INTRODUCTION
Amoebiasis is a parasitic infection caused by the protozoan Entamoeba histolytica, which principally affects the colon and liver. A majority of infected patients remain asymptomatic; however, the development of acute fulminant amoebic colitis is often fatal, with a very high mortality rate of 70%-100%[1,2] because of serious complications like colonic perforation[3,4] and necrotizing colitis[5]. On the other hand, the formation of a perianal ulcer due to acute fulminant amoebic colitis is very rare[6]. We describe the case of a 29-year-old homosexual man with acquired immunodeficiency syndrome (AIDS), who developed a perianal ulcer as a result of acute fulminant amoebic colitis.
CASE REPORT
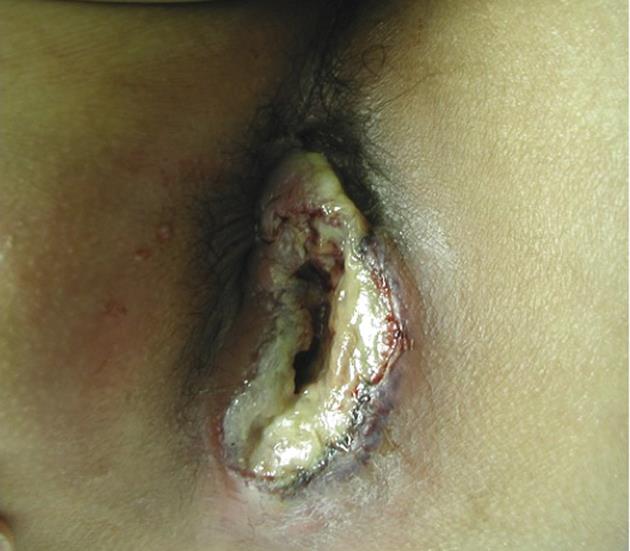
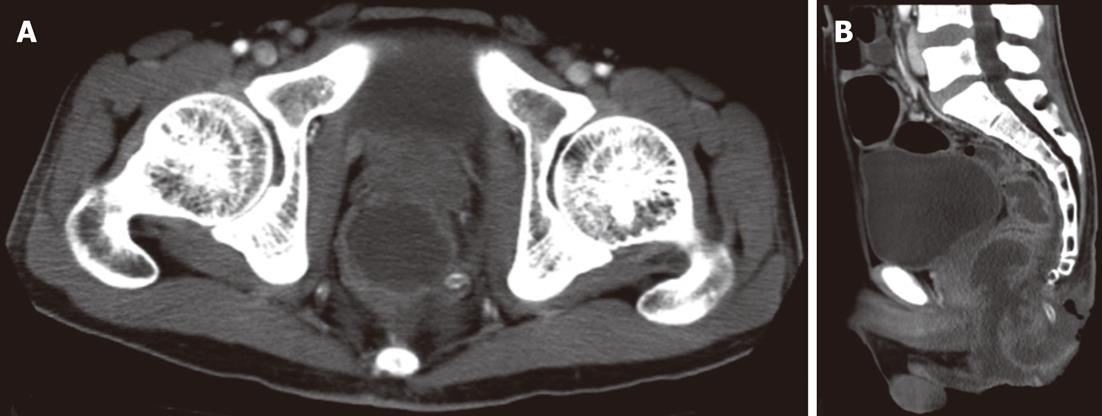
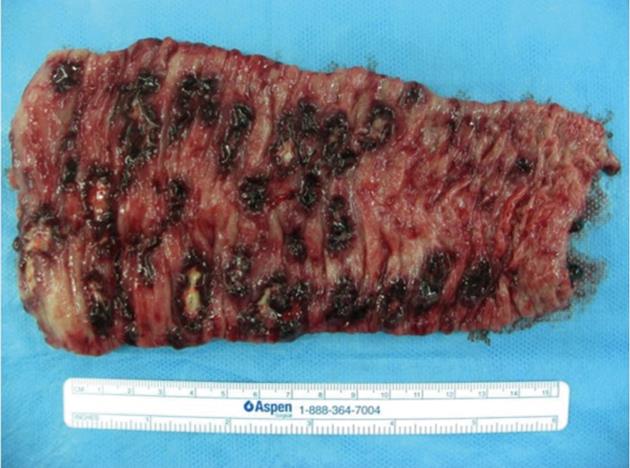
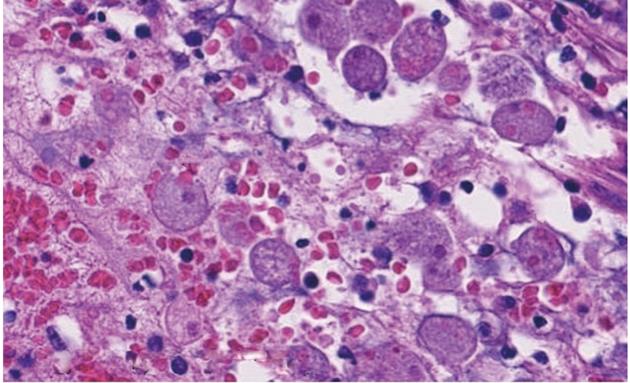
A 29-year-old Japanese homosexual man was admitted to our hospital with a complaint of spiking pyrexia and anal pain with a perianal abscess, which was refractory to antibiotics therapy administered at another hospital. On admission, we observed a giant ulcer in the perianal region (Figure 1). A diagnosis of AIDS was made on the basis of serum human immunodeficiency virus (HIV) antigen positivity and an increased HIV viral load (90 000 RNA copies/mL); his condition was further complicated by pneumocystis carinii infection. Moreover, cytomegalovirus (CMV) colitis was suspected from the occurrence of severe bloody diarrhea and the presence of serum antigenemia. Ganciclovir therapy was initiated without performing a colonoscopy because of patient refusal. However, he developed infected necrosis of the skin around the anus and over the perianal ulcer during therapy. Laboratory investigations revealed the following: white blood cell count, 7500/mm3; hemoglobin, 9.7 g/dL; platelets, 545 000/mm3; and serum C-reactive protein, 13.1 mg/dL. Abdominal computed tomography (CT) revealed an abscess formed due to necrosis and extending from the perianal region to the lower rectum (Figure 2). We therefore ruled out chances of anus preservation and performed only end-sigmoidostomy and necrotomy of the skin tissue from around the anus to the lower rectum. This was done to avoid excessive surgical invasion because of a significant decrease in the CD4-positive lymphocyte count (52 cells/mm3). Macroscopic findings of the resected colon revealed multiple deep ulcerations covered with yellow necrotic plaques (Figure 3). Histopathological examination of the surgical specimen revealed trophozoite amoebae that had ingested red blood cells in the resected colon and necrotic tissue, leading to in a final diagnosis of acute fulminant amoebic colitis (Figure 4). Furthermore, the serological test for Entamoeba histolytica was also positive. Typical cytomegalic intranuclear inclusion bodies were not observed in the endothelial cells of the resected specimen. Soon after receiving the pathological diagnosis of amoebiasis, metronidazole therapy was initiated. The patient’s postoperative course was uneventful because of intensive care. Antiretroviral therapy was also prescribed. The HIV viral load gradually decreased to below the detection limit of the HIV-RNA assay, and the CD4-positive lymphocyte count increased to 112 cells/mm3. On the basis of these findings, proctectomy of the residual rectum was performed 11 mo later.
Figure 1 A giant ulcer covered with necrotic tissue is identified in the perianal region.
Figure 2 Abdominal computed tomography revealing an abscess formation of the perianal region.
A: The axial view shows an abscess of the perianal region due to necrosis; B: The sagittal view shows an abscess formation extending from the perianal region to the lower rectum.
Figure 3 Macroscopic findings of the resected colon following end-sigmoidostomy show numerous deep ulcerations covered with yellow necrotic plaques.
Figure 4 Histopathological findings of the surgical specimen show trophozoite amoebae that have ingested red blood cells (arrows) (HE staining).
DISCUSSION
Amoebiasis, which is caused by the protozoan Entamoeba histolytica (E. histolytica), is the second most frequent parasitosis worldwide, particularly in tropical and subtropical areas[7]. Recently, the risk of amoebiasis has also increased in developed countries, particularly among travelers returning from endemic areas and homosexual men, including those with HIV infection[8,9]. Interestingly, 13.4%-20.4% Japanese homosexual men test positive for amoebic antibodies[10]. However, acute fulminant amoebic colitis is uncommon, and Adams et al[11] reported that acute fulminant colitis developed in only 3% of 3013 patients with amoebic colitis in South Africa. The outcome of this condition is very poor, with a high mortality rate of 70%-100%[1,2] because of serious complications like colonic perforation[3,4], necrotizing colitis[5], and toxic megacolon[12]. Perianal ulcers caused by acute fulminant amoebic colitis, such as that observed in the present case, are very rare and occur in only 0.04% patients[11]. Especially in homosexual men, the pathogen can easily invade the anal epithelium, thus resulting in the formation of a perianal ulcer.
Amoebic colitis can be diagnosed by the identification of E. histolytica in the stool or in colonic tissue removed by colonoscopy. Testing for serum antibodies against E. histolytica is also useful, with a high sensitivity of 85%[13]. In the present case, we could not perform colonoscopic examination because the patient did not consent to this procedure. He was initially treated by ganciclovir therapy after CMV colitis was suspected because of the occurrence of severe bloody diarrhea and the presence of serum antigenemia; however, he responded poorly to this treatment. Because the final diagnosis of amoebic colitis was only made after surgery, metronidazole therapy, which is the standard treatment for amoebic colitis, was delayed. It is very difficult to distinguish these two diseases by nonspecific clinical manifestations, such as diarrhea, abdominal pain, and gastrointestinal bleeding; moreover, a few cases of mixed infection with E. histolytica and CMV have been previously reported[14-16]. However, it should be considered that amoebic colitis is one of the causes of perianal ulcers in homosexual men. The primary treatment of amoebic colitis is metronidazole; nevertheless, surgical treatment is necessary in cases of perforative peritonitis[1,2], necrotizing colitis[5], and toxic megacolon[12] despite high mortality and poor prognosis. In the present case, the perianal ulcer may have developed as a result of acute fulminant amoebic proctitis, which may be attributed to homosexuality. Several reports have advocated less invasive surgical procedures, such as diverting colostomy or ileostomy and simple closure of the perforation, because of the high mortality rate following bowel resection surgery[17,18]. On the other hand, resection to resolve the septic focus and stoma creation should be considered one of the treatment options, depending on the general status of the patient[19]. We performed only end-sigmoidostomy and necrotomy of the skin tissue from around the anus to the lower rectum to avoid excessive surgical invasion because of a significant decrease in the CD4-positive lymphocyte count due to AIDS. Proctectomy of the residual rectum was performed after the recovery of his immunocompetence 11 mo later. The patient’s postoperative course following staged surgical treatment was favorable, and he has survived for 2 years since the first surgery.
Because of the rarity of this disease, the effectiveness of invasive surgical procedures for the treatment of acute fulminant amoebic colitis needs further investigation.
In summary, we described an extremely rare case of acute fulminant amoebic colitis that resulted in the development of a perianal ulcer in a homosexual man with AIDS. He was successfully treated by staged surgical treatment. Amoebiasis is the second most frequent parasitosis worldwide, but almost all of these infections are asymptomatic. However, acute fulminant amoebic colitis has a poor prognosis in immunocompromised patients, particularly those with AIDS. Early diagnosis, opportune surgical procedures, and intensive care for sepsis appear to decrease mortality. Therefore, amoebic colitis must be kept in mind when perianal ulcers manifest in immunocompromised patients; moreover, minimally invasive, staged surgical treatment should be considered in these patients.