Published online Sep 14, 2012. doi: 10.3748/wjg.v18.i34.4684
Revised: July 23, 2012
Accepted: August 14, 2012
Published online: September 14, 2012
AIM: To evaluate the protective properties of novel prostone ClC-2 agonist SPI-8811 in porcine model of gastric acid injury.
METHODS: Porcine gastric mucosa was mounted in Ussing chambers and injured by bathing mucosal tissues in an HCl Ringer’s solution (pH = 1.5) with or without SP1-8811 (1 μmol/L), cystic fibrosis transmembrane conductance regulator (CFTR) inhibitor (inhibitor 172, 10 μmol/L, apical) and ClC-2 inhibitor ZnCl2, 300 μmol/L, apical), on the apical surface of tissues. Transepithelial resistance and mucosal-to-serosal 3H-mannitol fluxes were measured over a 90-min period. Tissues were analyzed by morph metric techniques, Immunofluorescence and by western blots.
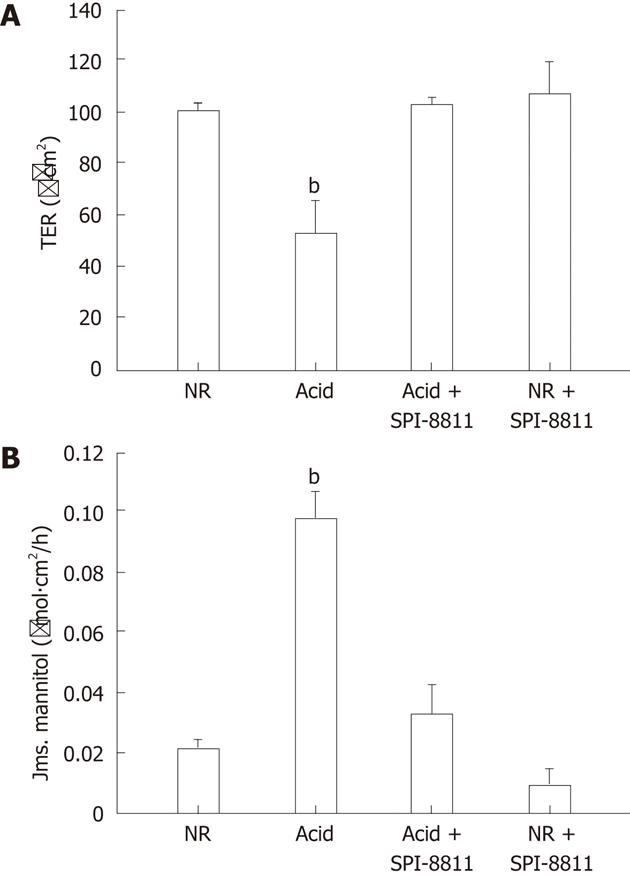
RESULTS: Compared with control tissues, acid exposure decreased transepithelial electrical resistance (TER) and increased 3H-mannitol flux. Pretreatment of gastric mucosa with SPI-8811 was protective against acid-induced decreases in TER (TER, 50 Ω.cm2vs 100 Ω.cm2) and abolished increases in flux (3H-mannitol flux, 0.10 μmol/L.cm2vs 0.04 μmol/L.cm2). Evidence of histological damage in the presence of acid was markedly attenuated by SPI-0811. Immunofluorescence and western analysis for occludin revealed enhanced localization to the region of the tight junction (TJ) after treatment with SPI-8811. Pretreatment with the ClC-2 inhibitor ZnCl2, but not the selective CFTR inhibitor 172, attenuated SPI-8811-mediated mucosal protection, suggesting a role for ClC-2. Prostone may serve both protective and reparative roles in injured tissues.
CONCLUSION: ClC-2 agonist SPI-8811 stimulated enhancement of mucosal barrier function by protecting TJ protein occludin in porcine gastric mucosa and thus protected the gastric acid injury in porcine stomach.
- Citation: Nighot M, Moeser A, Ueno R, Blikslager A. Gastro protective properties of the novel prostone SPI-8811 against acid-injured porcine mucosa. World J Gastroenterol 2012; 18(34): 4684-4692
- URL: https://www.wjgnet.com/1007-9327/full/v18/i34/4684.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i34.4684
It is becoming increasingly evident that many patients suffer from gastric ulcers, particularly in groups of patients such as those in intensive care facilities[1-4]. This suggests that medications that provide gastro protection have the potential to reduce morbidity associated with gastric ulceration. For decades, agents that suppress acid secretion[5-7] have been widely used for treatment of gastric ulcers[1,7-10]. Gastric ulcer disease and repair is complex, involving inflammation, cell proliferation, formation of granulation tissue, and angiogenesis[3,4,11]. However, gastro protection has also been studied in depth. For example, studies with rebamipide or misoprostol, geranylgeranyl hydrochloride (HSP70) have revealed that these compounds have gastro protective properties as well enhancing ulcer healing[2,7,12]. Nonetheless, our understanding of the mechanisms of gastro protection is incomplete and there is the prospect of novel pharmacological agents, aside from antacids, proton pump inhibitors, and prostanoid activators or analogs, which might protect the stomach[12,13].
