAJJ administration, this alternation was reversed to the normal state. Therefore, the observed improvement in the mechanical factors due to TWAJJ treatment in DGID may be through the inhibition of AGEs accumulation. However, in future studies we need to clarify this mechanism by immunohistochemistry of RAGE to demonstrate whether the change in mRNA expression actually translates to protein expression. Furthermore, it is also important to study whether specific knockdown of RAGE in the GI mucosa can improve biomechanical remodeling of the GI tract in diabetes and reverse DGID.
In conclusion, high dose TWAJJ treatment improves biomechanical and morphometric remodeling of the diabetic esophageal and intestinal wall. The mechanism may, at least partly, be explained by decreased RAGE mRNA levels in STZ-induced diabetic rats. Therefore, it seems feasible to develop Chinese herbs, such as TWAJJ, to improve the morphometric and biomechanical remodeling caused by diabetes. This may have an impact on GI dysfunction in diabetes and be used in clinical practice.
Diabetic gastrointestinal disorder (DGID) is a common complication of diabetes. However, neither the pathophysiology nor the pathogenesis of patients’ gastrointestinal (GI) symptoms associated with diabetes are well understood. Furthermore, many patients with DGID do not receive appropriate treatment. Although Western medicine can partially improve the clinical symptoms, it does not fundamentally reverse the diabetes-induced changes and results in a very high relapse rate. In the clinic, it was shown that Tangweian Jianji (TWAJJ) significantly improved DGID with a low relapse rate, however, the mechanism involved in this improvement is not fully understood.
The GI tract is functionally subjected to dimensional changes. Hence, biomechanical properties such as the stress-strain relationship are of particular importance. Several studies have demonstrated that diabetes induces GI morphological and biomechanical remodeling. The non-enzymatic glycation of the intestinal wall in diabetes is associated with remodeling. Therefore, the present study investigated whether TWAJJ treatment can improve morphometric and biomechanical remodeling of the GI tract in streptozotocin (STZ)-induced diabetic rats. Furthermore, the receptor of advanced glycation end-products (RAGE) mRNA level in the jejunal tissues was also investigated to explore the possible mechanism of TWAJJ in the treatment of DGID.
Compared with previous studies, this study is the first to use biomechanical test methods combined with RAGE detection to investigate the mechanism of the effect of the Chinese medicine, TWAJJ, on diabetic GI dysfunction. We showed that high dose TWAJJ improves the biomechanical and morphometric remodeling of the diabetic esophageal and intestinal walls. The mechanism may, at least partly, be related to the decreased RAGE mRNA levels in STZ-induced diabetic rats. As knowledge of GI biomechanics is new and was only recently developed and the non-enzymatic glycation of the GI tract is closely related to its biomechanical properties, determination of biomechanical properties and expression of advanced glycation end products and their receptor (AGE/RAGE) may be useful tools to investigate the mechanism of TWAJJ in DGID.
The findings of this study suggest that it seems feasible to develop Chinese herbs, such as TWAJJ, to improve the morphometric and biomechanical remodeling caused by diabetes. This may have an impact on GI dysfunction in diabetes and be used in clinical practice.
DGID is a common complication of diabetes related to the GI tract. Up to 76% of diabetic patients express GI symptoms including dysphagia, early satiety, reflux, constipation, abdominal pain, nausea, vomiting and diarrhea; GI biomechanical properties are used to define the GI wall deformation (strain) and force (stress), which can be described by the opening angle, residual stress and strain, stress-strain relationship, stress relaxation and creep; TWAJJ is a practical Chinese medicinal compound for diabetic gastrointestinal dysfunction and is composed of several herbs including Citrus aurantium, Codonopsis, fried Atractylodes and wine Rhubarb.
This is an interesting article. DGID is clearly a big topic especially in developed countries, thus this topic is interesting. Further, it was also fascinating to see that high dose TWAJJ was able to “treat” DGID without treating hyperglycemia. The manuscript is well written, the experiments are well planned, methods are adequately described and suitable to achieve the goals and results are appropriately described.
Peer reviewers: Dr. Kazuaki Takabe, MD, PhD, Surgical Oncology, Virginia Commonwealth University, West Hospital 7-402, 1200 East Broad Street, Richmond, VA 23298-0011, United States; Shivananda Nayak, PhD, Biochemistry Unit, Department of Preclinical Sciences, Faculty of Medical Sciences, The University of The West Indies, Building 36, Mount Hope EWMSC, Trinidad and Tobago; Islam Khan, Professor, Department of Biochemistry, Kuwait University, Jabrya 13110, Kuwait
S- Editor Gou SX L- Editor Webster JR E- Editor Zhang DN
| 1. | Icks A, Haastert B, Rathmann W, Wareham N. Prevalence of gastrointestinal symptoms in patients with type 2 diabetes: a population-based study. Arch Intern Med. 2002;162:1067-1069; author reply 1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Shakil A, Church RJ, Rao SS. Gastrointestinal complications of diabetes. Am Fam Physician. 2008;77:1697-1702. [PubMed] |
| 3. | Kashyap P, Farrugia G. Oxidative stress: key player in gastrointestinal complications of diabetes. Neurogastroenterol Motil. 2011;23:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Chandrasekharan B, Anitha M, Blatt R, Shahnavaz N, Kooby D, Staley C, Mwangi S, Jones DP, Sitaraman SV, Srinivasan S. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil. 2011;23:131-18, e26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 5. | Gregersen H. Biomechanics of the Gastrointestinal Tract. London: Springer-Verlag 2002; 1-268. |
| 6. | Lu X, Zhao J, Gregersen H. Small intestinal morphometric and biomechanical changes during physiological growth in rats. J Biomech. 2005;38:417-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Dou Y, Gregersen S, Zhao J, Zhuang F, Gregersen H. Morphometric and biomechanical intestinal remodeling induced by fasting in rats. Dig Dis Sci. 2002;47:1158-1168. [PubMed] |
| 8. | Zhao J, Liao D, Gregersen H. Biomechanics of the Gastrointestinal Tract in Health and Disease. Biomechanics: Principles, Trends and Applications. New York: Nova 2010; 163-206. |
| 9. | Zhao J, Yang J, Gregersen H. Biomechanical and morphometric intestinal remodelling during experimental diabetes in rats. Diabetologia. 2003;46:1688-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Zhao J, Liao D, Yang J, Gregersen H. Viscoelastic behavior of small intestine in streptozotocin-induced diabetic rats. Dig Dis Sci. 2003;48:2271-2277. [PubMed] |
| 11. | Zhao J, Frøkjaer JB, Drewes AM, Ejskjaer N. Upper gastrointestinal sensory-motor dysfunction in diabetes mellitus. World J Gastroenterol. 2006;12:2846-2857. [PubMed] |
| 12. | Zhao J, Liao D, Gregersen H. Biomechanical and histomorphometric esophageal remodeling in type 2 diabetic GK rats. J Diabetes Complications. 2007;21:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Zhao J, Nakaguchi T, Gregersen H. Biomechanical and histomorphometric colon remodelling in STZ-induced diabetic rats. Dig Dis Sci. 2009;54:1636-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Sims TJ, Rasmussen LM, Oxlund H, Bailey AJ. The role of glycation cross-links in diabetic vascular stiffening. Diabetologia. 1996;39:946-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 179] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Wolffenbuttel BH, Boulanger CM, Crijns FR, Huijberts MS, Poitevin P, Swennen GN, Vasan S, Egan JJ, Ulrich P, Cerami A. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc Natl Acad Sci USA. 1998;95:4630-4634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 310] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl). 2005;83:876-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 863] [Cited by in RCA: 954] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 17. | Stern DM, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev. 2002;1:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 184] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Wautier JL, Zoukourian C, Chappey O, Wautier MP, Guillausseau PJ, Cao R, Hori O, Stern D, Schmidt AM. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy. Soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J Clin Invest. 1996;97:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 373] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 19. | Dou Y, Fan Y, Zhao J, Gregersen H. Longitudinal residual strain and stress-strain relationship in rat small intestine. Biomed Eng Online. 2006;5:37. [PubMed] |
| 20. | Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2186] [Cited by in RCA: 2307] [Article Influence: 153.8] [Reference Citation Analysis (2)] |
| 21. | Jørgensen CS, Ahrensberg JM, Gregersen H, Flyvberg A. Tension-strain relations and morphometry of rat small intestine in experimental diabetes. Dig Dis Sci. 2001;46:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 62] [Reference Citation Analysis (1)] |
| 22. | Liao D, Zhao J, Gregersen H. Three-dimensional geometry analysis of the stomach in type II diabetic GK rats. Diabetes Res Clin Pract. 2006;71:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Sha H, Zhao JB, Zhang ZY, Zhou SP, Tong XL, Zhuang FY, Gregersen H. Effect of Kaiyu Qingwei Jianji on the morphometry and residual strain distribution of small intestine in experimental diabetic rats. World J Gastroenterol. 2006;12:7149-7154. [PubMed] |
| 24. | Liu WK, Dong L, Su H, Tong XL. Examples of professor Tong-xiao Lin in the treatment of diabetes gastrointestinal disorders. Sichuan Zhong Yi. 2010;28:4-7. |
| 25. | Zhou Q, Liu C, Li XY. Cases of professor Tong-xiao Lin in diabetic gastroparesis. Beijing Zhong Yi Yao. 2010;29:137-138. |
| 26. | Wang J, Tong XL. Diagnosis and treatment with Chinese medicine on diabetic gastroparesis. In: Tong XL, Liu TH, editors. Proceedings of the the 9th National Academic Conference of Chinese medicine and diabetes; 2006; 344-346. |
| 27. | Wang FX. Research progression of Atractylodes’ pharmacological and clinical applications in treatment of gastrointestinal diseases. Zhongguo Min Kang Yi Xue. 2008;20:1646. |
| 28. | Huang G, Sheng SD, Zhang QL, Cong H, Li Z. Zhishi on small intestinal smooth muscle function. Zhongguo Yi Ke Da Xue Xue Bao. 1993;1:49-52. |
| 29. | Liu Y, Ye F, Wang R, Qiu GQ, Cai Y, Sun Y. Effects of Zhishi (Fructus Aurantii Immaturus) and its medicinal serum on extracorporeal colonic muscle strips in rats with slow transit constipation. Beijing Zhong Yi Yao Da Xue Xue Bao. 2010;33:402-405. |
| 30. | Wu H, Cai BC, Rong GX, Ye DJ. The effect of Pinellia processed by ginger juice on gastric and intestinal function of animals. Zhongguo Zhong Yao Zazhi. 1994;19:535-537, 574. |
| 31. | Cho WC, Chung WS, Lee SK, Leung AW, Cheng CH, Yue KK. Ginsenoside Re of Panax ginseng possesses significant antioxidant and antihyperlipidemic efficacies in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2006;550:173-179. [PubMed] |
| 32. | Zhang Z, Li X, Lv W, Yang Y, Gao H, Yang J, Shen Y, Ning G. Ginsenoside Re reduces insulin resistance through inhibition of c-Jun NH2-terminal kinase and nuclear factor-kappaB. Mol Endocrinol. 2008;22:186-195. [PubMed] |
| 33. | Yang J, Zhao J, Zeng Y, Gregersen H. Biomechanical properties of the rat oesophagus in experimental type-1 diabetes. Neurogastroenterol Motil. 2004;16:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Yang J, Zhao J, Liao D, Gregersen H. Biomechanical properties of the layered oesophagus and its remodelling in experimental type-1 diabetes. J Biomech. 2006;39:894-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Zhao J, Liao D, Gregersen H. Tension and stress in the rat and rabbit stomach are location- and direction-dependent. Neurogastroenterol Motil. 2005;17:388-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Raab M, Neuhuber WL. Number and distribution of intraganglionic laminar endings in the mouse esophagus as demonstrated with two different immunohistochemical markers. J Histochem Cytochem. 2005;53:1023-1031. [PubMed] |
| 37. | Swithers SE, Baronowsky E, Powley TL. Vagal intraganglionic laminar endings and intramuscular arrays mature at different rates in pre-weanling rat stomach. Auton Neurosci. 2002;102:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Semba RD, Nicklett EJ, Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J Gerontol A Biol Sci Med Sci. 2010;65:963-975. [PubMed] |
| 39. | Yamagishi S, Matsui T. Soluble form of a receptor for advanced glycation end products (sRAGE) as a biomarker. Front Biosci (. Elite Ed). 2010;2:1184-1195. [PubMed] |
| 40. | Chen P, Zhao J, Gregersen H. Up-regulated expression of advanced glycation end-products and their receptor in the small intestine and colon of diabetic rats. Dig Dis Sci. 2012;57:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |