Published online Sep 14, 2012. doi: 10.3748/wjg.v18.i34.4693
Revised: March 21, 2012
Accepted: April 13, 2012
Published online: September 14, 2012
AIM: To examine whether the ob/ob mouse model of obesity is accompanied by enteric nervous system abnormalities such as altered motility.
METHODS: The study examined the distribution of the P2X2 receptor (P2X2R) in myenteric neurons of female ob/ob mice. Specifically, we used immunohistochemistry to analyze the co-expression of the P2X2R with neuronal nitric oxide synthase (nNOS), choline acetyltransferase (ChAT), and calretinin (CalR) in neurons of the small intestine myenteric plexus in ob/ob and control female mice. In these sections, we used scanning confocal microscopy to analyze the co-localization of these markers as well as the neuronal density (cm2) and area profile (μm²) of P2X2R-positive neurons. In addition, enteric neurons were labeled using the nicotinamide adenine dinucleotide (NADH) diaphorase method and analyzed with light microscopy as an alternate means by which to analyze neuronal density and area.
RESULTS: In the present study, we observed a 29.6% increase in the body weight of the ob/ob animals (OG) compared to the control group (CG). In addition, the average small intestine area was increased by approximately 29.6% in the OG compared to the CG. Immunoreactivity (IR) for the P2X2R, nNOS, ChAT and CalR was detectable in the myenteric plexus, as well as in the smooth muscle, in both groups. This IR appeared to be mainly cytoplasmic and was also associated with the cell membrane of the myenteric plexus neurons, where it outlined the neuronal cell bodies and their processes. P2X2R-IR was observed to co-localize 100% with that for nNOS, ChAT and CalR in neurons of both groups. In the ob/ob group, however, we observed that the neuronal density (neuron/cm2) of P2X2R-IR cells was increased by 62% compared to CG, while that of NOS-IR and ChAT-IR neurons was reduced by 49% and 57%, respectively, compared to control mice. The neuronal density of CalR-IR neurons was not different between the groups. Morphometric studies further demonstrated that the cell body profile area (μm²) of nNOS-IR, ChAT-IR and CalR-IR neurons was increased by 34%, 20% and 55%, respectively, in the OG compared to controls. Staining for NADH diaphorase activity is widely used to detect alterations in the enteric nervous system; however, our qualitative examination of NADH-diaphorase positive neurons in the myenteric ganglia revealed an overall similarity between the two groups.
CONCLUSION: We demonstrate increases in P2X2R expression and alterations in nNOS, ChAT and CalR IR in ileal myenteric neurons of female ob/ob mice compared to wild-type controls.
- Citation: Mizuno MS, Crisma AR, Borelli P, Castelucci P. Expression of the P2X2 receptor in different classes of ileum myenteric neurons in the female obese ob/ob mouse. World J Gastroenterol 2012; 18(34): 4693-4703
- URL: https://www.wjgnet.com/1007-9327/full/v18/i34/4693.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i34.4693
Obesity is a chronic heterogeneous disorder characterized by abnormal or excessive fat accumulation that presents a risk to health[1]. The leptin-deficient ob/ob mouse exhibits both obesity and peripheral diabetic neuropathy, traits characteristic of human patients, and thus represents a valuable animal model of obesity and type 2 diabetes-like pathology[2].
Obesity is associated with an increased risk of type 2 diabetes and is often accompanied by complications of the gastrointestinal tract such as gastroparesis, constipation, diarrhea, and fecal incontinence[1]. Disruptions in the function of the gastrointestinal tract have been associated with alterations in the enteric nervous system[3]. Enteric neurons comprise both the myenteric and submucosal plexuses, which control the motility of the intestine, the transport of fluids from the intestinal mucosa, and local blood flow[3]. The groups of neuronal cell bodies (ganglia) in these plexuses are interconnected by nerve fiber bundles[3]. The myenteric and submucosal plexuses also regulate gastrointestinal secretions important for the digestion of food particles, intestinal lubrication, nutrient uptake, regulation of pH, and the concentration of solutes and elimination of waste products[3]. Studies of chemical coding in neurons of the mouse enteric nervous system have been particularly important to our understanding of the function of enteric neurons[4,5]. Defects in enteric nervous system function have been reported in ob/ob mice, including alterations in intestinal motility[6] and in the enteric neurons[7,8]. Down-regulations in intestinal motility have been reported in obese humans[1]. Thus, the mouse is a valuable animal model for the study of obesity-related pathology in the enteric nervous system.
Adenosine 5’-triphosphate (ATP) has been well-established as a neurotransmitter and a ligand of the P2X receptor family, which is made up of seven known receptor subunits (PX1-7)[9]. These receptors play an important role in synaptic transmission within the neural pathways that mediate intestinal motility[10]. Previous immunohistochemical studies have documented the distribution of P2X2 receptors (P2X2Rs) in the enteric nervous system of the guinea pig[11-14], rat[15-18] and mouse[19-21]. However, the distribution of P2X2R expression in the enteric nervous system of obese mice remains unstudied.
It has been reported that the P2X2R is expressed in inhibitory, cholinergic, and intrinsic afferent neurons in the enteric nervous system[11]. The goal of the current study is to analyze the chemical coding, density, and area profile of neuron immunoreactivity (IR) for the P2X2R, neuronal nitric oxide synthase (nNOS), choline acetyltransferase (ChAT), calretinin (CalR), and positivity for nicotinamide adenine dinucleotide (NADH)-diaphorase activity in the small intestine enteric nervous system of female ob/ob and control mice.
Two groups of 11-mo-old female mice were compared in these studies. The obese group (OG) consisted of six homozygous (ob/ob) C57BL/6J mice, and the control group (CG) was comprised of six wild-type C57BL/6J mice. The animals were bred at the State University of Campinas Breeding Center. The animals were housed at five per cage in an artificially lit room (12 h/12 h, light/dark) and were fed a standard pellet diet (Nuvilab, São Paulo, Brazil) and water ad libitum. Animals were euthanized for experiments using a CO2 chamber. This study was conducted according to current legislation on animal experiments at the Biomedical Science Institute of the University of São Paulo.
Samples of blood were obtained via the puncture of the plexus axillary artery of mice previously anesthetized with xylazine (16 mg/kg) and ketamine (120 mg/kg). Following collection, the blood remained at rest for 2 h at room temperature for clot formation, and then was centrifuged at 2500 ×g for 20 min at 4 °C to obtain the serum. The serum supernatant was maintained at -40 °Cuntil being analyzed for glucose composition.