The gastric barrier is composed of a single layer of columnar epithelium, mucus, bicarbonate layer, and intraepithelial tight junctions (TJs) residing at the apical-most region of the paracellular space. The gastric barrier serves as the first line of defense against a hostile luminal environment. The mucus bicarbonate barrier is the only pre epithelial barrier between lumen and the epithelium[14,15]. When it is overwhelmed or breaks down in disease, the next series of protective mechanisms come into play, including intracellular neutralization of acid, rapid epithelial repair, and maintenance and distribution of mucosal blood flow. There are two components of this innate mucosal defense: mechanisms that reduce the ability of pathogens and their toxins to invade the mucosa, and the mechanisms that ensure rapid repair of defects in the epithelial monolayer[3,4,15-17]. The next line of mucosal defense is formed by a continuous layer of surface epithelial cells which secrete mucus and bicarbonate and generate prostaglandins, heat shock proteins, refoil factor peptides, and cathelicidins. Because of the presence of phospholipids on their surfaces, these cells are hydrophobic, repelling acid- and water-soluble damaging agents. Interconnected by TJs, the surface epithelial cells form a “barrier” preventing back diffusion of acid and pepsin. Once the epithelial barrier is disrupted, epithelial repair mechanisms must rapidly re-form a continuous epithelial monolayer in order to prevent entry of protons and bacteria[3,4,14,15,18]. The remarkable phenomenon of epithelial restitution during which epithelium re-seals mucosal defects in the presence of acidic environment has been a subject of several studies.
Acid injury is an important component of gastric ulcer disease, despite more recent findings on the role of Helicobacter pylori which also contributes to this troublesome problem. Aside from injuring epithelium directly, acid causes disruption of interepithelial TJ protein complexes thereby increasing epithelial permeability[10,14,16,19]. Models assessing the mechanisms of intestinal injury have demonstrated that the critical event defining disruption of barrier function is the loss of TJ architecture and redistribution of TJ proteins such as occludin from the apical region of the interepithelial space to the cytosol[10,20-22]. In our previous work, we have demonstrated a critical role for Cl- secretion in restoration of intestinal barrier function in ischemic-injured porcine ileum[21]. This appears to be attributable to activation of the Cl- channel ClC-2, which is localized to the TJ[21]. More specifically, prior studies have shown that the nonselective secretory agonist prostaglandin E2 triggered rapid recovery of transepithelial electrical resistance (TER) and reduced mucosal-to-serosal fluxes of 3H-mannitol in ischemic-injured intestinal mucus[20,22-24]. Previous studies have shown that activation of ClC-2 by the prostone Lubiprostone[22,25] enhanced recovery of barrier function in ischemic-injured porcine intestine. Cobiprostone (SPI-8811) is an investigational ClC-2 agonist prostone compound under development by Sucampo Pharmaceuticals Inc. (Bethesda, MD). Prostones are derived from fatty acids that are formed naturally within tissues. The present study was performed to evaluate the ability of the novel ClC-2 agonist SPI-8811 to provide gastro protection in acid-injured porcine gastric mucosa.
ZnCl2, Bumetanide, 3H-mannitol, and cystic fibrosis transmembrane conductance regulator (CFTR) inhibitor 172 were purchased from Sigma-Aldrich (St. Louis, MO). SPI-8811 was provided by Sucampo Pharmaceuticals Inc., Bethesda, MD.
All studies were approved by the North Carolina State University Institutional Animal Care and Use committee. Yorkshire crossbred pigs of either sex approximately 10-15 kg body weight were housed individually and maintained on a commercial pelleted feed. Pigs were held off feed, but had free access to water, for 12 h before each experiment. Anaesthesia was induced with xylazine (1.5 mg/kg, im) and ketamine (11 mg/kg, im), after which they were euthanized with pentobarbital (20 mg/kg, iv). The entire stomach was clamped proximally and distally with Doyen intestinal forceps.
After harvesting the entire stomach, it was sharply incised at the lesser curvature and washed in porcine Ringer’s (mmol/L: 154 Na+, 6.3 K+, 137 Cl-, 0.3 H2PO4, 1.2 Ca2+, 0.7 Mg2+, 24 HCO3-, pH 7.4) and maintained in oxygenated (95% O2/5% CO2) Ringer’s solution. The fundus portion was isolated for Ussing chamber studies. After stripping the mucosa from the seromuscular layer by using blunt scissors, mucosa was mounted in 3.14 cm2 aperture Ussing chambers. Gastric mucosa from individual pigs was mounted on multiple Ussing chambers and subjected to acid injury and select treatments. Tissues were initially bathed in 10 mL porcine Ringer’s on both mucosal and serosal sides. The serosal bathing solution contained 10 mmol/L glucose to maintain tissue viability, and this was osmotically balanced on the mucosal side with 10 mmol/L mannitol. Indomethacin (5 μmol/L) was added on the serosal and mucosal sides of gastric tissues to prevent prostaglandin production. Bathing solutions were oxygenated and maintained at 37 °C by water jacketed reservoirs. The spontaneous potential difference (PD) was measured via Ringer’s-agar bridges connected to calomel electrodes, and the PD was short-circuited via Ag-AgCl2 electrodes using voltage clamps that corrected for fluid resistance to measure short circuit current (Isc). Transepithelial resistance (Ω.cm2 was calculated from the spontaneous PD and Isc. If the spontaneous PD was between -1.0 mV and +1.0 mV, tissues were current clamped at ± 100 μA for 5 s and the PD was recorded. Isc and PD were recorded at 15 min intervals over a 90 min experimental period.
Once tissues were mounted on Ussing chambers, treatments aimed at inhibiting ClC-2 (ZnCl2, 300 μmol/L, apical), CFTR (inhibitor 172, 10 μmol/L, apical), or Na(+)-K(+)-2Cl(-) cotransporter (NKCC1) (bumetanide, 100 μmol/L, basolateral) were added. Alternatively, SPI-8811 was administered to the apical surface of tissues (1 μmol/L) to enhance epithelial Cl- secretion via ClC-2. Following a 30 min equilibration period, HCl (1 mol/L in Ringer’s) was added to the mucosal surface of the tissue to induce acid injury. The pH was monitored with a pH meter (Hanna Instruments Inc., Ann Arbor, MI, United States) and maintained the pH at 1.5 by adding 1 mol/L HCl as needed during the experiment.
To assess mucosal permeability, 0.2 Ci/mL 3H-mannitol was placed on the mucosal side of tissues after experimental treatments. After a 15 min equilibration period, standards were taken from the mucosal side of each chamber and a 30 min flux period was established by taking 0.5 mL samples from the serosal compartments. The presence of 3H was established by measuring emission in a liquid scintillation counter (Rack Beta, Perkin Elmer Life and Analytical Sciences, Boston, MA, United States). Unidirectional mucosal-to-serosal 3H-mannitol fluxes were calculated using a previously established spreadsheet[17].
Tissue samples were collected at 0, 30 and 90 min during the experimental period and fixed in 10% formalin for histological evaluation. Paraffin embedded samples were sectioned (5 μm) and stained with hematoxylin and eosin and periodic acid-schiff stain (PAS). For each tissue, mucosal epithelial lining and gastric pits (crypts) were identified to assess the damage caused by acid (pH 1.5).
For this procedure, tissues were embedded in optimal cutting temperature medium, frozen, and sectioned at 5 μm. Tissue sections were blocked with 2% Bovine serum albumin followed by incubation with rabbit anti-occludin polyclonal antibody (1:150, Zymed, San Francisco, CA, United States) overnight at 4 °C. Sections were washed with PBS and incubated for 45 min with FITC-conjugated anti-rabbit secondary antibody. Sections were mounted in fluorescent mounting medium, and well-orientated gastric pits were examined with a photomicroscope linked to a digital camera.
Following Ussing chamber experiments, gastric mucosal samples were snap frozen and stored at -70 °C prior to performing sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Tissue aliquots were thawed at 4 °C and added to chilled lysis buffer, including protease inhibitors (0.5 mmol/L Pefabloc, 0.1 mmol/L 4-nitrophenyl phosphate, 0.04 mmol/L glycerophosphate, 0.1 mmol/L Na3VO4, 40 μg/mL bestatin, 2 μg/mL aprotinin, 0.54 μg/mL eupeptic, and 0.7 μg/mL pepstatin A) (Sigma-Aldrich Inc., St. Louis, MO, United States) at 4 °C. This mixture was homogenized on ice and then centrifuged at 4 °C, and the supernatant was saved. Protein analysis of extract aliquots was performed (BCA Protein Assay Kit, Pierce, Rockford, IL, United States). Tissue extracts (amounts equalized by protein concentration) were mixed with an equal volume of 2 × SDS-PAGE sample buffers and boiled for 4 min. Lysates were loaded on an SDS polyacrylamide gradient gel, and electrophoresis was carried out according to standard protocols. Proteins were transferred to a polyvinylidene fluoride membrane (Immobilon®, Millipore, Billerica, MA, United States) by using an electro blotting minitransfer apparatus. Membranes were blocked at room temperature for 2 h in Tris-buffered saline plus 0.05% tris-buffered saline tween-20 and 5% dry powdered milk, and then incubated overnight in primary antibody at 4 °C in rabbit anti-rat ClC-2 (Alpha Diagnostics, San Antonio, TX, United States), rabbit anti-CFTR (Santa Cruz Biotech Inc., CA, United States) or rabbit anti-occludin (Zymed Laboratories Inc., San Francisco, CA, United States). After multiple washings in TBST, membranes were incubated with horseradish peroxidase conjugated secondary antibody, and developed for visualization of protein with luminol enhancer solution (Pierce, Rockford, IL, United States).
For preparation of detergent soluble and detergent insoluble fractions of gastric mucosa, the tissue samples were extracted in lysis buffer (20 mmol/L Tris, 5 mmol/L MgCl2, 0.3 mmol/L EGTA, 210 μg/mL sodium fluoride, 18.5 μg/mL sodium orthovanadate, 30 mmol/L sodium pyrophosphate, and complete mini Protease inhibitor cocktail tablet (Thermo Fisher Scientific, Rockford, IL, United States). Following brief centrifugation to remove debris, Triton X-100 soluble and insoluble fractions were collected by incubation and centrifugation (50 000 g for 30 min at 4 °C) with lysis buffer containing 0.5% Triton X-100 and 0.5% SDS, respectively. The samples were processed through an SDS sample preparation kit (Thermo Fisher Scientific, Rockford, IL, United States) and a protein assay was performed before proceeding with western blotting.
Data were reported as mean ± SE. For Ussing chamber experiments, an experimental number of 6 was used. Data was analyzed by using an analysis of variance and t-test using a commercial statistical package (Sigmastat, Systat Software, San Jose, CA, United States). P < 0.01 and P < 0.05 were considered significant.
To establish a gastric acid injury model, porcine gastric mucosa was mounted on Ussing chambers. After an equilibration period of 30 min the mucosal side was subjected to normal Ringer’s (NR) solutions (pH 7.4) or HCl Ringer’s (pH 1.5) for up to 180 min. Acid-bathed tissues had a significant decline in TER by 90 min and a further decline by 180 min (approximately 50% and 150% change in TER following 90 and 180 min of exposure to HCl, respectively, Figure 1A). Histological examination of the gastric mucosa exposed to acid for 90 min revealed injury that was limited to mucosal sloughing and partial damage to the gastric pits, resembling acute peptic ulcer disease. Alternatively, 180 min exposure to acid caused profound sloughing of the mucosal lining with damage extending into the gastric pits. Thus for further studies, gastric mucosa was exposed to HCl (pH 1.5) for 90 min to establish a more clinically relevant level of acid-induced gastric injury (data not shown).
As previously described, porcine gastric mucosa was mounted on Ussing chambers and the mucosal surface was exposed to acid Ringer’s (pH 1.5) for 90 min. Mucosa subjected to acid injury had significantly lower TER values when compared to the gastric mucosa bathed in NR, indicating electrophysiological evidence of disruption of epithelial barrier function. Alternatively, pretreatment of the apical side of gastric mucosa with the ClC-2 agonist SPI-8811 (1 μmol/L) nullified decreases in TER due to acid injury (Figure 1A). Epithelial permeability was assessed by mucosal-to-serosal fluxes of 3H-mannitol in control and acid-injured tissues in the presence or absence of apical SPI-8811. In agreement with TER responses, acid-injured tissues exhibited increases in permeability to 3H-mannitol as compared to control uninjured tissue. Pretreatment of the apical surface of mucosal tissues SPI-8811 ablated changes in permeability noted in untreated acid-injured tissues (Figure 1B).
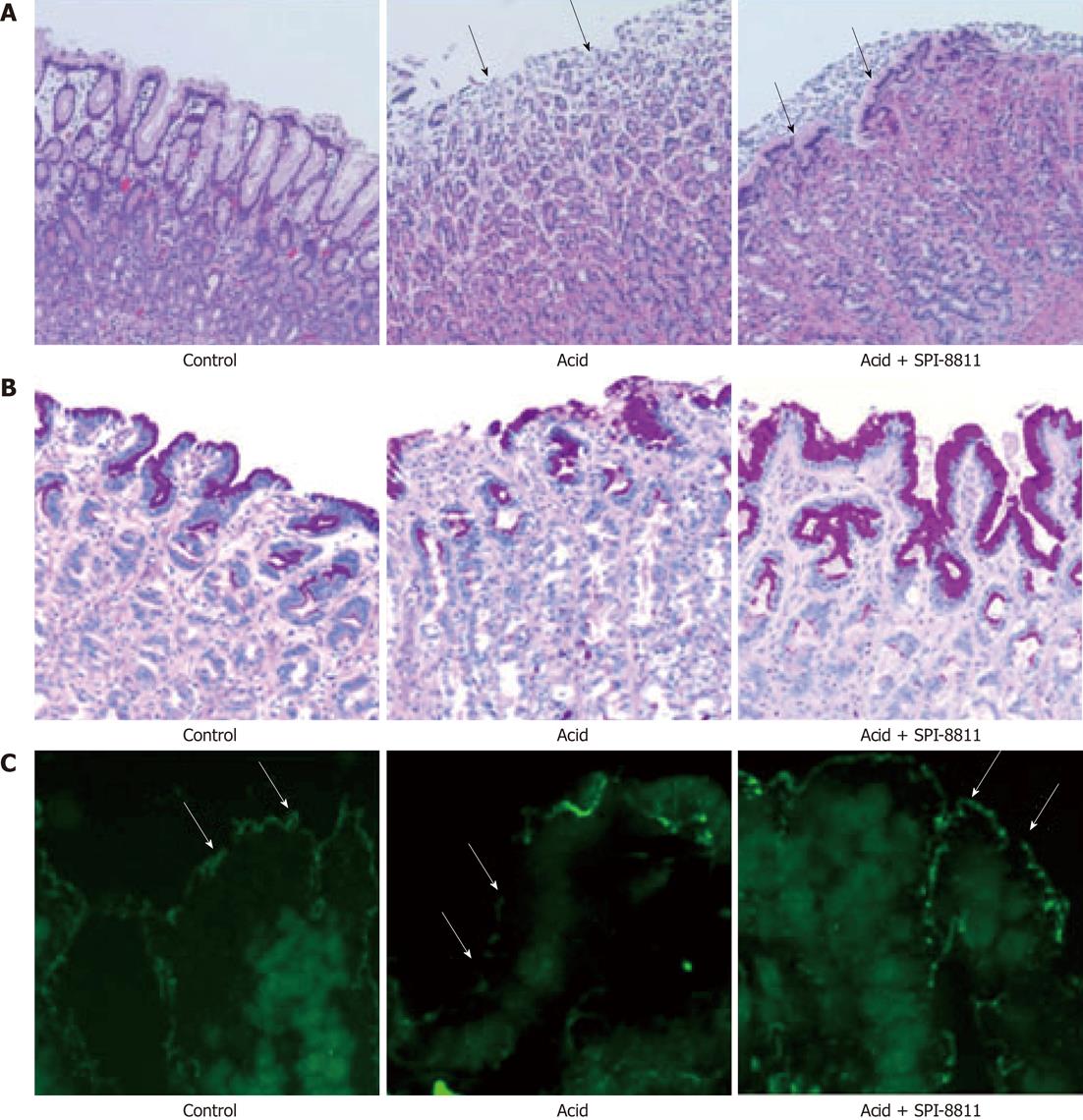
Gastric mucosa from Ussing chambers after 90 min in NR revealed intact gastric pits with intact epithelial lining. Alternatively, when the gastric mucosa was exposed to acid (pH 1.5) over a 90 min period, extensive epithelial sloughing and erosion extending into the glandular region of the gastric mucosa and sub-epithelium was noted. Tissues pretreated with SPI-8811 had far lesser evidence of mucosal injury in response to acid, although the epithelium appeared to have flattened to maintain the barrier and the gastric pits appeared dilated (Figure 2A).
Using PAS staining for mucus we observed that the loss of mucus by acid injury was seen restored by pretreatment of ClC-2 agonist (Figure 2B). In further experiments, we performed Immunofluorescence of occludin to assess the gastric mucosal TJs. As compared to apical immunolocalization of occludin in the control uninjured gastric mucosal lining epithelium, acid-injured tissues revealed loss of occludin immunofluorescence (Figure 2C). In contrast, gastric mucosa pretreated with SPI-8811 and subsequently exposed to acid for 90 min had evidence of localization of occludin to the TJ.
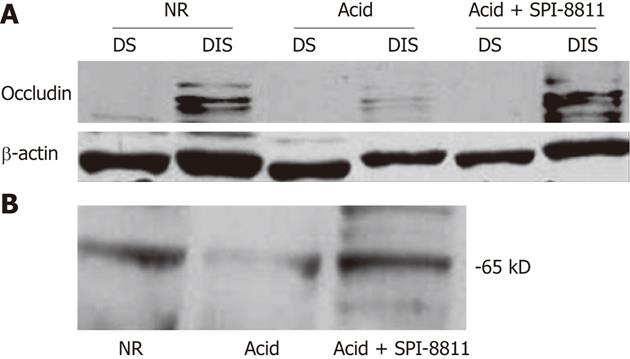
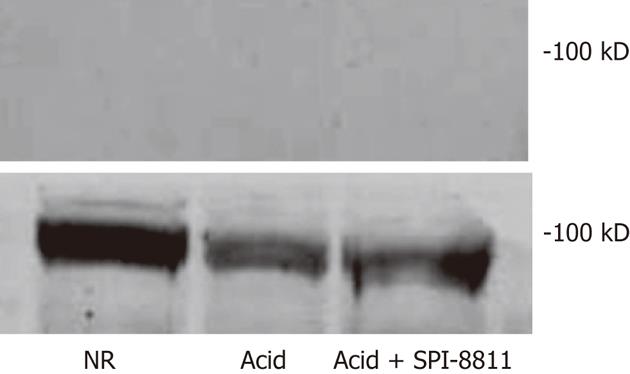
Since SPI-8811 prevented increases in paracellular permeability in response to acid, based on reduced 3H-mannitol fluxes and apical epithelial localization of occludin, we evaluated expression of occludin. Occludin expression was studied in detergent soluble and detergent insoluble gastric mucosal fractions, using-actin expression as a loading control. Tissues exposed to NR had expression of multiple bands clustered at 65 kD in the detergent insoluble fraction. The finding of several bands for occludin at its expected molecular weight has been attributed to multiple occludin phosphorylation states[26]. The presence of occludin solely in the insoluble fraction was interpreted as an indication of this protein’s propensity to localize to the TJ. In contrast to tissues bathed in NR, there was very little expression of occludin in acid-treated tissues, possibly because acid injury was severe enough to cause surface epithelium and interepithelial TJs. On the other hand, occludin expression in the detergent insoluble fraction of tissues treated with SPI-8811 appeared similar to tissues treated with Ringer’s solution alone, suggesting this prostone prevented loss of occludin. Cellular expression of occludin as a whole was also seen markedly reduced compared to control tissue and this loss was prevented with pretreatment with SPI-8811(Figure 3).
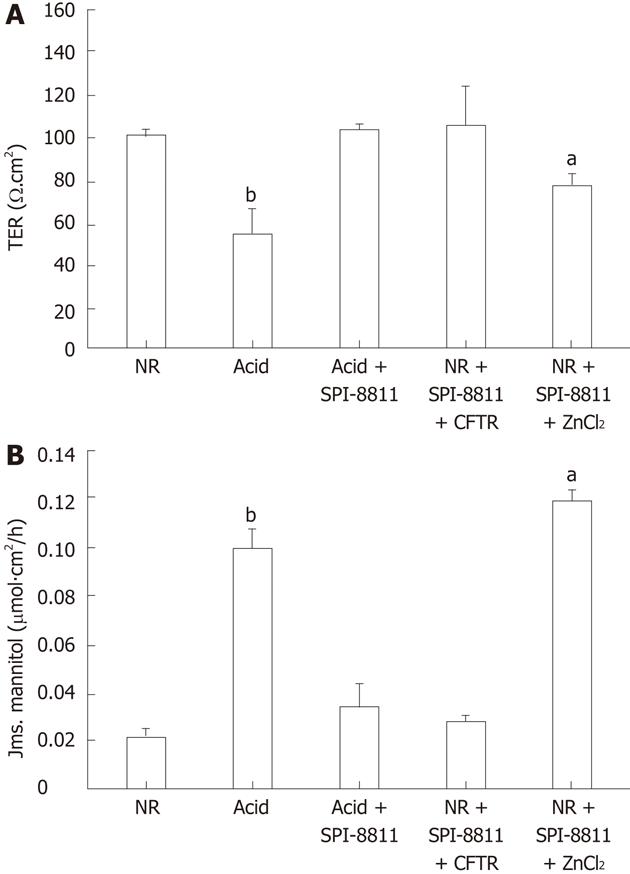
To explore the potential role of Cl- secretion in SPI-8811-associated gastro protection, mucosa was treated with the NKCC1 inhibitor bumetanide (100 μmol/L, basolateral) and SPI-8811 (1 μmol/L, apical), which blocked the ability of SPI-8811 to reduce acid injury (data not shown). In more targeted studies in tissues pretreated with SPI-8811, tissues were pretreated with pharmacological inhibitors of the apical chloride channels ClC-2 and CFTR. Pretreatment of acid-injured mucosa with the ClC-2 inhibitor ZnCl2 (300 μmol/L, apical) abolished the gastro protective properties of SPI-8811 as determined by change in TER and mannitol fluxes. On the other hand, pretreatment with CFTR inhibitor 172 (10 μmol/L, apical) had no effect (Figure 4).
The literature is not clear about the expression of ClC-2 and CFTR in the porcine gastric mucosa, aside from a few studies that describe gastric mucosal changes in CFTR mouse mutants[19]. Therefore, western analyses were performed to study the expression of ClC-2 and CFTR in porcine gastric mucosa. As shown in Figure 5, ClC-2 was expressed approximate 98 kD in porcine gastric homogenates. ClC-2 expression was observed in porcine gastric mucosa which confirmed the expression of ClC-2 in porcine stomach (Figure 5). Western analyses showed no evidence of CFTR in gastric mucosa, whereas CFTR was clearly present in porcine jejunum as a positive control (data not shown).
Prior studies have provided evidence for a critical role of ClC-2 in the recovery of mucosal barrier function in ischemic-injured intestine[20,21,27,28]. For example, Lubiprostone has been shown to interact with ClC-2 channels and enhance repair of the epithelial barrier after acute injury by ischemia[22,25,29,30]. The aim of the present study was to determine if an alternate prostone, SPI-8811, had a protective role against mucosal injury, and we chose to investigate this in the stomach. Acid plays an integral role in gastric mucosal ulceration[1,3,4,8,15,24,31]. Considering the physiological presence of acid to aid digestion in the stomach, mechanisms to provide gastro protection against injury in predisposed patients are critical. To date, this has principally included agents that ameliorate acid secretion. However, according to the results of the present study, prostones appear to provide protection in the face of low levels of pH, which would allow for continued acid secretion for normal digestive processes. Acid causes injury to the gastric mucosa in part by disrupting the TJ protein complexes, thereby increasing epithelial permeability. This in turn allows further injury by permitting permeability to luminal acid, ultimately causing erosion and then ulceration as the mucosa becomes progressively disrupted[3,14,24,32]. Among the established methods to study gastro protection and acute gastric injury are the direct application of acid or ethanol to gastric mucosa[8,31].
The first aim of our study was to produce acute acid injury resembling ulceration in clinical patients in order to study gastro protection. Accordingly, acid Ringer’s solution was applied to the mucosal surface as previously described[33]. To mimic peptic ulcer disease, the body of the porcine stomach was used in this study. Optimum gastric injury was achieved (based on TER and histological examination) with a pH of 1.5, over a time period of 90 min. In each of the experiments, SPI-8811 was applied to the gastric mucosa prior to acid injury on the apical surface. Earlier studies have shown that Lubiprostone activates Cl- secretion by a mechanism associated with recruitment of the TJ occludin in porcine intestine, thereby aiding recovery of barrier function[9,22,34,35]. Gastric mucosa was pretreated with SPI-8811 prior to induction of acid injury and showed evidence of gastro protection as evidenced by blockade of changes in TER and mannitol fluxes in tissues exposed to acid. In addition, histological changes in response to acid were ameliorated by SPI-8811. Because of the important role of TJs in maintenance of barrier function, we also examined the localization of occludin in tissues pretreated with SPI-8811 and exposed to acid. Tissues injured by acid alone appeared to have complete loss of apical epithelial occludin, possibly because of loss of cells due to severe injury, whereas tissues pretreated with SPI-8811 had evidence of apical epithelial occludin similar to that of control tissues. Further studies evaluating detergent soluble and insoluble fractions using western analyses confirmed the loss of occludin in tissues treated with acid. The reason for the loss of occludin, rather than intracellular movement of occludin, is unknown. The simplest explanation would be that the extensive reduction in pH resulted in loss of TJ proteins into the lumen, followed by erosion of epithelium and ultimately ulceration. Tissues pretreated with SPI-8811 evaluated by immunofluorescence were protected from this effect, with localization of occludin to the apical epithelium similar to that of control tissues. Additionally, occludin remained in the detergent insoluble fraction in western analyses under the influence of SPI-8811 indicating localization to the TJs.
The next aim of the study was to investigate the role of Cl- channels in gastro protection, given that SPI-8811 is a purported Cl- secretagogue. Because ClC-2 has been shown to be localized to interepithelial TJs in intestinal studies[22], and in gastric mucosa in the parietal cells[29,36] is a target for the prostones, we were particularly interested in this channel. The CFTR served as an alternate possibility. Our data demonstrated that SPI-8811-mediated gastro protection against acid injury was attenuated by the ClC-2 inhibitor ZnCl2 whereas the selective CFTR inhibitor 172 had no effect. Although CFTR is difficult to pharmacologically inhibit, our Western analyses showed a lack of CFTR expression in porcine gastric mucosa as compared to robust expression in jejunal mucosa. In previous studies, investigators have shown that CFTR knockout mice are vulnerable to gastric ulcers due to loss of bicarbonate secretion. The lack of CFTR in porcine gastric mucosa especially in the fundus of stomach might partially explain susceptibility to acid injury in the present studies[32]. Taken together, these studies suggest that activation of ClC-2 by SPI-8811 is integral to the mechanism of gastro protection. Further studies will be required to understand the mechanisms underlying these findings.
There are opposing views regarding the role of ClC-2 in gastric chloride secretion[29,36,37] suggested involvement of ClC-2 in gastric acid secretion while another other group[38], Indicated that ClC-2 chloride secretion plays no role in production of HCl. It is also noteworthy that the apical ClC-2 channel has been suggested to serve as a route for both bicarbonate and Cl- exit into the lumen[29,39]. This raises the possibility that ClC-2-mediated bicarbonate secretion attenuates acid injury to some extent by raising the pH. However in our studies, constant monitoring and adjustment of pH to a level of 1.5 would nullify this possibility. We also found that SPI-8811 produces a gastro protective effect not only via increasing gastric pH, but also by protecting and restoring the gastric mucus level (Figure 2B). The importance of gastro protection via mucus protection has been previously shown[7,40,34].
ClC-2 localizes to the TJ in the intestine[21,27,28,41] and this localization facilitates the interactions with TJ proteins and associated regulatory molecules. For example, recent studies have shown that ClC-2 is required for rapid reassembly of TJ proteins after ischemic injury in murine intestine[27]. The rapid process of repair is a hallmark of gastric mucosal barrier function, and may involve rapid restoration of TJs during restitution. Although this study does not completely answer questions as to the mechanism of SPI-8811, it does provide convincing evidence for the ability of SPI-8811 to protect the gastric mucosa against acid, and it does suggest this activity is at least in part attributable to a mechanism related to ClC-2 regulation.
Important gastric diseases such as peptic ulcer disease are aggravated by acid secretion. Injury caused by acid is characterized by damage to the gastric epithelium lining the gut. Mechanisms are in place to rapidly repair epithelial defects, and prevent epithelial barrier dysfunction and thus protect the gastric mucosa and further acid injury. Currently studies have shown the importance of the interepithelial tight junctions (TJs) in recovery of the epithelial barrier. Studies, particularly in the intestine, have shown particularly in that prostones increase the rate of epithelial recovery via re-assembly of TJs.
The prostone lubiprostone, a new medication on the market indicated for the treatment of chronic constipation and irritable bowel syndrome has its effect on the chloride channel ClC-2. This channel is unusual in that it is located within TJs. These channels are involved in secretion of chloride in the intestine, but until now very little was about the role of ClC-2 in the stomach. Recent studies have shown that ClC-2, when activated by prostones, play an important role in re-assembly of TJs, resulting in increased rate of epithelial repair in the intestine. This study was performed to see if a prostone (cobiprostone, SPI-8811) hastened repair in the gastric mucosa via a mechanism involving TJs.
Activation of one of the chloride channels, ClC-2, is an innovative way to induce reassembly of TJs during repair of the epithelial mucosal barrier. Gastric epithelium is continuously exposed to gastric acid and in the case of peptic ulcer disease the gastric acid secretion disrupts the barrier and thus aggravates the condition. In such cases, reducing acid secretion by using various acid secretory antagonists is the most common way to prevent gastric injury. Other alternatives to prevent breakdown of the gastric barrier function are limited. The present data show an innovative breakthrough in the treatment of acid induced gastric epithelium. The authors’ findings were consistent with prior studies showing enhanced repair with prostones in the intestine at the level of TJs.
By understanding the role of prostone activation of ClC-2 in acid injured gastric porcine mucosa, this study might represents a future strategy to therapeutic intervention in patients with non-steroidal anti-inflammatory drugs injury and peptic ulcer disease. Further basic science research followed by clinical trials will be needed to determine the validity of these findings.
The term ClC-2 is used to describe a chloride channel in the gut epithelium that is localized to interepithelial TJs. The term prostone refers to a new group of compounds which are distinct from prostaglandins and specifically activate ClC-2.
The present study was performed using porcine tissues; it demonstrates that the novel ClC-2 agonist SPI-8811 stimulates recovery of barrier function in acid injured porcine gastric mucosa by preventing loss of the TJ protein occludin. The data were appropriately analyzed and indicate SPI-8811 has an impact on protecting epithelial barrier function by preventing breakdown of TJs and thus prevent further deep injury. Additional molecular studies need to be performed to understand how ClC-2 interacts with prostones to protect the epithelial TJs.
Peer reviewers: Wojciech Blonski, MD, PhD, University of Pennsylvania, GI Research-Ground Centrex, 3400 Spruce St, PA 19104, United States; Nikolaus Gassler, Professor, Institute of Pathology, University Hospital RWTH Aachen, Pauwelsstrasse 30, 52074 Aachen, Germany
S- Editor Gou SX L- Editor A E- Editor Xiong L
.
| 1. | Montrose MH. Choosing sides in the battle against gastric acid. J Clin Invest. 2001;108:1743-1744. [PubMed] |
| 2. | Park SH, Cho CS, Lee OY, Jun JB, Lin SR, Zhou LY, Yuan YZ, Li ZS, Hou XH, Zhao HC. Comparison of Prevention of NSAID-Induced Gastrointestinal Complications by Rebamipide and Misoprostol: A Randomized, Multicenter, Controlled Trial-STORM STUDY. J Clin Biochem Nutr. 2007;40:148-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Tarnawski A. Molecular mechanisms of ulcer healing. Drug News Perspect. 2000;13:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Tarnawski AS. Cellular and molecular mechanisms of gastrointestinal ulcer healing. Dig Dis Sci. 2005;50 Suppl 1:S24-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 289] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 5. | Romano M, Razandi M, Ivey KJ. Protection of gastric epithelial cell monolayers from a human cell line by omeprazole in vitro. Scand J Gastroenterol. 1989;24:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Romano M, Razandi M, Ivey KJ. Role of sulphydryl compounds in the defense of rat gastric epithelial cells against oxygen reactive metabolite-induced damage. Ital J Gastroenterol. 1991;23:55-59. [PubMed] |
| 7. | Romano M, Razandi M, Ivey KJ. Effect of cimetidine and ranitidine on drug induced damage to gastric epithelial cell monolayers in vitro. Gut. 1989;30:1313-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Okabe S, Amagase K. An overview of acetic acid ulcer models--the history and state of the art of peptic ulcer research. Biol Pharm Bull. 2005;28:1321-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 163] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Okabe S, Amagase K. [An overview of acetic acid ulcer models and their utility for drug screening]. Nihon Yakurigaku Zasshi. 2003;122:73-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Suzuki T, Yoshida N, Nakabe N, Isozaki Y, Kajikawa H, Takagi T, Handa O, Kokura S, Ichikawa H, Naito Y. Prophylactic effect of rebamipide on aspirin-induced gastric lesions and disruption of tight junctional protein zonula occludens-1 distribution. J Pharmacol Sci. 2008;106:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Wallace JL, Granger DN. The cellular and molecular basis of gastric mucosal defense. FASEB J. 1996;10:731-740. [PubMed] |
| 12. | Aihara E, Hayashi S, Amagase K, Kato S, Takeuchi K. Prophylactic effect of rebamipide against the irritative and healing impairment actions of alendronate in rat stomachs. Inflammopharmacology. 2007;15:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Arakawa T, Kobayashi K, Yoshikawa T, Tarnawski A. Rebamipide: overview of its mechanisms of action and efficacy in mucosal protection and ulcer healing. Dig Dis Sci. 1998;43:5S-13S. [PubMed] |
| 14. | Allen A, Flemström G, Garner A, Kivilaakso E. Gastroduodenal mucosal protection. Physiol Rev. 1993;73:823-857. [PubMed] |
| 15. | Allen A, Flemström G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Cell Physiol. 2005;288:C1-19. [PubMed] |
| 16. | Bi LC, Kaunitz JD. Gastroduodenal mucosal defense: an integrated protective response. Curr Opin Gastroenterol. 2003;19:526-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Rainsford KD. Mechanisms of gastrointestinal damage by NSAIDS. Agents Actions Suppl. 1993;44:59-64. [PubMed] |
| 18. | Ito S, Lacy ER, Rutten MJ, Critchlow J, Silen W. Rapid repair of injured gastric mucosa. Scand J Gastroenterol Suppl. 1984;101:87-95. [PubMed] |
| 19. | Matter K, Balda MS. Occludin and the functions of tight junctions. Int Rev Cytol. 1999;186:117-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev. 2007;87:545-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 422] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 21. | Moeser AJ, Haskell MM, Shifflett DE, Little D, Schultz BD, Blikslager AT. ClC-2 chloride secretion mediates prostaglandin-induced recovery of barrier function in ischemia-injured porcine ileum. Gastroenterology. 2004;127:802-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Moeser AJ, Nighot PK, Engelke KJ, Ueno R, Blikslager AT. Recovery of mucosal barrier function in ischemic porcine ileum and colon is stimulated by a novel agonist of the ClC-2 chloride channel, lubiprostone. Am J Physiol Gastrointest Liver Physiol. 2007;292:G647-G656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Blikslager AT, Roberts MC, Rhoads JM, Argenzio RA. Prostaglandins I2 and E2 have a synergistic role in rescuing epithelial barrier function in porcine ileum. J Clin Invest. 1997;100:1928-1933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 113] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Takezono Y, Joh T, Oshima T, Suzuki H, Seno K, Yokoyama Y, Alexander JS, Itoh M. Role of prostaglandins in maintaining gastric mucus-cell permeability against acid exposure. J Lab Clin Med. 2004;143:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Bao HF, Liu L, Self J, Duke BJ, Ueno R, Eaton DC. A synthetic prostone activates apical chloride channels in A6 epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008;295:G234-G251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Wong V. Phosphorylation of occludin correlates with occludin localization and function at the tight junction. Am J Physiol. 1997;273:C1859-C1867. [PubMed] |
| 27. | Nighot PK, Blikslager AT. ClC-2 regulates mucosal barrier function associated with structural changes to the villus and epithelial tight junction. Am J Physiol Gastrointest Liver Physiol. 2010;299:G449-G456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Nighot PK, Moeser AJ, Ryan KA, Ghashghaei T, Blikslager AT. ClC-2 is required for rapid restoration of epithelial tight junctions in ischemic-injured murine jejunum. Exp Cell Res. 2009;315:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Malinowska DH. Cl- channel blockers inhibit acid secretion in rabbit parietal cells. Am J Physiol. 1990;259:G536-G543. [PubMed] |
| 30. | Cuppoletti J, Malinowska DH, Tewari KP, Li QJ, Sherry AM, Patchen ML, Ueno R. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol. 2004;287:C1173-C1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 183] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Morris GP, Wallace JL. The roles of ethanol and of acid in the production of gastric mucosal erosions in rats. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;38:23-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 102] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Akiba Y, Furukawa O, Guth PH, Engel E, Nastaskin I, Sassani P, Dukkipatis R, Pushkin A, Kurtz I, Kaunitz JD. Cellular bicarbonate protects rat duodenal mucosa from acid-induced injury. J Clin Invest. 2001;108:1807-1816. [PubMed] |
| 33. | Argenzio RA, Eisemann J. Mechanisms of acid injury in porcine gastroesophageal mucosa. Am J Vet Res. 1996;57:564-573. [PubMed] |
| 34. | Romano M, Razandi M, Ivey KJ. Effect of ranitidine on taurocholate-, ethanol-, and indomethacin-induced damage to gastric epithelial cells in vitro. Digestion. 1989;43:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Romano M, Razandi M, Sekhon S, Krause WJ, Ivey KJ. Human cell line for study of damage to gastric epithelial cells in vitro. J Lab Clin Med. 1988;111:430-440. [PubMed] |
| 36. | Sherry AM, Malinowska DH, Morris RE, Ciraolo GM, Cuppoletti J. Localization of ClC-2 Cl- channels in rabbit gastric mucosa. Am J Physiol Cell Physiol. 2001;280:C1599-C1606. [PubMed] |
| 37. | Kosiek O, Busque SM, Föller M, Shcheynikov N, Kirchhoff P, Bleich M, Muallem S, Geibel JP. SLC26A7 can function as a chloride-loading mechanism in parietal cells. Pflugers Arch. 2007;454:989-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Hori K, Takahashi Y, Horikawa N, Furukawa T, Tsukada K, Takeguchi N, Sakai H. Is the ClC-2 chloride channel involved in the Cl- secretory mechanism of gastric parietal cells? FEBS Lett. 2004;575:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Hogan DL, Crombie DL, Isenberg JI, Svendsen P, Schaffalitzky de Muckadell OB, Ainsworth MA. Acid-stimulated duodenal bicarbonate secretion involves a CFTR-mediated transport pathway in mice. Gastroenterology. 1997;113:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Rainsford KD, Willis C. Relationship of gastric mucosal damage induced in pigs by antiinflammatory drugs to their effects on prostaglandin production. Dig Dis Sci. 1982;27:624-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 110] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Gyömörey K, Yeger H, Ackerley C, Garami E, Bear CE. Expression of the chloride channel ClC-2 in the murine small intestine epithelium. Am J Physiol Cell Physiol. 2000;279:C1787-C1794. [PubMed] |