Published online Sep 14, 2012. doi: 10.3748/wjg.v18.i34.4677
Revised: December 5, 2011
Accepted: March 28, 2012
Published online: September 14, 2012
Chronic hepatitis B virus (HBV) infection affects about 350 million individuals worldwide. Management of HBV infection in pregnancy is difficult because of several peculiar and somewhat controversial aspects. The aim of the present review is to provide a tool that may help physicians to correctly manage HBV infection in pregnancy. This review focuses on (1) the effect of pregnancy on HBV infection and of HBV infection on pregnancy; (2) the potential viral transmission from mother to newborn despite at-birth prophylaxis with immunoglobulin and vaccine; (3) possible prevention of mother-to-child transmission through antiviral drugs, the type of antiviral drug to use considering their efficacy and potential teratogenic effect, and the timing of their administration and discontinuation; and (4) the evidence for the use of elective caesarean section vs vaginal delivery and the possibility of breastfeeding.
- Citation: Borgia G, Carleo MA, Gaeta GB, Gentile I. Hepatitis B in pregnancy. World J Gastroenterol 2012; 18(34): 4677-4683
- URL: https://www.wjgnet.com/1007-9327/full/v18/i34/4677.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i34.4677
Chronic hepatitis B virus (HBV) infection affects about 350 million individuals worldwide: half of them acquired the infection either perinatally or in early childhood, especially in endemic areas[1,2]. The estimated burden of death associated with HBV infection is 500 000-1.2 million per year. Progression of the disease towards cirrhosis, liver failure or hepatocellular carcinoma occurs in 15%-40% of infected subjects. The risk of chronic infection is inversely proportional to the age at which the infection was acquired[3].
HBV infection in pregnancy has several peculiar aspects among which are the effect of pregnancy on HBV infection, the potential viral transmission from mother to newborn and its possible prevention through antiviral drugs, and the potential teratogenic effect of these drugs. These elements can complicate the management of HBV infection in the setting of pregnancy[4]. The aim of this review was to examine recent studies devoted to the clinical, therapeutic, and prognostic aspects of HBV infection during pregnancy in order to extrapolate and interpret data that can help physicians in the management of HBV infection in this setting.
In most cases, acute or chronic HBV infection in pregnancy is similar to that in the general adult population: HBV infection does not increase the mortality and it does not yield teratogenic effects. However, a higher incidence of low birth weight and prematurity has been reported during acute infection than in the general population[1], whereas gestational diabetes mellitus, antepartum hemorrhage and preterm delivery are more frequent in chronic maternal HBV infection than in the general population[1]. Acute infection must be differentiated from other acute liver diseases that occur during pregnancy such as intrahepatic cholestasis[1].
Differently, the management of more advanced disease (cirrhosis) may be troublesome. Patients with advanced cirrhosis usually are amenorrheic and infertile due to hypothalamic-pituitary dysfunction, but successful pregnancy may be completed in those with well-compensated disease[5]. In these cases, increased maternal and fetal problems can be expected in about 50% cases, with increased fetal loss[5]. The main risk to the mother is rupture of esophageal varices and consequent bleeding (20%-25%) especially during the second trimester or during labor. Other risks include hepatic decompensation, jaundice, and rupture of splenic aneurysms[5]. In the presence of known esophageal varices, women planning a pregnancy should be considered for endoscopic therapy, shunt surgery, or even liver transplantation before pregnancy. Even if varices are absent before pregnancy, all patients should undergo upper endoscopy for assessment of varices in the second trimester and, if large varices are present, beta blocker therapy must be administered despite occasional fetal effects[5]. Acute variceal bleeding is managed endoscopically, as in the non-pregnant setting, though vasopressin is contraindicated. Ascites and hepatic encephalopathy are treated in the usual way (i.e., use of diuretics, rifaximin, lactulose, etc.). Except in patients with known large varices, a vaginal delivery is preferable to avoid abdominal surgery[5].
Pregnancy in a liver transplant recipient represents a unique clinical situation: with the success of liver transplantation, more pregnancies are being reported in liver recipients, and a carefully planned pregnancy in a stable healthy patient, beyond the first 2 years after orthotopic liver transplantation, can have an excellent outcome for fetus, mother, and the graft. However, this is still a high-risk pregnancy with increased fetal prematurity and dysmaturity[5]. In addition, it entails some risk to the allograft from acute cellular rejection or recurrent viral hepatitis[5,6].
During pregnancy there are several modifications in the maternal immune system, namely, a shift in the Th1-Th2 balance towards a Th2 response, increased amounts of regulatory T cells, etc., that contribute to a depressed immune response against HBV. The aim of these modifications is to prevent the rejection of the fetus who is partially allogenic for the mother’s immune system. These modifications result in an increase of HBV DNA and a reduction of aminotransferase levels. After delivery the immune system is restored thereby causing opposite consequences; namely, there is a significant increase in alanine aminotransferase (ALT) and a reduction of HBV DNA in this period[7].
ter Borg et al[7] studied the course of the liver disease in 38 HBV-positive pregnancies before, during and after delivery. In 13 pregnancies, antiviral therapy with lamivudine was started during the last trimester of pregnancy due to high viremia to reduce the risk of mother-to-child transmission (see later) and was stopped immediately after delivery. Forty-five per cent of untreated women experienced a flare (defined as a three-fold increase in ALT) within 6 mo after delivery, compared to 62% of women who received lamivudine. No clinical decompensation occurred in these women. However, the authors recommend monitoring closely and, if necessary, administering treatment to women with chronic HBV shortly after delivery.
Perinatal transmission is a common mode of HBV transmission worldwide[1]. HBV infection in newborns is defined as hepatitis B surface antigen (HBsAg) positivity 6 mo after birth. Antibody to hepatitis B e antigen (anti-HBe) and anti-hepatitis B core antigen cross the placental barrier and disappear in nearly all babies before 12 and 24 mo of age, respectively[8]. Therefore, they simply represent the transplacental maternal antibodies and are not indicators of HBV infection status[8].
Without prophylaxis the risk of mother-to-child transmission is very high. It varies with the HBeAg/anti-HBe status of mothers, being 70%-90% for HBeAg-positive mothers, 25% for HBeAg-negative/HBeAb-negative mothers and 12% for HBeAg-negative/anti-HBe-positive mothers[9-12]. Maternal screening programs aimed at identifying HBsAg-positive mothers are part of pregnancy routine examinations in most countries. Once HBsAg-positive mothers are identified, their babies receive passive-active immunoprophylaxis at birth to reduce vertical HBV transmission[3]. Passive immunoprophylaxis consists of the administration of hepatitis B immune globulin (HBIG) whereas active immunoprophylaxis is the administration of hepatitis B vaccine[1,3].
Although this prophylaxis is effective in blocking HBV maternal transmission, a small but not negligible proportion of children (3%-13%) born from HBsAg-positive mothers, especially those carrying HBeAg, become HBsAg carriers despite correct passive-active immunoprophylaxis[1,10,12-14].
The biological explanation for the higher risk of transmission for HBeAg-positive mothers is that, differently from HBsAg, maternal HBeAg could pass through placenta from mother to fetus and induce T-cell tolerance in utero[8]. The intra-uterine infection of HBV, whose mechanism remains unclear, is the major cause of unsuccessful immunological blockade. High levels of serum HBV DNA in pregnant women is the main risk factor for the occurrence of HBV intra-uterine infection: it correlates with the cord blood HBV DNA level and HBsAg titer[12,15]. It has also been demonstrated that HBV can infect all kinds of cells in the placenta (decidual, trophoblastic, villous mesenchymal, villous capillary endothelial cells) and that HBV DNA is present in all generations of spermatogenic cells and sperms in HBV-infected males, in follicular fluid and in ovary (cellular transfer)[15,16]. The presence of the virus in the spermatogenic cells can have a role in the transmission of HBV infection to the newborn, as a high homology between the father’s and the child’s viral sequences in discordant couples has been found[16].
The maternal HBeAg-positive serological status and high viral load are associated positively with intrauterine transmission of HBV, especially through villous capillary endothelial cells. In fact, HBeAg can pass through the placenta via partial placental leakage or via the “cellular route”[17]. The absence of HBeAg expression is associated with lower levels of viral replication and with a significantly lower risk of intrauterine transmission of HBV[17].
All decisions about the treatment of HBV in pregnancy must include an analysis of the risks and benefits for the mother and fetus. The major issue regarding the mother is the consequences of the treatment on short- and long-term liver disease outcomes. The major concern for the fetus is the risk of exposure to potentially teratogenic drugs during early embryogenesis[18].
Seven drugs have been approved by the United States Food and Drug Administration (FDA) for the treatment of hepatitis B: PEG-interferon alpha 2a, interferon alpha 2b, lamivudine, adefovir, entecavir, telbivudine and tenofovir[3] (Table 1).
| Drug | Pregnancy category |
| Lamivudine | C |
| Entecavir | C |
| Telbivudine | B |
| Adefovir | C |
| Tenofovir | B |
| Interferon alpha 2b | C |
| Pegylated-Interferon alpha 2a | C |
Interferon, contraindicated during pregnancy, can be used in women of childbearing age because it is usually given for a defined period (48-96 wk). The administration of interferon must be accompanied by the recommendation to use contraception during treatment[3,18].
The oral antiviral agents, namely nucleoside or nucleotide analogues that inhibit viral polymerase, are generally used for long periods. However, they also interfere with replication of mitochondrial DNA thereby resulting in potential mitochondrial toxicity; effects of which are poorly known in the developing fetus[18].
The FDA classifies drugs in five categories (A, B, C, D and X) according to their possible teratogenic effects in humans or animal models. The 5 oral nucleos(t)ide analogues for HBV treatment are classified as either a category B or a category C agent. Category C drugs, namely lamivudine, adefovir and entecavir, are those that exert teratogenic or embryocidal effects in animals and for which there are no controlled studies in humans[16]. Lamivudine is highly toxic in rabbits with first trimester exposure; however, because it was the first oral agent approved for the treatment of HBV, extensive clinical experience indicates a general lack of teratogenetic effects in humans[18].
Category B drugs, namely telbivudine and tenofovir, are those that, according to the results of animal studies, carry no teratogenic or embryogenic risk and for which there have been no controlled human studies or for which animal studies may indicate a risk but controlled human studies refute these findings[19]. Tenofovir has both a high power and a high genetic barrier to resistance. Telbivudine has a high power, but a low barrier to resistance[18].
Safety data on HBV antivirals during pregnancy come from two major sources: the Antiretroviral Pregnancy Registry (APR) and the Development of Antiretroviral Therapy Study (DART)[20,21], due to the fact that some analogues are active both against HBV and against human immunodeficiency virus (HIV). The APR, an international, voluntary, prospective registry, has analyzed as of January 2010 a cohort of 11 867 women exposed to antiretroviral therapies, most of whom are HIV-1 monoinfected and only 112 of whom are HBV monoinfected. The results indicate that the rate of birth defects among women exposed to HBV therapy (2.7% of live births) is similar to that in the general population (2.72% rate) as reported by the Centers for Disease Control and Prevention (CDC) birth defect surveillance system. No significant difference was reported in the rate of adverse outcomes if the initial exposure of any HBV drug occurred in the first trimester (2.7%) compared to the second or third trimester (2.5%) of pregnancy. Lamivudine and tenofovir are the two agents with the most in vivo experience in the first trimester, and these appear to be safe. For telbivudine and entecavir, only 5 and 12 pregnancies with exposure in the first trimester are recorded in this registry, with no adverse outcomes reported. The APR has some limitations: short follow-up and recording only defects identified at birth. Therefore, developmental anomalies (e.g., cardiac or neurologic defects) identified at a later date may be omitted[19].
The DART study is a 6-year, multicenter, randomized trial of antiretroviral therapy among adults with symptomatic HIV-1 infection or advanced disease/AIDS in Africa. The rate of congenital anomalies reported in this study is 3% and compares favorably to the 2.72% reported by the CDC birth defect surveillance system[21].
It is difficult to decide what anti-HBV therapy to administer in pregnant women. In addition to parameters usually considered in decision-making about hepatitis B therapy (age, stage of disease, comorbidity, viral load, genotype, power of the agent, genetic barrier to resistance, etc.), specific issues in the treatment choice for women of childbearing age include safety of drugs in pregnancy and breastfeeding, and proposed length of therapy[1].
In the case of untreated women who are “planning” a pregnancy, it may be prudent to delay therapy until after the child is born. For example, if the woman is in the immune-tolerant phase of infection (high HBV DNA levels with normal ALT and inactive liver biopsy), treatment can usually be postponed until after delivery. However, should the woman be HBeAg-positive with a high viral load, a 3rd trimester prophylaxis to reduce transmission could be initiated in case of pregnancy (see later)[3].
For pregnant women already in treatment, in the case of significant fibrosis, therapy should be continued to reduce the risk of decompensation of liver disease. This would have a negative impact on the health of the fetus. In this case, it is preferable to switch to an agent that is safe in pregnancy and which has a long in vivo experience in this setting[5].
In summary, the choice of the anti-HBV drug to administer in a pregnant woman depends on whether the aim of treatment is to treat an active liver disease for which therapy cannot be delayed, or to prevent transmission of infection to the fetus from high viremia mothers without significant liver disease. A third decision-requiring condition is that of women who become pregnant while on anti-HBV therapy in whom the drug can be continued, stopped or changed into a class B drug.
There is no consensus about the therapy choice for pregnant women who are HBsAg-positive and highly viremic in the third trimester to prevent perinatal transmission[18].
In a pilot study published in 2003, eight women with HBV DNA levels greater than 109 copies/mL were given 150 mg of lamivudine daily during the last month of gestation: babies received passive and active immunization and only one became infected, compared to 7 of 25 (28%) cases of transmission in a matched historical control population[22].
A randomized, double-blind, placebo-controlled trial conducted with 150 HBsAg-positive highly viremic pregnant women evaluated whether lamivudine administered from the 32nd week of gestation to 4th week post-partum prevented HBV transmission to newborns who received standard prophylaxis with HBIG and vaccination. All but one woman were HBeAg-positive. At 1 year of age, 18% of babies from lamivudine-treated mothers were HBsAg-positive compared to 39% in the placebo-treated arm in intention-to-treat analysis (P = 0.014). Based on these results, Xu et al[23] recommended treatment in the third trimester for women with high viral loads. However, these data should be interpreted with caution due to a high dropout rate (13% in the lamivudine group and 31% in the placebo group). No safety concerns were noted in the lamivudine-treated mothers and their infants.
A recent meta-analysis of 10 randomized controlled trials for a total of 951 HBV-positive mothers evaluated the efficacy of lamivudine in reducing in utero transmission of HBV[24]. The results confirm the safety of lamivudine and its efficacy in preventing HBV intrauterine infection and mother-to-child transmission. However, the limitation of meta-analysis is the different quality of the studies included and the heterogeneity of the cut-off values for high viral load that prompted therapy[24].
A non-randomized, controlled trial that included 190 HBsAg-positive, HBeAg-positive pregnant women with high viremia (> 106 copies/mL) evaluated the efficacy and safety of telbivudine vs placebo. In both groups, newborns were treated with standard active/passive prophylaxis within 24 h from birth. Telbivudine was given to mothers from 20 to 32 wk before delivery and was continued for 4 wk after delivery in the case of inactive disease and for 28 wk in the case of active chronic hepatitis. At birth, a significant reduction of HBsAg positivity rate was observed in babies born from telbivudine-treated mothers compared to the control group (6.3% vs 30.4%). Twenty-eight weeks after birth, the rate of HBsAg or HBV DNA positivity was significantly reduced in babies born from telbivudine-treated mothers compared to placebo-treated ones (2% vs 13%). Post-partum ALT flares occurred in 7.5% of telbivudine-treated women and in 18.5% of placebo-treated women. No safety concerns were observed for mothers or children in either group. No cases of severe hepatitis were observed in women who discontinued telbivudine at week 4 post-partum. These data confirm the need of antiviral therapy in HBeAg-positive pregnant women with high viremia (> 106 copies/mL) and the efficacy and safety of telbivudine in this setting[14].
Few data are available about the efficacy of tenofovir in pregnant women. Some information regarding toxicity of tenofovir comes from studies on animals, such as gravid rhesus monkeys, which found a reduction of circulating insulin-like growth factor in newborns and severe growth reduction in approximately 25% of the infant monkeys within 2 mo of maternal treatment[25-27]. However, given its potency and its high barrier to resistance, tenofovir would be expected to be at least equally as effective as lamivudine in reducing perinatal transmission; it is an attractive agent to consider in the third trimester[18].
Regarding the issue of an HBV-infected male spouse who is planning a baby, no data are available in terms of drug safety to the fetus.
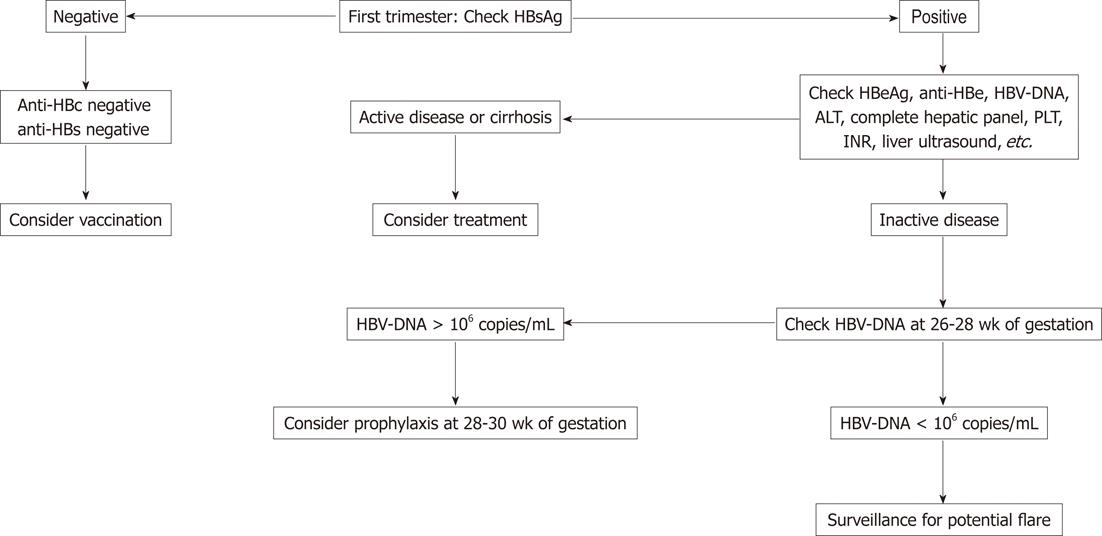
A proposed strategy in summarized in Figure 1. Briefly, every woman in the first trimester should be screened for HBV infection. If she is negative, she does not have to be routinely vaccinated during pregnancy, although it is considered safe and should therefore be administered to those with high risk behavior for acquisition. Her child will be vaccinated for hepatitis B together with other routine vaccines. If the pregnant woman is positive in early pregnancy, it is necessary to understand the status of the disease. If she has very active HBV (significantly elevated ALT with a high viral load), or if cirrhosis is suspected, therapy should be initiated regardless of trimester. If the woman has inactive disease (low ALT and low viral load), therapy is not warranted and continued surveillance is suggested because of the risk of a flare of hepatitis B later in pregnancy and for several months postpartum[3,18].
Quantification of HBV DNA is recommended in all infected women at the end of the second trimester (at 26-28 wk of gestation): if the viral load is >106 copies/mL, antiviral prophylaxis of HBV transmission to newborn can be initiated early in the third trimester (28-30 wk), even though there is no consensus or guidelines on this topic, with consideration to the potential mitochondrial toxicity of all nucleoside/nucleotide analogues. Similarly, there is no consensus regarding discontinuation of the drug and the time of discontinuation. The decision should take into account the pre-pregnancy stage of liver disease because of the potential risk of flare. In the absence of active disease or cirrhosis at baseline, treatment discontinuation 4 wk after delivery is reasonable. If the patient has advanced liver disease we do not recommend discontinuation. It should be stressed that breastfeeding while on treatment for HBV is not recommended (see later)[3,18].
A meta-analysis of four randomized trials, involving 789 pregnant women, assessed the efficacy and safety of elective caesarean section (ECS, caesarean section before labor or before ruptured membranes) vs vaginal delivery in preventing mother-to-child HBV transmission. It was found that ECS vs vaginal delivery significantly reduced the rate of maternal transmission of HBV (ECS: 10.5%; vaginal delivery: 28.0%) (relative risk: 0.41, 95% CI 0.28 to 0.60, P < 0.000 001)[28]. No data regarding maternal morbidity or infant morbidity according to mode of delivery were available. However, the conclusions of the meta-analysis must be viewed with great caution given the high risk of bias in each study included in the analysis. The role of ECS in preventing mother-to-child transmission of HBV is uncertain. Currently, there is no convincing evidence that ECS reduces the rate of mother-to-child transmission of HBV compared with vaginal delivery[28].
In 1975, before the availability of neonatal immunization, Beasley et al[29] reported a rate of HBV acquisition of 53% in breast-fed and of 60% in formula-fed babies born to HBsAg-positive mothers. With the introduction of immunoprophylaxis, a similar rate of infection in breast-fed and formula-fed infants (0% and 3%) was found[30]. Although the high vertical transmission rates confounded the true rate of acquisition from breastfeeding, the current guidelines state that breastfeeding is not contraindicated in HBV-infected mothers who are not on antiviral therapy and whose infants receive immunoprophylaxis[31]. For mothers on antiviral therapy with lamivudine or tenofovir, breastfeeding is not recommended because few data are available about the safety of antiviral exposure during breastfeeding[18].
Testing for HBsAg is recommended for every pregnant woman, regardless of previous testing or vaccination. Identification of HBV-positive pregnant women remains the most effective way to prevent HBV transmission to newborns thanks to a very effective passive/active prophylaxis at birth. However, in the case of women with very high viremia, a non-negligible proportion of newborns can acquire the infection (probably through in utero transmission) despite the use of passive/active prophylaxis. For this reason, antiviral treatment in the third trimester can be considered for those women. The choice of antiviral should be restricted to those drugs considered safe in this setting. Decisions regarding the time of eventual discontinuation should consider the stage and activity of liver disease and of infection, taking into account also the risk of post-partum hepatitis flare. Breastfeeding is not contraindicated for HBV patients. However, it is not recommended for women taking antiviral drugs. Finally, there is no clear evidence that ECS reduces the risk of mother-to-child transmission compared to vaginal delivery.
The authors thank Jean Ann Gilder for text editing.
Peer reviewers: Byung Chul Yoo, MD, PhD, Professor, Division of Gastroenterology, Department of Medicine, Samsung Medical Center, SungKyunKwan University School of Medicine, 50 Irwon-dong, Gangnam-gu, Seoul 135-710, South Korea; Seong Gyu Hwang, MD, Professor, Department of Internal Medicine, CHA Bundang Medical Center, CHA University, No. 351, Yatap-Dong, Bundang-Gu, Seongnam, Gyeonggi-Do 463-712, South Korea; Arezoo Aghakhani, MD, PhD, Assistant Professor, Clinical Research Department Pasteur Institute of Iran, No 69, Pasteur Ave., Tehran 13164, Iran
S- Editor Shi ZF L- Editor Logan S E- Editor Xiong L
.
| 1. | Jonas MM. Hepatitis B and pregnancy: an underestimated issue. Liver Int. 2009;29 Suppl 1:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1734] [Cited by in RCA: 1750] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 3. | Tran TT. Management of hepatitis B in pregnancy: weighing the options. Cleve Clin J Med. 2009;76 Suppl 3:S25-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Nguyen G, Garcia RT, Nguyen N, Trinh H, Keeffe EB, Nguyen MH. Clinical course of hepatitis B virus infection during pregnancy. Aliment Pharmacol Ther. 2009;29:755-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Hay JE. Liver disease in pregnancy. Hepatology. 2008;47:1067-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Russell MA, Craigo SD. Cirrhosis and portal hypertension in pregnancy. Semin Perinatol. 1998;22:156-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | ter Borg MJ, Leemans WF, de Man RA, Janssen HL. Exacerbation of chronic hepatitis B infection after delivery. J Viral Hepat. 2008;15:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Wang JS, Chen H, Zhu QR. Transformation of hepatitis B serologic markers in babies born to hepatitis B surface antigen positive mothers. World J Gastroenterol. 2005;11:3582-3585. [PubMed] |
| 9. | Beasley RP, Trepo C, Stevens CE, Szmuness W. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol. 1977;105:94-98. [PubMed] |
| 10. | Wong VC, Ip HM, Reesink HW, Lelie PN, Reerink-Brongers EE, Yeung CY, Ma HK. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin. Double-blind randomised placebo-controlled study. Lancet. 1984;1:921-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 285] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Okada K, Kamiyama I, Inomata M, Imai M, Miyakawa Y. e antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. N Engl J Med. 1976;294:746-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 450] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Bai H, Zhang L, Ma L, Dou XG, Feng GH, Zhao GZ. Relationship of hepatitis B virus infection of placental barrier and hepatitis B virus intra-uterine transmission mechanism. World J Gastroenterol. 2007;13:3625-3630. [PubMed] |
| 13. | Wiseman E, Fraser MA, Holden S, Glass A, Kidson BL, Heron LG, Maley MW, Ayres A, Locarnini SA, Levy MT. Perinatal transmission of hepatitis B virus: an Australian experience. Med J Aust. 2009;190:489-492. [PubMed] |
| 14. | Han GR, Zhao W, Cao K, Jiang HX, Pan C. A prospective and open-label study for the efficacy and safety of telbivudine (LTD) in pregnancy for the prevention of perinatal transmission of hepatitis B virus (HBV) to the infants. . |
| 15. | Xu DZ, Yan YP, Choi BC, Xu JQ, Men K, Zhang JX, Liu ZH, Wang FS. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol. 2002;67:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 197] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Zhang SL, Yue YF, Bai GQ, Shi L, Jiang H. Mechanism of intrauterine infection of hepatitis B virus. World J Gastroenterol. 2004;10:437-438. [PubMed] |
| 17. | Papadakis MA, Elefsiniotis IS, Vlahos G, Daskalakis G, Barbatis C, Antsaklis A. Intrauterine-transplacental transmission of hepatitis B virus (HBV) from hepatitis B e antigen negative (precore mutant, G1896A) chronic HBV infected mothers to their infants. Preliminary results of a prospective study. J Clin Virol. 2007;38:181-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Bzowej NH. Hepatitis B Therapy in Pregnancy. Curr Hepat Rep. 2010;9:197-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Terrault NA, Jacobson IM. Treating chronic hepatitis B infection in patients who are pregnant or are undergoing immunosuppressive chemotherapy. Semin Liver Dis. 2007;27 Suppl 1:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Available from: http: //www.apregistry.com. |
| 21. | Munderi P, Wilkes H, Tumukunde D. Pregnancy and outcomes among women on triple-drug antiretroviral therapy (ART) in the DART trial. Curr Hepat Rep. 2010;9:197-204. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | van Zonneveld M, van Nunen AB, Niesters HG, de Man RA, Schalm SW, Janssen HL. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat. 2003;10:294-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 184] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Xu WM, Cui YT, Wang L, Yang H, Liang ZQ, Li XM, Zhang SL, Qiao FY, Campbell F, Chang CN. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: a multicentre, randomized, double-blind, placebo-controlled study. J Viral Hepat. 2009;16:94-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 279] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 24. | Shi Z, Yang Y, Ma L, Li X, Schreiber A. Lamivudine in late pregnancy to interrupt in utero transmission of hepatitis B virus: a systematic review and meta-analysis. Obstet Gynecol. 2010;116:147-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Tarantal AF, Castillo A, Ekert JE, Bischofberger N, Martin RB. Fetal and maternal outcome after administration of tenofovir to gravid rhesus monkeys (Macaca mulatta). J Acquir Immune Defic Syndr. 2002;29:207-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Van Rompay KK, Durand-Gasselin L, Brignolo LL, Ray AS, Abel K, Cihlar T, Spinner A, Jerome C, Moore J, Kearney BP. Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects. Antimicrob Agents Chemother. 2008;52:3144-3160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Foster C, Lyall H, Olmscheid B, Pearce G, Zhang S, Gibb DM. Tenofovir disoproxil fumarate in pregnancy and prevention of mother-to-child transmission of HIV-1: is it time to move on from zidovudine? HIV Med. 2009;10:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Yang J, Zeng XM, Men YL, Zhao LS. Elective caesarean section versus vaginal delivery for preventing mother to child transmission of hepatitis B virus--a systematic review. Virol J. 2008;5:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Beasley RP, Stevens CE, Shiao IS, Meng HC. Evidence against breast-feeding as a mechanism for vertical transmission of hepatitis B. Lancet. 1975;2:740-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 117] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Hill JB, Sheffield JS, Kim MJ, Alexander JM, Sercely B, Wendel GD. Risk of hepatitis B transmission in breast-fed infants of chronic hepatitis B carriers. Obstet Gynecol. 2002;99:1049-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 31. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2171] [Article Influence: 135.7] [Reference Citation Analysis (0)] |