Following CO2 euthanasia, fresh segments of distal ileum were removed from each animal and placed in phosphate-buffered saline (PBS: 0.15 mol/L NaCl in 0.01 mol/L sodium phosphate buffer, pH 7.2) containing nicardipine (10-6 mol/L; Sigma, United States) to inhibit tissue contraction. The dissected segments were sliced open along the mesenteric border and cleaned of their contents using PBS. They were then pinned out tautly, mucosa side down, onto a balsa-wood board and fixed overnight at 4 °C in paraformaldehyde in 0.2 mol/L sodium phosphate buffer (pH 7.3). The next day, the segments of ileum tissue were cleared of fixative with three 10-min washes in 100% dimethyl sulfoxide followed by three 10-min washes in PBS. All tissue was stored at 4 °C in PBS containing sodium azide (0.1%). The fixed tissue was then dissected and the mucosal, submucosal, and circular layers were removed to obtain whole-mounts of the longitudinal muscle-myenteric plexus. In a second type of preparation, the mucosa and muscularis externa were removed to leave the intact submucosal layer. Whole-mount preparations of the myenteric and submucosa of the ileum were then pre-incubated in blocking buffer, 10% normal horse serum in PBS containing 1.5% Triton X-100 for 45 min at room temperature to reduce non-specific antibody binding and to permeabilize the tissue, respectively (Table 1). To localize P2X2R IR, we used a rabbit antiserum raised against amino acids 457-472 of the rat P2X2R, with a single Cys extension at the N-terminal (AB5244 from Chemicon, Temecula, CA, United States). Tissues were incubated with the antibody at a dilution of 1:120 in blocking buffer for 48 h at 4 °C. Dual immunohistochemistry was achieved using combinations of antisera (Table 1). Following incubation in primary antisera, the tissue samples received three 10-min washes in PBS and were then incubated in a mixture of secondary antibodies (Table 1). Following three further 10-min washes in PBS, the tissue samples were mounted onto microscope slides in glycerol buffered with 0.5 mol/L sodium carbonate buffer (pH 8.6). Tissues preparations were examined using both Leica and Nikon epifluorescent microscopes. Images were captured using a digital camera coupled to Image-Pro Plus software. Preparations were also analyzed by confocal microscopy using a Zeiss confocal scanning laser system installed on a Zeiss Axioplan 2 microscope. The image dimensions were 512 × 512 pixels, with an optical section thickness of 0.5 μm. Immunoreactive cells were scanned as a series of optical sections, with a center spacing of 0.2 μm, and the images were collected using LSM 5 Image Zeiss processing software, and further processed using Corel Photo Paint and Corel Draw software programs.
| Antigen | Host | Dilution | Code and reference |
| P2X2 receptor | Rabbit | 1:120 | AB5244, Chemicon |
| Nitric oxide synthase | Sheep | 1:2000 | H205 (Williamson et al[31], 1996) |
| Choline acetyltransferase | Goat | 1:50 | Chemicon |
| Calretinin | Goat | 1:100 | CG1 (Swant) |
| Secondary antibodies | |||
| Donkey anti-rabbit IgG Alexa 488 | Donkey | 1:500 | Molecular probes |
| Donkey anti-sheep IgG Alexa 594 | Donkey | 1:100 | Molecular probes |
Antigen colocalization was determined by examining preparations immunolabeled with two fluorescent secondary antibodies. Neurons of interest were identified by immunofluorescence for one antigen; the filter was switched, and labeling for the second antigen was determined. In this way, the proportion of neurons immunoreactive for both antigens was determined. For each antigen pair, the cohort size was 100 neurons. Co-IR for the P2X2R with that of the other antigens (nNOS, ChAT, or CalR) was assessed. The percentage of double-immunoreactive neurons was calculated and expressed as mean ± SE (n = number of tissue preparations). The number of neurons immunoreactive for the P2X2R, nNOS, CalR, and ChAT, as well as the neuronal profile (area of cell body), were measured by examining the whole-mount preparations under a binocular microscope at 100 × magnification. All neurons present in each cm2 were counted. The profile areas of 50 nerve cell perikarya from each animal were measured using Image-Pro Plus software. Data were compared by one-way analysis of variance (ANOVA) and Student’s t-tests; P < 0.05 was considered to be statistically significant.
A separate cohort of 12 animals was weighed and euthanized in a CO2 chamber prior to the opening of the anterior abdominal wall. The distal ileum was removed and washed in Krebs solution. The surface area of the entire small intestine was measured using a planimeter. Each piece of ileum was then ligated with cotton thread at the proximal end and gently distended with Krebs solution introduced with a syringe into the distal end. When the intestine was sufficiently distended, the syringe needle was withdrawn and the ligature was simultaneously tightened. The following steps were then performed to label neurons using the NADH diaphorase histochemical technique[22,23]. Following incubation in Krebs solution at room temperature for 15-30 min, the small intestine was transferred to a permeabilizing agent (0.3% Triton-X 100 in Krebs solution) for 15-90 s and then submitted to three 10-min washes in Krebs solution. The specimens were incubated for 30-45 min at 20 °C in 20 mL of incubation medium that contained 0.5 mg/mL of nitro blue tetrazolium (Sigma Chemical Co, St. Louis, MO) in distilled water (25 parts), 0.1 mol/L sodium phosphate buffer, pH 7.3 (25 parts), distilled water (50 parts), and 0.5 mg/mL of the reduced form of β-NADH (Sigma, United States). The reaction was stopped by immersion in 10% buffered formalin solution, in which the tissue samples were fixed for a minimum of 24 h at room temperature. Whole mount preparations were then prepared as follows: the small intestine was opened, the mucosa was removed, and the longitudinal muscle, with the myenteric plexus attached, was lifted at one corner and gently removed from the entire strip. After several washes in distilled water, fragments (2 cm2) of the proximal, middle, and distal portions of the large intestine were prepared as whole mounts in glycerol on microscope slides and sealed with Entellan (Merck).
Myenteric neurons were identified by the presence of intense formazan reaction product filling the perikaryon, as well as by large, round, and unstained nuclei. The number of neurons and the profile areas of the nerve cell bodies were measured by examining the whole mount preparations under a binocular microscope at a magnification of 400 ×. All labeled neurons in each fragment were counted. The profiles of 100 nerve cell perikarya from each portion were obtained using a semi-automatic morphometry device (Image-Pro Plus Program 3.1)[17,18].
Results are expressed as mean ± SE. Data were compared by ANOVA with Student’s t-tests for multiple comparisons, as appropriate. P < 0.05 was considered statistically significant.
In the present study, we found a significant (29.6%, P < 0.05) increase in the body weight of the ob/ob animals (OG) compared to controls (Table 2). In addition, the average small intestine area was increased by approximately 29.6% in the OG as compared to the CG (P < 0.05; Table 2). Plasma glucose measurements revealed levels of 195.75 ± 58 mg/dL in control mice and 322.3 ± 58 mg/dL in the OG mice (P < 0.05). Mice with blood glucose levels above 300 mg/dL were considered to be diabetic (Table 2).
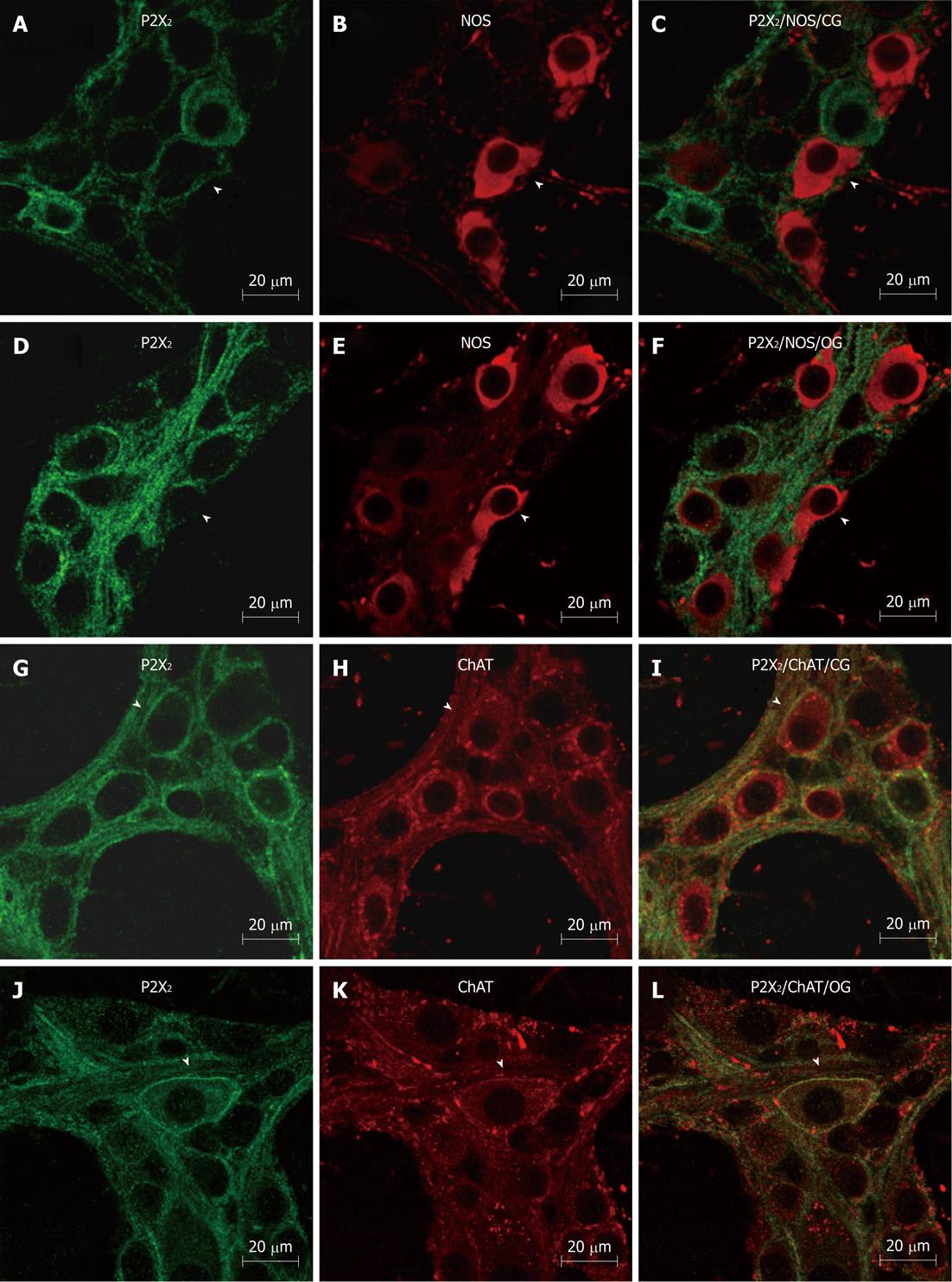
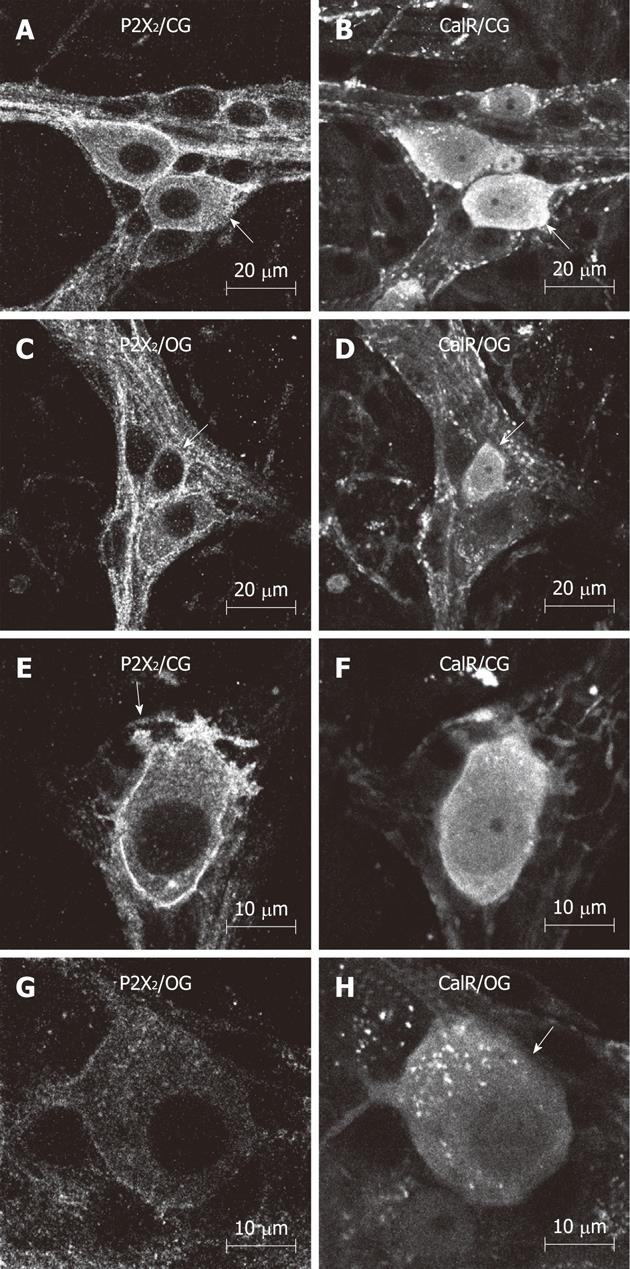
IR for the P2X2R, nNOS, ChAT and CalR was detectable in the myenteric plexus, as well as in the smooth muscle, in both groups. This IR mainly occurred throughout the cytoplasm and on cell surface and nuclear membranes, and effectively outlined the neuronal cell bodies and their processes in the myenteric plexus (Figure 1). nNOS-IR neurons were detected in both groups. Some nNOS-IR neurons displayed short dendritic processes, characteristic of Dogiel type I morphology. The primary and secondary fiber tracts were also immunoreactive for nNOS (Figure 1). ChAT-IR neuronal cell bodies were abundant in the myenteric ganglia; ChAT-IR was present in the cytoplasm of neurons from both groups. Nearly all of the ChAT-IR neurons displayed Dogiel type I morphology. We also observed a small number of large, smooth-surfaced ChAT-IR cell bodies. In addition, varicose ChAT-IR fibers were observed surrounding the ganglia, and constituted the primary and secondary fiber tracts of the ileum myenteric plexus (Figure 2). IR for CalR was detected in neuronal cell bodies and axonal/dendritic processes in both the control and the OG. Some neurons showed typical Dogiel type I morphology, with short dendritic processes, while others displayed long axonal projections characteristic of Dogiel type II morphology (Figure 2).
Double immunolabeling for the P2X2R in combination with anti-nNOS, ChAT, and CalR IR was conducted to identify different chemical classes (chemical coding) of neurons in the myenteric plexus. In the CG, 99.5% ± 0.6% of NOS-IR neurons exhibited P2X2R-IR and, in the OG, the colocalization score was similar (99.1% ± 1.1%). The colocalization of ChAT-IR and CalR-IR with P2X2-IR was 100% in neurons from both the control and the OGs (Table 3). In summary, these analyses did not show any statistically significant differences in the colocalization of P2X2R-IR with that of the other antigens between the two groups (Table 3).
| Control group | Obese group | |
| nNOS-IR+/P2X2-IR+ | 99.5 ± 0.6 | 99 ± 1.0 |
| ChAT-IR+/P2X2-IR+ | 100 | 100 |
| CalR-IR+/ P2X2-IR+ | 100 | 100 |
| P2X2-IR+/nNOS-IR+ | 19.5 ± 3.6 | 19.5 ± 3.6 |
| P2X2-IR+/ChAT-IR+ | 23.6 ± 4.2 | 24.6 ± 4.37 |
| P2X2-IR+/CalR-IR+ | 23.7 ± 3.75 | 21.6 ± 1.20 |
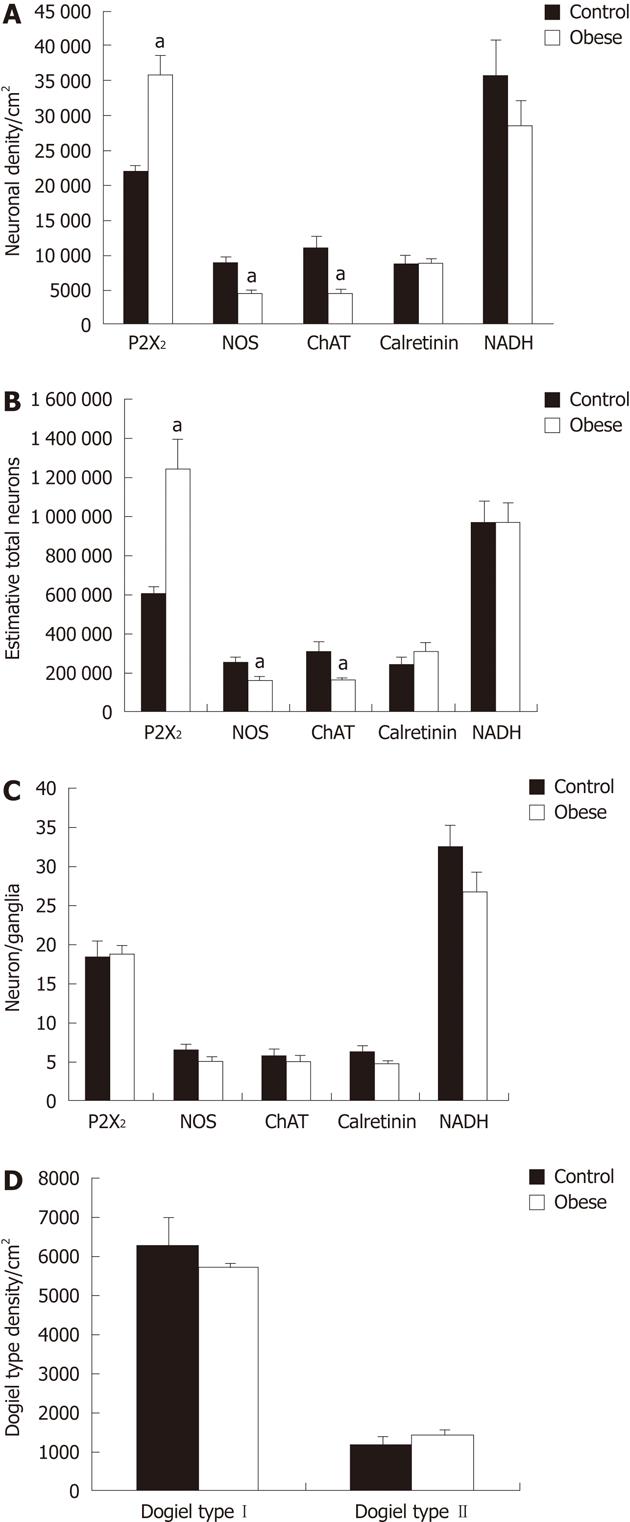
Upon examination of neuronal density in the myenteric plexus, we found an increase of 34.3% in the density of P2X2R-IR neurons in the OG compared to controls (P < 0.05). In addition, the density of nNOS-IR and ChAT-IR neurons decreased by 42.6% and 53.4%, respectively, in the OG compared to controls (P < 0.05). There were no differences between the groups in the density of CalR-IR neurons (Figure 3). These data suggest that the density of P2X2R-IR neurons increases, while that of nNOS-IR and ChAT-IR neurons decreases, in the OG compared to controls. We then estimated the total number of neuron IR for the P2X2R and found an increase of 105% in the OG compared to controls, while the total number of nNOS-IR and ChAT-IR neurons was decreased 35% and 47%, respectively, compared to controls (P < 0.05). The total number of CalR-IR neurons was not significantly different between the two groups (Figure 3).
We then evaluated the number of P2X2R-IR neurons per myenteric ganglion in both groups. The number of P2X2-IR neurons was 19 ± 1.8 neurons/ganglion in the OG vs 18.6 ± 3.9 neurons/ganglion in the CG. In addition, the number of nNOS-IR neurons per ganglion did not significantly differ between the groups, and was 6.6 ± 1.4 neurons/ganglion in the CG, and 5.2 ± 0.7 neurons/ganglion in the OG. The number of ChAT-IR neurons per ganglion also did not differ between the groups (5.9 ± 1.8 for CG, 5.1 ± 1.4 for OG). The number of CalR-IR neurons per ganglion also did not differ between groups (6.5 ± 1.2 for CG, 5.0 ± 0.2 for OG). Therefore, these data show that there were no significant differences between the control and OGs in the numbers of P2X2R-, nNOS-, ChAT- or CalR-IR neurons in the myenteric ganglia (Figure 3).
Neurons with typical Dogiel type I and II morphologies were observed in whole mounts immunolabeled for CalR-IR. The density of Dogiel type I neurons in the CG and OG was 6.3 ± 1.4 neurons/cm2 and 5.7 ± 0.3 neurons/cm2, respectively. The density of Dogiel type II neurons was 1.3 ± 0.2 neurons/cm2 in the CG and 1.5 ± 0.2 neurons/cm2 in the OG. These data show that there were no significant differences in the density of CalR-IR Dogiel type I and II neurons between the CG and OG (Figure 3).
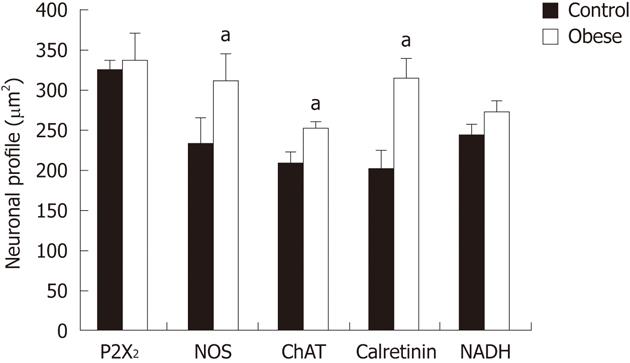
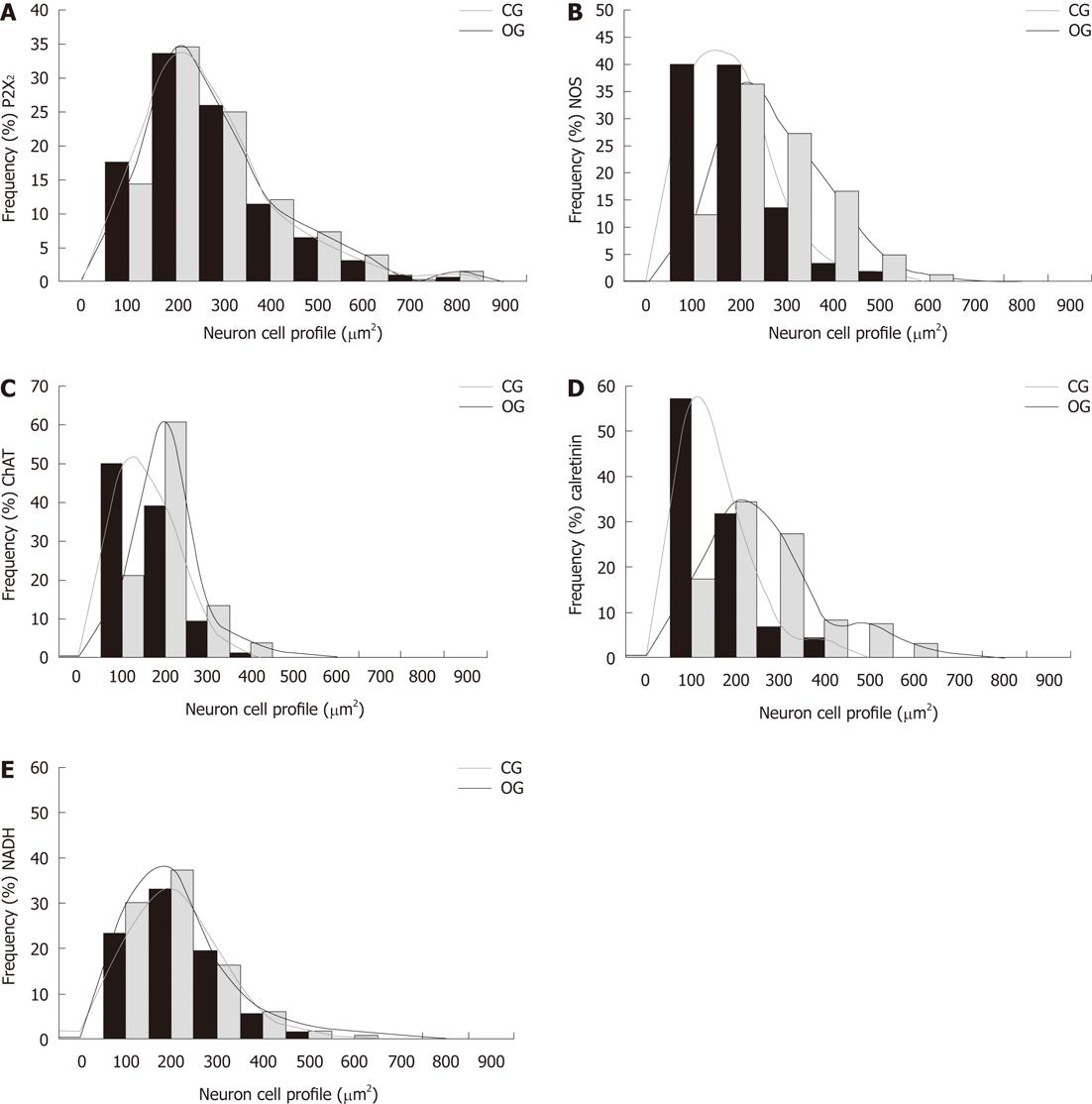
We then measured the cell body area of neurons in the myenteric plexus from both control and OGs. Although there was no change in the size of P2X2R-IR neurons between the groups, we observed a significant increase in the cell body areas of nNOS-IR (34%), ChAT-IR (17%), and CalR-IR neurons (35%) in the OG compared to controls (P < 0.05; Figure 4). The size distribution of neurons in the myenteric plexus of CG and OG mice are shown in Figure 5.
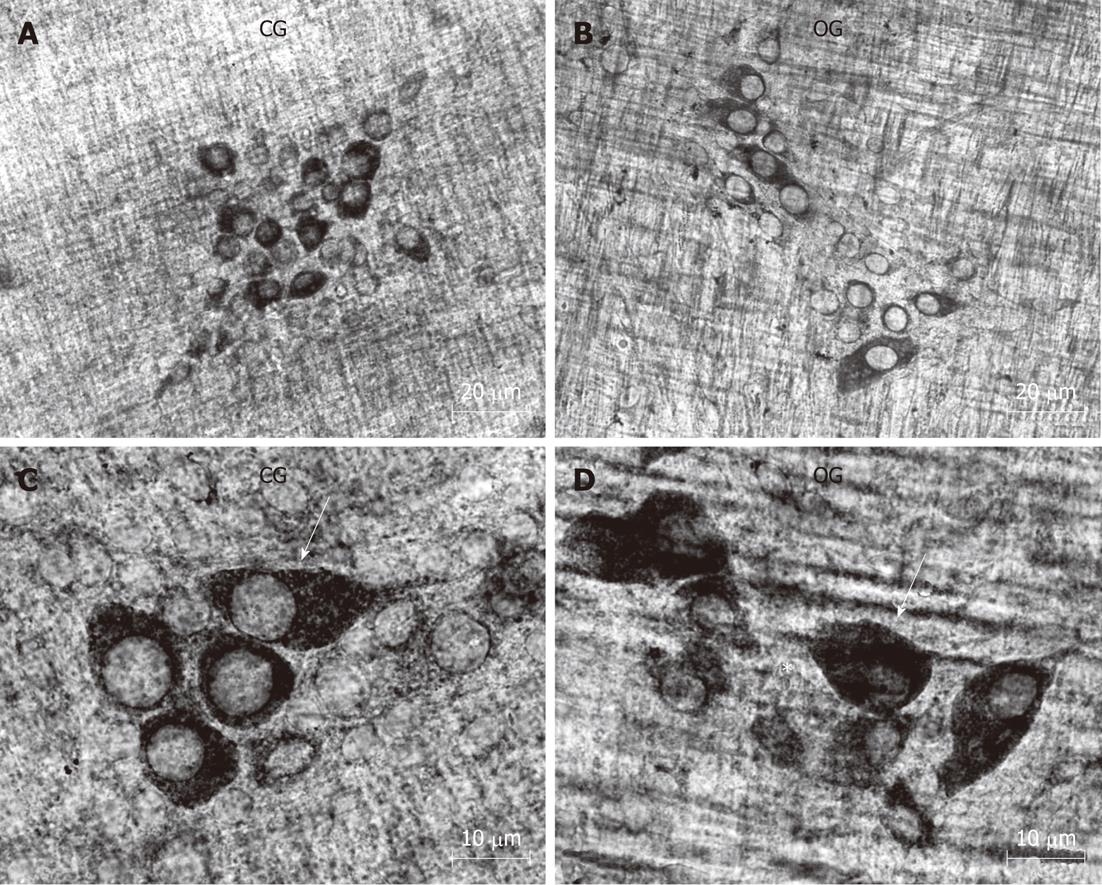
Our qualitative examination of NADH-diaphorase positive neurons in the myenteric ganglia revealed an overall similarity between the two groups. With this method, the formazan reaction product labels the neuronal cytoplasm with a varying intensity, while neuronal nuclei appear translucent. We did observe that the myenteric neurons appeared to be more widely-spaced (less dense, or grouped) in the myenteric ganglia of the OB group compared to controls (Figure 6).
This histochemical analysis provided some potentially useful general information about the myenteric neuronal population in the mouse ileum. The density of NADH-diaphorase-positive neurons was reduced by about 20% in the OG compared to controls; however, this reduction was not statistically significant (Figure 3). The total surface area of the small intestine in both groups was measured, and the total number of neurons was estimated to be 971 558 ± 205 000 in the CG and 974 042 ± 187 000 in the OG (Figure 3). In addition, an estimate of the number of neurons per myenteric ganglion did not reveal any significant differences between the groups (Figure 3). Cell body area profile measurements of NADH-diaphorase-positive neurons also showed no differences in overall neuronal size between the control and OGs (Figure 4).
Gastrointestinal disorders arising from alterations in intestinal motility in obese individuals have been related to alterations in the enteric nervous system[1]. In the current report, we demonstrate changes in the population of enteric neurons of the myenteric plexus in the ob/ob mouse that may explain, in part, impairments in intestinal motility suffered by obese individuals[1]. The current study demonstrates, for the first time, the presence of P2X2R IR in the myenteric plexus, and alterations in the chemical identity, or coding, of myenteric neurons in the ileum of obese female ob/ob mice compared to controls. Previous studies have revealed the presence of P2X2,3,7 receptor-containing neurons in the enteric nervous systems of various species[11-21]. In the current study, we show that IR for the P2X2R is present in both the cytoplasm and membrane of myenteric plexus neurons in control and obese animals. We confirm that IR for the P2X2R colocalizes with that for nNOS, ChAT and CalR in myenteric neurons. This finding is consistent with previous work demonstrating the presence of P2X2R in inhibitory neurons, as well as in intrinsic excitatory and secretomotor/vasodilator primary afferent neurons in the guinea pig[11] and rat[16-18]. We did not observe any differences in the colocalization of P2X2R with IR for these markers (nNOS, ChAT, CalR) between the control and obese mice, suggesting that the neurochemical coding of these neurons is not altered in the myenteric plexus of obese female ob/ob female mice compared to wild-type controls.
The creation of the ob/ob mouse on the C57BL/6J background, which followed the cloning and sequencing of the mouse ob gene and its human homologue, provides an excellent model for the study of obesity[2]. These mice present with obesity that is accompanied by the manifestation of motor and sensory nerve conduction deficits, small sensory nerve fiber neuropathy, intraepidermal sensory nerve fiber loss, and oxidative nitrosative stress in peripheral nerves, features that are characteristic of human subjects with obesity and diabetes[2]. Previous studies have demonstrated that mice with blood glucose levels above 300 mg/dL are useful as a mouse model of diabetes[2]. In the current study, the obese ob/ob mice had average blood glucose levels of 322.3 ± 58 mg/dL, clearly within the diabetic range. Consistent with previous reports, the body weights of the ob/ob mice were twice those of the control mice in our study. This increase in body weight was accompanied by a similar increase in the area of the ileum in the obese animals, in accordance with previous findings[6]. Obesity in humans is associated with increased risk of type 2 diabetes and is often accompanied by complications of the gastrointestinal tract. In the current work, we found high levels of glucose, well within the diabetic range, accompanied by alterations in the myenteric neurons in the obese ob/ob mice compared to controls, providing a possible explanation for the dysfunctions in intestinal motility that can accompany obesity. In keeping with this idea, animals with type 1 diabetes have been observed to have gastrointestinal complications[2].
We examined nNOS IR as a marker of inhibitory motor neurons, as well as ChAT and CalR-IR to identify excitatory motor neurons and interneurons, respectively. The results we obtained are consistent with the current literature[4]. For example, our morphological analysis showed that nNOS-IR neurons exhibit Dogiel type I morphologies, while CalR-IR neurons exhibit both Dogiel type I and II morphologies. Qu et al[4] in 2008 showed that about 15% of all myenteric neurons are Dogiel type II and are immunoreactive for CalR. Our current findings show that 14% of all the CalR-IR neurons in the myenteric plexus were Dogiel type II in the CG, and that a similar percentage (16%) existed in the OG.
ChAT is the synthesizing enzyme for the excitatory neurotransmitter acetylcholine, a transmitter not found in inhibitory motor neurons[3,4]. In the current work, we show that numerous ChAT-IR neurons are present in the myenteric ganglia, as has been described in other species. In addition, we find that all the CalR-IR neurons are also immunoreactive for ChAT, confirming their identity as excitatory cholinergic motor neurons[4].
Changes in neuronal density have been observed in autonomic neurons[24], and various regions of the gastrointestinal tract in rat models of malnutrition and re-feeding[22,23,25] as well as following intestinal ischemia/reperfusion (I/R-i)[18]. In the ob/ob group of the current study, the density of nNOS-IR and ChAT-IR neurons was decreased by 42.6% and 53.4% compared to controls; in addition, the total number of nNOS-IR and ChAT-IR neurons was reduced by 35% and 47%, respectively, in the OG compared to controls. No statistically significant difference was found in the density of CalR-IR neurons between the groups. The observed decrease in neuronal density may be explained by the 29% increase in the area of the small intestine that we measured in the obese female mice. However, the estimated overall decrease in the number of neurons in the ob/ob intestine implies an actual loss of enteric neurons in these mice. Another enzyme, NADH-diaphorase, has been shown to decrease during malnourishment in neurons of the small and large intestines[22,23]. In the current study, however, we did not observe any alteration in the density of NADH-diaphorase-positive neurons in the OG compared to controls.
In the obese female mouse small intestine, we observed increases in both the density and estimated number of P2X2R-IR neurons compared to controls. This increase could be due to the presence of P2X2R IR in another type of enteric nervous system cell, one which is not immunoreactive for nNOS, CalR or ChAT; namely, enteric glial cells. The presence of P2X and P2Y receptors has been described in astrocytes and microglia of the central nervous system (CNS)[9] as well as in enteric glial cells[26]. However, the increase of P2X2R IR following undernourishment has been show to occur specifically in enteric neurons[17], making it likely that our observations of increased P2X2R-IR also result from increased neuronal expression.
Changes in the central neuronal expression of P2X1-7 purinoceptors frequently occur not only during maturation and neuronal differentiation, but also following various types of acute insults to the CNS. Prolonged stimulation of ATP receptors has been shown to result in changes in the location and density of P2 receptors in the cell membrane[27].
The NADH-diaphorase method is widely used in studies of alterations in the enteric nervous system and other autonomic plexus that relate to undernourishment, re-feeding, and age[22,23,28]. Our current data indicate that the density of NADH-diaphorase positive neurons showed a tendency to decrease in the obese mice compared to controls; however, these differences were not statistically significant.
Previous immunohistochemical and histochemistry studies have shown that under-nutrition[22,23] and I/R-i[18] affect the size profiles of neurons in the gastrointestinal tract. When using NADPH-diaphorase histochemistry to label myenteric neurons, we did not observe differences between the control and OGs, although the range of profile areas using this technique was consistent with that estimated from measurements of nNOS-, ChAT- and CalR-IR. Using an immunohistochemistry technique we were unable to verify exactly which neuronal classes demonstrated alterations in cell size in the obese mouse intestine. We show that cell area was increased for nNOS-, ChAT-, and CalR-IR neurons in the obese mouse, compared to controls. In agreement with these findings, modest (4.4%) increases in the profile area of myenteric neurons have been demonstrated in diabetic rats[29].
Epidemiological studies have suggested that excess body weight may be a major risk factor for colon and breast cancer. In an animal model of colon carcinogenesis, mice fed a high-fat diet exhibited a greater number of colon tumors than lean animals. Their increased abdominal fat was associated with higher concentrations of leptin, insulin, and insulin-like growth factor 1, which possibly mediate tumor growth. These data suggest that the metabolic burden created by excess adiposity accelerates uncontrolled cell growth and survival, thereby increasing the risk of developing breast and colon cancer[30].
Our understanding of the anatomical and molecular consequences of obesity becomes even more complex when we take into account the very complicated nature of the innervation of the gut. We believe that the current study provides a positive step in our knowledge of the relationship between obesity, diabetes, and gastrointestinal disease. The results of this study suggest that there are significant changes in the intestinal ileum of obese mice, findings that further promote our understanding of obesity and the enteric nervous system.
We would like to thank Professors Edson Aparecido Liberti, Jackson Cioni Bittencourt, Carol Fuzeti Elias, Lício A Velloso, and Rosana Prisco for statistical analysis.
Obesity is a chronic heterogeneous disorder characterized by abnormal or excessive fat accumulation that presents a risk to health. Obesity is associated with increased risk of type 2 diabetes and is often accompanied by complications of the gastrointestinal tract, such as gastroparesis, constipation, diarrhea, and fecal incontinence. In addition, defects in enteric nervous system function have been reported in ob/ob mice, including alterations in intestinal motility and in the enteric neurons.
The myenteric plexus is affected in obesity. The chemical coding of enteric neurons, as well as P2X2 receptor (P2X2R) expression, has not been characterized in the ob/ob mice.
The present study showed the effects of obesity in ob/ob mice on the morphology of the P2X2R-, nitric oxide synthase-, calretinin- and choline acetyltransferase-immunoreactivity (IR) neurons of the myenteric plexus.
The present study suggests that obesity may change the chemical coding of the P2X2R-IR neurons in the myenteric plexus, as well as that of inhibitory, cholinergic, and intrinsic primary afferent neurons on the enteric nervous system.
The enteric nervous system is composed of the myenteric and submucosal plexuses, which control the motility of the intestine. Adenosine 5’-triphosphate is a well-known neurotransmitter and a ligand of the P2X receptor family, which is made up of seven known receptor subunits PX1-7.
This study fulfills its purpose and provides good results both qualitatively and quantitatively.
Peer reviewers: Romeu R Souza, Professor, Department of Morphology, Laboratorio de Biodinamica, Universidade Sao Judas Tadeu, Rua Afonso de Freitas, 451 ap 122, São Paulo 04006052, Brazil; Dr. Maria Raquel Marçal Natali, Department of Morphology Science, State University of Maringá, Av, Colombo 5790, Maringá 87020-900, Brazil
S- Editor Wu X L- Editor Logan S E- Editor Zheng XM
| 1. | Bessesen DH. Update on obesity. J Clin Endocrinol Metab. 2008;93:2027-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 2. | Drel VR, Mashtalir N, Ilnytska O, Shin J, Li F, Lyzogubov VV, Obrosova IG. The leptin-deficient (ob/ob) mouse: a new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes. 2006;55:3335-3343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 3. | Furness JB. The enteric nervous system. New York: Wiley-Blackwell publishing 2006; . |
| 4. | Qu ZD, Thacker M, Castelucci P, Bagyánszki M, Epstein ML, Furness JB. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008;334:147-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 249] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 5. | Mongardi Fantaguzzi C, Thacker M, Chiocchetti R, Furness JB. Identification of neuron types in the submucosal ganglia of the mouse ileum. Cell Tissue Res. 2009;336:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Kiely JM, Noh JH, Graewin SJ, Pitt HA, Swartz-Basile DA. Altered intestinal motility in leptin-deficient obese mice. J Surg Res. 2005;124:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Spångéus A, El-Salhy M. Myenteric plexus of obese diabetic mice (an animal model of human type 2 diabetes). Histol Histopathol. 2001;16:159-165. [PubMed] |
| 8. | Surendran S, Kondapaka SB. Altered expression of neuronal nitric oxide synthase in the duodenum longitudinal muscle-myenteric plexus of obesity induced diabetes mouse: implications on enteric neurodegeneration. Biochem Biophys Res Commun. 2005;338:919-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 627] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 10. | Galligan JJ. Ligand-gated ion channels in the enteric nervous system. Neurogastroenterol Motil. 2002;14:611-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 102] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Castelucci P, Robbins HL, Poole DP, Furness JB. The distribution of purine P2X(2) receptors in the guinea-pig enteric nervous system. Histochem Cell Biol. 2002;117:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Poole DP, Castelucci P, Robbins HL, Chiocchetti R, Furness JB. The distribution of P2X3 purine receptor subunits in the guinea pig enteric nervous system. Auton Neurosci. 2002;101:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 13. | Van Nassauw L, Brouns I, Adriaensen D, Burnstock G, Timmermans JP. Neurochemical identification of enteric neurons expressing P2X(3) receptors in the guinea-pig ileum. Histochem Cell Biol. 2002;118:193-203. [PubMed] |
| 14. | Hu HZ, Gao N, Lin Z, Gao C, Liu S, Ren J, Xia Y, Wood JD. P2X(7) receptors in the enteric nervous system of guinea-pig small intestine. J Comp Neurol. 2001;440:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Xiang Z, Burnstock G. P2X2 and P2X3 purinoceptors in the rat enteric nervous system. Histochem Cell Biol. 2004;121:169-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Yu Q, Zhao Z, Sun J, Guo W, Fu J, Burnstock G, He C, Xiang Z. Expression of P2X6 receptors in the enteric nervous system of the rat gastrointestinal tract. Histochem Cell Biol. 2010;133:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Misawa R, Girotti PA, Mizuno MS, Liberti EA, Furness JB, Castelucci P. Effects of protein deprivation and re-feeding on P2X2 receptors in enteric neurons. World J Gastroenterol. 2010;16:3651-3663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Paulino AS, Palombit K, Cavriani G, Tavares-de-Lima W, Mizuno MS, Marosti AR, da Silva MV, Girotti PA, Liberti EA, Castelucci P. Effects of ischemia and reperfusion on P2X2 receptor expressing neurons of the rat ileum enteric nervous system. Dig Dis Sci. 2011;56:2262-2275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Giaroni C, Knight GE, Ruan HZ, Glass R, Bardini M, Lecchini S, Frigo G, Burnstock G. P2 receptors in the murine gastrointestinal tract. Neuropharmacology. 2002;43:1313-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Castelucci P, Robbins HL, Furness JB. P2X(2) purine receptor immunoreactivity of intraganglionic laminar endings in the mouse gastrointestinal tract. Cell Tissue Res. 2003;312:167-174. [PubMed] |
| 21. | Ruan HZ, Burnstock G. The distribution of P2X5 purinergic receptors in the enteric nervous system of mouse. Cell Tissue Res. 2005;319:191-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Castelucci P, de Souza RR, de Angelis RC, Furness JB, Liberti EA. Effects of pre- and postnatal protein deprivation and postnatal refeeding on myenteric neurons of the rat large intestine: a quantitative morphological study. Cell Tissue Res. 2002;310:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Gomes OA, Castelucci P, de Vasconcellos Fontes RB, Liberti EA. Effects of pre- and postnatal protein deprivation and postnatal refeeding on myenteric neurons of the rat small intestine: a quantitative morphological study. Auton Neurosci. 2006;126-127:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Gomes SP, Nyengaard JR, Misawa R, Girotti PA, Castelucci P, Blazquez FH, de Melo MP, Ribeiro AA. Atrophy and neuron loss: effects of a protein-deficient diet on sympathetic neurons. J Neurosci Res. 2009;87:3568-3575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Greggio FM, Fontes RB, Maifrino LB, Castelucci P, de Souza RR, Liberti EA. Effects of perinatal protein deprivation and recovery on esophageal myenteric plexus. World J Gastroenterol. 2010;16:563-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Vanderwinden JM, Timmermans JP, Schiffmann SN. Glial cells, but not interstitial cells, express P2X7, an ionotropic purinergic receptor, in rat gastrointestinal musculature. Cell Tissue Res. 2003;312:149-154. [PubMed] |
| 27. | Burnstock G, Krügel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol. 2011;95:229-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Mizuno MS, Pompeu E, Castelucci P, Liberti EA. Age-related changes in urinary bladder intramural neurons. Int J Dev Neurosci. 2007;25:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | De Freitas P, Natali MR, Pereira RV, Miranda Neto MH, Zanoni JN. Myenteric neurons and intestinal mucosa of diabetic rats after ascorbic acid supplementation. World J Gastroenterol. 2008;14:6518-6524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Sung MK, Yeon JY, Park SY, Park JH, Choi MS. Obesity-induced metabolic stresses in breast and colon cancer. Ann N Y Acad Sci. 2011;1229:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Williamson S, Pompolo S, Furness JB. GABA and nitric oxide synthase immunoreactivities are colocalized in a subset of inhibitory motor neurons of the guinea-pig small intestine. Cell Tissue Res. 1996;284:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |