Published online Sep 14, 2012. doi: 10.3748/wjg.v18.i34.4651
Revised: March 27, 2012
Accepted: March 29, 2012
Published online: September 14, 2012
The liver is an important site for iron and lipid metabolism and the main site for the interactions between these two metabolic pathways. Although conflicting results have been obtained, most studies support the hypothesis that iron plays a role in hepatic lipogenesis. Iron is an integral part of some enzymes and transporters involved in lipid metabolism and, as such, may exert a direct effect on hepatic lipid load, intrahepatic metabolic pathways and hepatic lipid secretion. On the other hand, iron in its ferrous form may indirectly affect lipid metabolism through its ability to induce oxidative stress and inflammation, a hypothesis which is currently the focus of much research in the field of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH). The present review will first discuss how iron might directly interact with the metabolism of hepatic lipids and then consider a new perspective on the way in which iron may have a role in the two hit hypothesis for the progression of NAFLD via ferroportin and the iron regulatory molecule hepcidin. The review concludes that iron has important interactions with lipid metabolism in the liver that can impact on the development of NAFLD/NASH. More defined studies are required to improve our understanding of these effects.
- Citation: Ahmed U, Latham PS, Oates PS. Interactions between hepatic iron and lipid metabolism with possible relevance to steatohepatitis. World J Gastroenterol 2012; 18(34): 4651-4658
- URL: https://www.wjgnet.com/1007-9327/full/v18/i34/4651.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i34.4651
The liver is a major site for the storage of iron and the metabolism of lipids and is therefore an important site for interaction between these two metabolic pathways. One role of iron in the pathogenesis of diseases associated with hyperlipidemia and lipid deposition is likely to include an ability to induce oxidative stress and inflammation in the liver. In the setting of lipid deposition in the liver, iron overload has been associated with increased fibrosis and progression of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis (NAFLD/NASH)[1-3]. Iron in its ferrous form can generate free radicals via the Fenton reaction, resulting in oxidative stress and lipid peroxidation. These effects can, in turn, stimulate hepatic stellate cells to increase production of collagen and progression of fibrosis in patients with NAFLD and NASH. Notwithstanding these indirect effects, there are also recent and archival studies to show that iron has direct effects on lipid metabolism. Variations in hepatic iron stores result in inappropriate lipogenesis for lipid storage and secretion. For example, iron deficiency is associated with increased hepatic lipogenesis and lipemia[4,5]. Since the liver co-ordinates iron and lipid metabolism, and under conditions of stress is the site of excessive deposition of both these nutrients, this review will focus on the role of iron in the metabolism of hepatic lipids. In particular, it examines the role of different enzymes, receptors and transporters, as well as the effects of proteins involved in lipogenesis and lipoprotein secretion as sites for interaction between iron and lipids. The review then addresses the way in which iron may function in the progression of NAFLD. To begin, a brief overview of normal hepatic lipid metabolism is provided.
Hepatic steatosis occurs whenever there is an imbalance among the uptake, synthesis, oxidative and secretory pathways of fatty acid metabolism. Sources of fatty acids for hepatic triglyceride (TG) synthesis are those derived from the plasma non-esterified fatty acids (NEFA) pool and those from within the liver through de novo lipogenesis (DNL), mainly from glucose (Figure 1). The hepatic uptake of fatty acid from the plasma is not regulated and is therefore directly related to the concentration of plasma NEFAs. Adipose tissue by its TG lipolysis is the main contributor to the plasma NEFA pool. The remaining NEFAs come from peripheral lipolysis of lipoproteins [chylomicrons (CM), very low density lipoprotein (VLDL)] and dietary fatty acids in the circulation. The hepatic uptake of fatty acids occurs by fatty acid binding protein and fatty acid translocase. Once in the hepatocyte, fatty acids undergo oxidation or they may be detoxified by re-esterification with glycerol and cholesterol to form TG and cholesteryl esters (CE), respectively. The TG and CE are then secreted as VLDL into the plasma or, if in excess, are stored in the cytoplasm as lipid droplets. Adipose differentiation-related protein (ADRP) is expressed in hepatocytes and is involved in cytosolic lipid storage[6].
The partitioning of hepatic TG into different pools under normal conditions of fasting/feeding and dietary variations, i.e. carbohydrate or fat diet, involves different hormonal and metabolic regulatory mechanisms. Insulin is a fat-sparing hormone and a major metabolic factor in the regulation of fat metabolism. In the liver, insulin increases TG synthesis by the hepatocytes and restricts its secretion by partitioning the newly synthesized TG into cytosolic stores[7]. In addition, insulin also inhibits lipolysis in the hepatocyte[8], decreases the assembly of VLDL by impairing the association of apoB-100 with TG[9], inhibits the translation of apoB-100 mRNA in human hepatocellular carcinoma (HepG2) cells[10] and stimulates the degradation of newly synthesized apoB[11], which collectively decrease VLDL-TG secretion. These actions coupled with insulin-stimulated expression of ADRP promotes lipid droplet assembly[12]. Furthermore, insulin also activates sterol regulatory element binding protein-1c, which transcriptionally activates nearly all genes involved in DNL[13].
There is evidence to suggest that lipoprotein uptake pathways are affected by iron.
Anaemia/hypoxia is associated with decreased fat uptake by the liver, but increased fat accumulation by the liver. Decreased fat uptake may either be due to impaired peripheral lipolysis or decreased uptake of CM and VLDL remnants, leaving the lipoproteins to accumulate in the circulation. Rat models of chronic anemia/hypoxia in which iron deficiency is present show decreased lipoprotein lipase and hepatic lipase activities[14-18]. This may be due to decreased synthesis[18], defects in the structure or conversion from precursor to the functional form, or in the activation of these enzymes[14,16]. It may be that iron itself can impact on hepatic uptake pathways. Supporting this idea, Brown et al[19] have shown that the binding of low density lipoprotein to its receptors requires a divalent cation. Although speculative, one of the divalent cations may include ferrous-iron. Anaemic rats produced by experimentally induced chronic renal failure show down- regulation of the expression of the VLDL-receptor in a non-erythropoietin dependent manner[20]. Furthermore, the plasma ratio of cholesteryl ester to cholesterol is increased with iron deficiency in rats. This cannot be explained by increased lecithin cholesterol acyl transferase (LCAT) activity, since LCAT is reduced in iron deficiency[21,22].
On the other hand, iron deficiency in rats has been shown to increase hepatic lipogenesis, leading to cellular TG accumulation and steatosis[4,5,23,24]. Given that hepatic uptake pathways are down regulated, the increased lipogenesis could be due to decreased utilization of fatty acids or increased DNL. In iron deficient rats, it is proposed that beta-oxidation of fatty acids is decreased, allowing the fatty acids to be diverted towards TG synthesis[24]. Supporting this, the level of hepatic carnitine, the long chain fatty acid transporter required for β-oxidation of fatty acids is reduced in the iron deficient state. Since this enzyme requires iron as a cofactor, its activity might be expected to decrease with iron deficiency[24,25]. However, others report that beta-oxidation of fatty acids remains unchanged[4], while hepatic DNL increases[4,23]. The increased hepatic lipogenesis seen in iron-deficient rats is supported by genomic studies that show a concurrent decrease in gene expression related to hepatic β-oxidation and an increase in gene expression related to lipogenesis[26]. Therefore, some of the changes in lipid metabolism appear to be related to effects of iron-deficiency on transcriptional/post-transcriptional mechanisms, as well as to effects on the kinetics/activity of enzymes that depend upon iron as a cofactor.
In contrast to the studies that show an increase in hepatic lipogenesis with iron deficiency, others have reported a decrease in hepatic lipid synthesis. In rats with moderate iron deficiency, glucose-fueled hepatic lipogenesis fell[27] which was attributed to decreased activity of either the rate limiting enzymes [fatty acid synthase (FAS)] or enzymes involved in providing the redox potential for lipogenesis (glucose 6 phosphate dehydrogenase, malic enzyme)[28-30].
In chicks, anemia depresses levels of mitochondrial cytochromes[31-33]. This finding is consistent with that found in yeast in which iron-deficiency down-regulates transcription of certain respiratory cytochromes that require heme as prosthetic groups for activity[34], while the heme degradation enzyme, heme oxygenase, is upregulated[35]. It is proposed that this decrease in the activity of hepatic heme pathways in iron-deficiency preserves the availability of iron for other essential sites/pathways when cellular iron levels are low[36]. Supporting this idea is the observation that when lipid metabolism is affected by iron deficiency in yeast, amino acid homeostasis remains unaltered[36].
Iron deficiency can affect the function of many hepatic enzymes involved in cholesterol metabolism[28]. In a mouse model of iron-deficiency induced by genetic mutation of hephaestin (sla mice), lack of the copper-dependent ferroxidase in the enterocyte results in impaired iron absorption and anaemia[37]. Under these conditions, hepatic cholesterol synthesis is reduced in association with decreased mevalonic acid levels. HMG CoA reductase activity, the rate limiting enzyme in de novo cholesterol synthesis, is increased in this model, arguing that this enzyme is not responsible for the decreased cholesterol production[37]. Similarly, acetyl-CoA thiolase and HMG-CoA synthase involved in cholesterol production are not affected by iron deficiency[37]. All these enzymes act upstream in the production of mevalonic acid, suggesting that this intermediate is shunted to a non-steroidal pathway.
Other studies show that the cholesterol degradation pathway is also affected. One study in dogs shows that iron deficiency can decrease the activity of hepatic 7α hydroxylase, the rate limiting enzyme in the conversion of cholesterol into bile acids[38]. In this study, bile contained cholesterol crystals, suggesting that reduced conversion of cholesterol to bile acids may impair biliary micellar formation and explain the cholesterol precipitates in the bile. However, hepatic lipids were not measured in this study.
Nutritional iron depletion in rats also leads to alterations in the fatty acid composition of hepatic phospholipids, indicating impaired desaturation of saturated and essential fatty acids[29,39,40]. Steroyl CoA desaturase (SCD) is required for the conversion of saturated fatty acids to the unsaturated form and this enzyme is dependent on iron for its activity. Lower activity of SCD in iron deficiency may explain the increased saturated composition of fatty acids in the liver in this condition[29]. The effect of iron on the various steps in lipid metabolism is summarised in Figure 1.
It is likely that some of the reasons for the conflicting observations reported here as a result of iron deficiency can be explained in part by the specifics of the model system, by the means of inducing iron deficiency through phlebotomy or dietary restriction, or by the extent of the iron deficiency. Severe iron deficiency will produce anaemia and hypoxia which are likely to activate additional transcription factors to those stimulated during mild iron deficiency, and in turn, affect the transcription of more genes encoding enzymes/proteins[41]. Analysis of the reports cited in this review for correlations between the extent of iron deficiency and alterations in lipid metabolism was not possible, since the level of iron deficiency was not measured in these studies. It is recommended that future studies exploring effects of iron deficiency need to incorporate measurements of iron into their experimental design.
Another reason for the conflicting observations reported here might be the model systems themselves. Some animal model systems are designed to develop iron-deficiency from birth and others induce iron deficiency in a mature animal[4,23]. There are studies to suggest that metabolic conditions in development can impact on adult metabolism. For example, epidemiological data show that under normal birth weight conditions, there is an inverse correlation between the weight at birth and adult obesity[42,43]. Experimental data show that changing the diets during pregnancy or during the newborn period may permanently change the rate of lipid metabolism[44]. Therefore, it is important to recognize the possibility of fetal programming on hepatic lipid metabolism as an explanation for some of the differences reported.
Iron overload can generate oxidative stress[45,46] and lipid peroxidation, which can modify the fatty acid profile of cellular membranes, leading to their disruption, damage to cell organelles[47-49] and impairment of mitochondrial oxidative metabolism[50,51]. It is suggested that the free radicals that form may cause a change in the ratio of saturated to unsaturated membrane phospholipids, leading to alterations in membrane fluidity[52]. This in turn may affect the activity of the embedded enzymes[53-56]. Enzymes respond to oxidative stress differently by altering their activity[55]. It is also known that the formation of peroxidation products by hepatocytes increases with the proportion of unsaturated fatty acids. Thus, polyunsaturated fatty acids in the presence of iron overload may exert an inhibitory effect on lipogenic genes (e.g., FAS) by generating a peroxidative cytotoxic effect[57] .
Iron overload also has direct effects on hepatic lipid metabolism, although studies report conflicting results based on different experimental models. Specific enzymes in hepatic cholesterol metabolism show variable responses to iron overload. In rats with dietary iron overload, an increase in the activity of acyl-CoA cholesterol acyltransferase (ACAT) coupled with reduced activities of HMG CoA reductase and 7 α-hydroxylase correlates with hypercholesterolemia and unaltered hepatic cholesterol content[55]. Since ACAT increases intrahepatic cholesterol esterification and contributes to VLDL-cholesterol secretion, these findings suggest that the cholesterol synthetic and excretory pathways are not affected while the secretory pathways maybe upregulated in this model system[55,58]. On the other hand, a recent study in mice by Graham et al[59], shows that transcripts of seven enzymes, including the rate limiting enzyme HMG CoA reductase, are up-regulated with increasing hepatic iron, suggesting that hepatic iron loading increases liver cholesterol synthesis. Although valuable, genomic analysis does not provide insight into enzyme kinetics and substrate concentration which may also be altered by the level of iron. This may explain some of the differences between these studies as well as different feeding regimes employed. Hepatic iron overload using carbonyl iron in the methionine-choline deficient rat model of NAFLD is associated with decreased hepatic TG and decreased hepatic steatosis; however, an improvement in liver injury is not seen due to increased necroinflammation and a trend towards increased perivenular fibrosis in iron loaded animals[58]. In carbonyl-iron and iron dextran models of iron overload in mice, studies show an upregulation of the mRNA and enzyme activity of SCD. SCD increases the biosynthesis of unsaturated fatty acids at 8 but not 2 mo of feeding[60]. In contrast, feeding ferric citrate for 3 mo has no effect on hepatic fatty acid composition or desaturase activity[40]. These findings suggest that the experimental model used, the level and duration of iron overload, as well as cellular repartition of iron storage in the liver may play a role in SCD induction[60]. The effect of iron on the various steps in lipid metabolism is summarised in Figure 1.
In vitro studies in HepG2 cells show that iron overload increases intracellular lipid droplet formation by increasing the expression of cluster of differentiation 1d, an unconventional major histocompatibility complex class 1 molecule reported to monitor intracellular and plasma membrane lipid metabolism[61]. Both of these changes are also associated with increased expression of phosphatidylserine in the outer leaflet of the plasma membrane, a feature associated with apoptosis and cell death[62,63]. Hepatosteatosis has also been associated with an increased likelihood of apoptosis[64]. Cell death of mature hepatocytes disproportionate to their ability to regenerate has been referred to as a “third hit” in the pathogenesis of NAFLD/NASH leading to progression of fibrosis[65,66].
Several important factors may contribute to the inconsistencies described here in studies exploring effects of iron overload. These include differences in experimental designs, diets, age, sex, weight and strain of animals; differences in methods for generating iron overload; and also maternal status and its effect on fetal programming, as discussed for models of iron deficiency above.
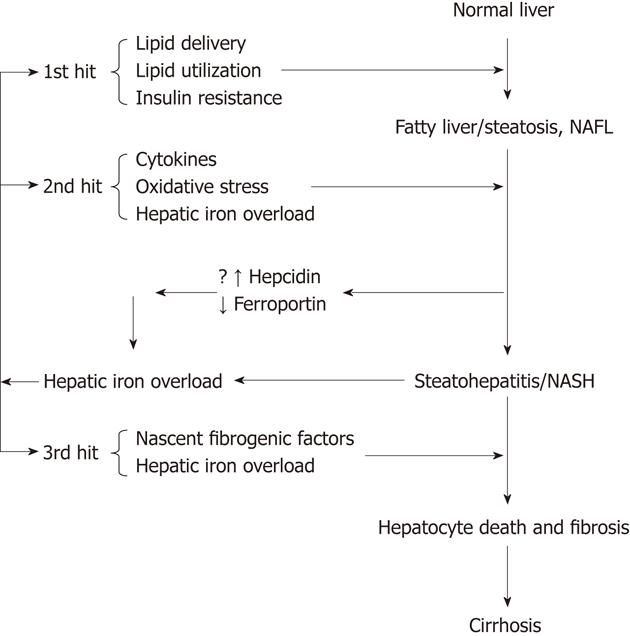
NAFLD is now accepted as an hepatic component of the metabolic syndrome[67]. It represents a spectrum of disease ranging from a benign state of non-alcoholic fatty liver to severe fibrosis or cirrhosis. NASH is the intermediate progressive stage between the two conditions. The exact mechanism for this progression is not known but a “two hit theory” is generally accepted. The first hit is considered to be insulin resistance, which leads to excessive accumulation of fat in the liver. The second hit is the generation of oxidative stress by a number of factors, including hepatic iron overload, which lead to cytotoxicity and necroinflammation. A number of clinical and animal studies have shown variable results concerning the effects of iron in NAFLD and NASH. This has lead to controversy about its role in the disease progression (Figure 2).
As already mentioned, excess iron is directly toxic to cells[47-49]. Furthermore, iron accumulation has a proinflammatory and profibrogenic role by activating Kupffer cells to release inflammatory cytokines[68,69] and by activating hepatic stellate cells, which can culminate in the replacement of parenchymal tissue with connective tissue[70]. Bacon et al[1], in 1994 first showed abnormal iron parameters (serum ferritin and transferrin saturation) and increased hepatic iron concentration in cases of NASH. Other clinical studies have also shown an increase of hepatic iron deposition in NASH/NAFLD and an increased prevalence of the hemochromatosis gene (HFE) mutation[2,3,71-73]. One study showed an association between increased hepatic iron and severity of fibrosis in NASH[2]. In contrast to the work of Bacon and others, however, there are also clinical studies that fail to find an increase in hepatic iron deposition in NASH[74-78] or an increase in HFE mutation[79-81], casting doubt on iron as a causative agent in the progression of the disease.
On the other hand, several clinical studies of NAFLD/NASH show an improvement in liver enzymes and insulin sensitivity with phlebotomies, even in cases without increased levels of iron[82-84]. One case report of a patient with NASH shows complete histologic resolution of steatohepatitis in the liver with phlebotomy-induced iron depletion[85]. However, it must be kept in mind that repeated phlebotomies might predispose to anemia, which could lead to tissue hypoxia and subsequent peripheral vasodilatation. Cardiac output would be expected to increase in response to reduced peripheral resistance, which in turn would increase tissue perfusion and peripheral lipoprotein clearance, reducing the lipid load on the liver. This proposed sequence of events is supported by hemodynamic studies in the anaemic state[86-88] and clearance studies using adrenergic agonists[89,90]. Taken together, an improvement in NAFLD/NASH observed with repeated phlebotomies might be more a physiological response to anaemia/tissue hypoxia rather than a direct effect of reducing iron toxicity. Care must be taken to choose this procedure in cardiac compromised patients.
It is a generally accepted hypothesis that iron overload may play a role in the progression of NAFLD via promoting the 2nd hit (Figure 2). However, it is also possible that the steatosis, cytokines and oxidative stress that are integral to NASH pathophysiology secondarily lead to iron overload, which then works in a feed-forward manner to generate more oxidative stress and inflammation, making the environment more favourable for the 3rd hit, injury that overwhelms the capacity of dying hepatocytes to regenerate and increases scar, as proposed by Diehl[65,91]. Supporting the idea, increased hepatic iron deposition is also seen in non-biliary causes of cirrhosis, such as alcoholic liver disease and chronic viral hepatitis as well as NASH[92]. The process of hepatocellular injury may itself result in increased iron uptake from the intestine[93]. The expression of transferrin receptor might be increased in regenerating hepatocytes after liver injury, which could contribute to increased hepatic iron loading[94]. Another mechanism of increased iron accumulation in liver in NAFLD and NASH might be due to changes in levels of ferroportin and the iron regulatory peptide hepcidin[95,96]. Rats developing insulin resistance on a high fat, high energy diet show decreased hepatic mRNA expression of hepcidin[97]. Similarly, mice developing steatosis on a choline deficient diet show a negative correlation of hepcidin and ferroportin (iron export molecule) mRNA with hepatic lipid concentration, suggesting that enhanced dietary intake and reduced hepatic iron efflux may lead to increased hepatic iron content[98]. In patients with NAFLD, there is also reduced expression of ferroportin and the iron-sensing molecule, hemojuvelin, which is associated with an increase of tumor necrosis factor-α, hepatic iron accumulation and increased hepcidin expression[99]. Hepcidin expression and iron regulatory molecules need to be further investigated in the livers of patients with metabolic syndrome and NAFLD.
It may also be the case that factors inducing localized iron deposition in the microenvironment of the liver are as, or more, important than total hepatic iron or systemic levels of iron. Iron in the microenvironment can promote regional oxidative stress that can lead to changes in microcirculation/capillarization, inflammation and fibrosis in NAFLD. In a rabbit model of steatohepatitis, Otogawa et al[100] have shown an increased phagocytosis of erythrocytes by Kupffer cells that is associated with increased hepatic iron deposition, suggesting that iron may accumulate locally as part of heme degradation. This finding is supported by a clinical study of patients with NASH in which aggregations of erythrocytes are seen in inflammed hepatic sinusoids[100].
In the aggregate, the findings reviewed here suggest the hypothesis that hepatic iron overload itself may be a critical event in the progression of NAFLD/NASH by exacerbating the 2nd hit of inflammation or perhaps by providing a critical 3rd hit that can ultimately lead to liver cell loss, failure of hepatocyte replication and eventual cirrhosis. Further studies are required to clearly establish the relationship between NASH/NAFLD, insulin resistance and iron overload.
Peer reviewers: Dr. Tamir Miloh, Phoenix Children’s Hospital, 1919 E Thomas Rd, Phoenix, AZ 85016, United States; Manuel Romero-Gomez, Professor, Department of Medicine, University of Sevilla, Avda. de Bellavista, 41014 Sevilla, Spain
S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103-1109. [PubMed] |
| 2. | George DK, Goldwurm S, MacDonald GA, Cowley LL, Walker NI, Ward PJ, Jazwinska EC, Powell LW. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998;114:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 447] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 3. | Fargion S, Mattioli M, Fracanzani AL, Sampietro M, Tavazzi D, Fociani P, Taioli E, Valenti L, Fiorelli G. Hyperferritinemia, iron overload, and multiple metabolic alterations identify patients at risk for nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96:2448-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 163] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 4. | Sherman AR, Guthrie HA, Wolinsky I, Zulak IM. Iron deficiency hyperlipidemia in 18-day-old rat pups: effects of milk lipids, lipoprotein lipase, and triglyceride synthesis. J Nutr. 1978;108:152-162. [PubMed] |
| 5. | Sherman AR, Bartholmey SJ, Perkins EG. Fatty acid patterns in iron-deficient maternal and neonatal rats. Lipids. 1982;17:639-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res. 1997;38:2249-2263. [PubMed] |
| 7. | Sparks JD, Sparks CE. Insulin regulation of triacylglycerol-rich lipoprotein synthesis and secretion. Biochim Biophys Acta. 1994;1215:9-32. [PubMed] |
| 8. | Debeer LJ, Beynen AC, Mannaerts GP, Geelen MJ. Lipolysis of hepatic triacylglycerol stores. FEBS Lett. 1982;140:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Patsch W, Franz S, Schonfeld G. Role of insulin in lipoprotein secretion by cultured rat hepatocytes. J Clin Invest. 1983;71:1161-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 142] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Adeli K, Theriault A. Insulin modulation of human apolipoprotein B mRNA translation: studies in an in vitro cell-free system from HepG2 cells. Biochem Cell Biol. 1992;70:1301-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Sparks JD, Sparks CE. Insulin modulation of hepatic synthesis and secretion of apolipoprotein B by rat hepatocytes. J Biol Chem. 1990;265:8854-8862. [PubMed] |
| 12. | Phillips SA, Choe CC, Ciaraldi TP, Greenberg AS, Kong AP, Baxi SC, Christiansen L, Mudaliar SR, Henry RR. Adipocyte differentiation-related protein in human skeletal muscle: relationship to insulin sensitivity. Obes Res. 2005;13:1321-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Shimomura I, Bashmakov Y, Ikemoto S, Horton JD, Brown MS, Goldstein JL. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci USA. 1999;96:13656-13661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 601] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 14. | Lau BW, Klevay LM. Postheparin plasma lipoprotein lipase in copper-deficient rats. J Nutr. 1982;112:928-933. [PubMed] |
| 15. | Muratsubaki H, Enomoto K, Ichijoh Y, Yamamoto Y. Hypertriglyceridemia associated with decreased post-heparin plasma hepatic triglyceride lipase activity in hypoxic rats. Arch Physiol Biochem. 2003;111:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Mochizuki S, Murase T, Yamaoka H, Ishiki M, Tada N, Nagano M. Lipoprotein lipase activity in ischaemic and anoxic myocardium. Basic Res Cardiol. 1987;82 Suppl 1:45-52. [PubMed] |
| 17. | Lewis M, Iammarino RM. Lipemia in rodent iron-deficiency anemia. J Lab Clin Med. 1971;78:546-554. [PubMed] |
| 18. | Vaziri ND, Liang K. Down-regulation of tissue lipoprotein lipase expression in experimental chronic renal failure. Kidney Int. 1996;50:1928-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Brown MS, Kovanen PT, Goldstein JL. Regulation of plasma cholesterol by lipoprotein receptors. Science. 1981;212:628-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 650] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 20. | Vaziri ND, Liang K. Down-regulation of VLDL receptor expression in chronic experimental renal failure. Kidney Int. 1997;51:913-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Rao GA, Crane RT, Larkin EC. Reduced plasma lecithin cholesterol acyl transferase activity in rats fed iron-deficient diets. Lipids. 1983;18:673-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Jain SK, Yip R, Pramanik AK, Dallman PR, Shohet SB. Reduced plasma cholesterol esterifying activity in iron-deficient rats: its possible role in the lipemia of iron deficiency. J Nutr. 1982;112:1230-1232. [PubMed] |
| 23. | Sherman AR. Lipogenesis in iron-deficient adult rats. Lipids. 1978;13:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Bartholmey SJ, Sherman AR. Carnitine levels in iron-deficient rat pups. J Nutr. 1985;115:138-145. [PubMed] |
| 25. | Hulse JD, Ellis SR, Henderson LM. Carnitine biosynthesis. beta-Hydroxylation of trimethyllysine by an alpha-ketoglutarate-dependent mitochondrial dioxygenase. J Biol Chem. 1978;253:1654-1659. [PubMed] |
| 26. | Davis MR, Rendina E, Peterson SK, Lucas EA, Smith BJ, Clarke SL. Enhanced expression of lipogenic genes may contribute to hyperglycemia and alterations in plasma lipids in response to dietary iron deficiency. Genes Nutr. 2012;7:415-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Amine EK, Desilets EJ, Hegsted DM. Effect of dietary fat on lipogenesis in iron deficiency anemic chicks and rats. Journal of Nutrition. 1976;106:405-411. |
| 28. | Bailey-Wood R, Blayney LM, Muir JR, Jacobs A. The effects of iron deficiency on rat liver enzymes. Br J Exp Pathol. 1975;56:193-198. [PubMed] |
| 29. | Stangl GI, Kirchgessner M. Different degrees of moderate iron deficiency modulate lipid metabolism of rats. Lipids. 1998;33:889-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Sochor M, Baquer NZ, McLean P. Bio-inorganic regulation of pathways of carbohydrate and lipid metabolism. II. The effect of iron-deficiency on the profile of enzymes in the developing rat adrenal gland. Enzyme. 1982;27:149-155. [PubMed] |
| 31. | Dallman PR, Goodman JR. The effects of iron deficiency on the hepatocyte: a biochemical and ultrastructural study. J Cell Biol. 1971;48:79-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Amine EK, Hegsted DM. Iron deficiency lipemia in the rat and chick. J Nutr. 1971;101:1575-1582. [PubMed] |
| 33. | Floch MH, Shearman DJ, Herskovic T, Levine RJ, Spiro HM. Iron deficiency anemia and hepatic lesions in weanling rats. Arch Pathol. 1969;87:526-532. [PubMed] |
| 34. | Puig S, Askeland E, Thiele DJ. Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell. 2005;120:99-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 322] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 35. | Protchenko O, Philpott CC. Regulation of intracellular heme levels by HMX1, a homologue of heme oxygenase, in Saccharomyces cerevisiae. J Biol Chem. 2003;278:36582-36587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Shakoury-Elizeh M, Protchenko O, Berger A, Cox J, Gable K, Dunn TM, Prinz WA, Bard M, Philpott CC. Metabolic response to iron deficiency in Saccharomyces cerevisiae. J Biol Chem. 2010;285:14823-14833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 37. | Au YP, Schilling RF. Relationship between anemia and cholesterol metabolism in 'sex-linked anemic' (gene symbol, sla) mouse. Biochim Biophys Acta. 1986;883:242-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Johnston SM, Murray KP, Martin SA, Fox-Talbot K, Lipsett PA, Lillemoe KD, Pitt HA. Iron deficiency enhances cholesterol gallstone formation. Surgery. 1997;122:354-61; discussion 361-2. [PubMed] |
| 39. | Rao GA, Crane RT, Larkin EC. Reduction of hepatic stearoyl-CoA desaturase activity in rats fed iron-deficient diets. Lipids. 1983;18:573-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Cunnane SC, McAdoo KR. Iron intake influences essential fatty acid and lipid composition of rat plasma and erythrocytes. J Nutr. 1987;117:1514-1519. [PubMed] |
| 41. | Li J, Grigoryev DN, Ye SQ, Thorne L, Schwartz AR, Smith PL, O'Donnell CP, Polotsky VY. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J Appl Physiol. 2005;99:1643-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 42. | Barker DJ. Fetal growth and adult disease. Br J Obstet Gynaecol. 1992;99:275-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 124] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Barker DJ, Martyn CN, Osmond C, Hales CN, Fall CH. Growth in utero and serum cholesterol concentrations in adult life. BMJ. 1993;307:1524-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 388] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 44. | Lucas A, Baker BA, Desai M, Hales CN. Nutrition in pregnant or lactating rats programs lipid metabolism in the offspring. Br J Nutr. 1996;76:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 125] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Kruidenier L, Verspaget HW. Review article: oxidative stress as a pathogenic factor in inflammatory bowel disease--radicals or ridiculous? Aliment Pharmacol Ther. 2002;16:1997-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 286] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 46. | Moldovan L, Moldovan NI. Oxygen free radicals and redox biology of organelles. Histochem Cell Biol. 2004;122:395-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 311] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 47. | Bacon BR, O'Neill R, Park CH. Iron-induced peroxidative injury to isolated rat hepatic mitochondria. J Free Radic Biol Med. 1986;2:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Högberg J, Bergstrand A, Jakobsson SV. Lipid peroxidation of rat-liver microsomes. Its effect on the microsomal membrane and some membrane-bound microsomal enzymes. Eur J Biochem. 1973;37:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 121] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Myers BM, Prendergast FG, Holman R, Kuntz SM, LaRusso NF. Alterations in the structure, physicochemical properties, and pH of hepatocyte lysosomes in experimental iron overload. J Clin Invest. 1991;88:1207-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Bacon BR, Britton RS. The pathology of hepatic iron overload: a free radical--mediated process? Hepatology. 1990;11:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 268] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 51. | Bacon BR, O'Neill R, Britton RS. Hepatic mitochondrial energy production in rats with chronic iron overload. Gastroenterology. 1993;105:1134-1140. [PubMed] |
| 52. | Ohyashiki T, Ohtsuka T, Mohri T. A change in the lipid fluidity of the porcine intestinal brush-border membranes by lipid peroxidation. Studies using pyrene and fluorescent stearic acid derivatives. Biochim Biophys Acta. 1986;861:311-318. [PubMed] |
| 53. | Curtis MT, Gilfor D, Farber JL. Lipid peroxidation increases the molecular order of microsomal membranes. Arch Biochem Biophys. 1984;235:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Schachter D. Fluidity and function of hepatocyte plasma membranes. Hepatology. 1984;4:140-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 176] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Brunet S, Thibault L, Delvin E, Yotov W, Bendayan M, Levy E. Dietary iron overload and induced lipid peroxidation are associated with impaired plasma lipid transport and hepatic sterol metabolism in rats. Hepatology. 1999;29:1809-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 56. | Whittaker P, Chanderbhan RF. Effect of increasing iron supplementation on blood lipids in rats. Br J Nutr. 2001;86:587-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Foretz M, Foufelle F, Ferré P. Polyunsaturated fatty acids inhibit fatty acid synthase and spot-14-protein gene expression in cultured rat hepatocytes by a peroxidative mechanism. Biochem J. 1999;341:371-376. [PubMed] |
| 58. | Kirsch R, Sijtsema HP, Tlali M, Marais AD, Hall Pde L. Effects of iron overload in a rat nutritional model of non-alcoholic fatty liver disease. Liver Int. 2006;26:1258-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Graham RM, Chua AC, Carter KW, Delima RD, Johnstone D, Herbison CE, Firth MJ, O'Leary R, Milward EA, Olynyk JK. Hepatic iron loading in mice increases cholesterol biosynthesis. Hepatology. 2010;52:462-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 60. | Pigeon C, Legrand P, Leroyer P, Bouriel M, Turlin B, Brissot P, Loréal O. Stearoyl coenzyme A desaturase 1 expression and activity are increased in the liver during iron overload. Biochim Biophys Acta. 2001;1535:275-284. [PubMed] |
| 61. | Cabrita M, Pereira CF, Rodrigues P, Cardoso EM, Arosa FA. Altered expression of CD1d molecules and lipid accumulation in the human hepatoma cell line HepG2 after iron loading. FEBS J. 2005;272:152-165. [PubMed] |
| 62. | Iguchi K, Hirano K, Hamatake M, Ishida R. Phosphatidylserine induces apoptosis in adherent cells. Apoptosis. 2001;6:263-268. [PubMed] |
| 63. | Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10243] [Cited by in RCA: 9604] [Article Influence: 533.6] [Reference Citation Analysis (0)] |
| 64. | Imeryuz N, Tahan V, Sonsuz A, Eren F, Uraz S, Yuksel M, Akpulat S, Ozcelik D, Haklar G, Celikel C. Iron preloading aggravates nutritional steatohepatitis in rats by increasing apoptotic cell death. J Hepatol. 2007;47:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 65. | Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:370-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 352] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 66. | Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM. 2010;103:71-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 472] [Cited by in RCA: 525] [Article Influence: 35.0] [Reference Citation Analysis (4)] |
| 67. | Bugianesi E, Moscatiello S, Ciaravella MF, Marchesini G. Insulin resistance in nonalcoholic fatty liver disease. Curr Pharm Des. 2010;16:1941-1951. [PubMed] |
| 68. | She H, Xiong S, Lin M, Zandi E, Giulivi C, Tsukamoto H. Iron activates NF-kappaB in Kupffer cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G719-G726. [PubMed] |
| 69. | Xiong S, She H, Tsukamoto H. Signaling role of iron in NF-kappa B activation in hepatic macrophages. Comp Hepatol. 2004;3 Suppl 1:S36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Ramm GA, Crawford DH, Powell LW, Walker NI, Fletcher LM, Halliday JW. Hepatic stellate cell activation in genetic haemochromatosis. Lobular distribution, effect of increasing hepatic iron and response to phlebotomy. J Hepatol. 1997;26:584-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | Bonkovsky HL, Jawaid Q, Tortorelli K, LeClair P, Cobb J, Lambrecht RW, Banner BF. Non-alcoholic steatohepatitis and iron: increased prevalence of mutations of the HFE gene in non-alcoholic steatohepatitis. J Hepatol. 1999;31:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 236] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 72. | Nelson JE, Bhattacharya R, Lindor KD, Chalasani N, Raaka S, Heathcote EJ, Miskovsky E, Shaffer E, Rulyak SJ, Kowdley KV. HFE C282Y mutations are associated with advanced hepatic fibrosis in Caucasians with nonalcoholic steatohepatitis. Hepatology. 2007;46:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 73. | Sumida Y, Nakashima T, Yoh T, Furutani M, Hirohama A, Kakisaka Y, Nakajima Y, Ishikawa H, Mitsuyoshi H, Okanoue T. Serum thioredoxin levels as a predictor of steatohepatitis in patients with nonalcoholic fatty liver disease. J Hepatol. 2003;38:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 155] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 74. | Duseja A, Das R, Nanda M, Das A, Garewal G, Chawla Y. Nonalcoholic steatohepatitis in Asian Indians is neither associated with iron overload nor with HFE gene mutations. World J Gastroenterol. 2005;11:393-395. [PubMed] |
| 75. | Deguti MM, Sipahi AM, Gayotto LC, Palácios SA, Bittencourt PL, Goldberg AC, Laudanna AA, Carrilho FJ, Cançado EL. Lack of evidence for the pathogenic role of iron and HFE gene mutations in Brazilian patients with nonalcoholic steatohepatitis. Braz J Med Biol Res. 2003;36:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 76. | Younossi ZM, Gramlich T, Bacon BR, Matteoni CA, Boparai N, O'Neill R, McCullough AJ. Hepatic iron and nonalcoholic fatty liver disease. Hepatology. 1999;30:847-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 155] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 77. | Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1091] [Article Influence: 42.0] [Reference Citation Analysis (1)] |
| 78. | Chitturi S, Weltman M, Farrell GC, McDonald D, Kench J, Liddle C, Samarasinghe D, Lin R, Abeygunasekera S, George J. HFE mutations, hepatic iron, and fibrosis: ethnic-specific association of NASH with C282Y but not with fibrotic severity. Hepatology. 2002;36:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 79. | Valenti L, Fracanzani AL, Bugianesi E, Dongiovanni P, Galmozzi E, Vanni E, Canavesi E, Lattuada E, Roviaro G, Marchesini G. HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2010;138:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 80. | Duseja A, Das A, Das R, Dhiman RK, Chawla Y, Bhansali A, Kalra N. The clinicopathological profile of Indian patients with nonalcoholic fatty liver disease (NAFLD) is different from that in the West. Dig Dis Sci. 2007;52:2368-2374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 81. | Yamauchi N, Itoh Y, Tanaka Y, Mizokami M, Minami M, Morita A, Toyama T, Yamaguchi K, Fujii H, Okanoue T. Clinical characteristics and prevalence of GB virus C, SEN virus, and HFE gene mutation in Japanese patients with nonalcoholic steatohepatitis. J Gastroenterol. 2004;39:654-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 82. | Facchini FS, Hua NW, Stoohs RA. Effect of iron depletion in carbohydrate-intolerant patients with clinical evidence of nonalcoholic fatty liver disease. Gastroenterology. 2002;122:931-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 83. | Fargion S, Dongiovanni P, Guzzo A, Colombo S, Valenti L, Fracanzani AL. Iron and insulin resistance. Aliment Pharmacol Ther. 2005;22 Suppl 2:61-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 84. | Sumida Y, Kanemasa K, Fukumoto K, Yoshida N, Sakai K, Nakashima T, Okanoue T. Effect of iron reduction by phlebotomy in Japanese patients with nonalcoholic steatohepatitis: A pilot study. Hepatol Res. 2006;36:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Riquelme A, Soza A, Nazal L, Martínez G, Kolbach M, Patillo A, Arellano JM, Duarte I, Martínez J, Molgó M. Histological resolution of steatohepatitis after iron depletion. Dig Dis Sci. 2004;49:1012-1015. [PubMed] |
| 86. | González-Alonso J, Mortensen SP, Dawson EA, Secher NH, Damsgaard R. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: role of erythrocyte count and oxygenation state of haemoglobin. J Physiol. 2006;572:295-305. [PubMed] |
| 87. | Duke M, Abelmann WH. The hemodynamic response to chronic anemia. Circulation. 1969;39:503-515. [PubMed] |
| 88. | Roy SB, Bhatia ML, Mathur VS, Virmani S. Hemodynamic effects of chronic severe anemia. Circulation. 1963;28:346-356. [PubMed] |
| 89. | Mackintosh VS, Elsegood CL, Redgrave TG. Effects of adrenoreceptor antagonists and agonists on clearance of emulsion models of triacylglycerol-rich lipoproteins from plasma in rats. Clin Exp Pharmacol Physiol. 1991;18:775-788. [PubMed] |
| 90. | Mackintosh V, Redgrave TG. Effects of adrenaline and noradrenaline on clearance of triacylglycerol-rich lipoproteins from plasma: studies with chylomicron-like lipid emulsions in rats. Clin Exp Pharmacol Physiol. 1991;18:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 91. | Diehl AM. Lessons from animal models of NASH. Hepatol Res. 2005;33:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 92. | Ludwig J, Hashimoto E, Porayko MK, Moyer TP, Baldus WP. Hemosiderosis in cirrhosis: a study of 447 native livers. Gastroenterology. 1997;112:882-888. [PubMed] |
| 93. | Batey RG, Johnston R. Effects of alcohol, carbon tetrachloride, and choline deficiency on iron metabolism in the rat. Alcohol Clin Exp Res. 1993;17:931-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 94. | Lee AW, Oates PS, Trinder D. Effects of cell proliferation on the uptake of transferrin-bound iron by human hepatoma cells. Hepatology. 2003;38:967-977. [PubMed] |
| 95. | Sharp PA. New insights into the role of iron in the development of nonalcoholic fatty liver disease. Hepatology. 2010;52:408-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 96. | Fujita N, Takei Y. Iron overload in nonalcoholic steatohepatitis. Adv Clin Chem. 2011;55:105-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 97. | Le Guenno G, Chanséaume E, Ruivard M, Morio B, Mazur A. Study of iron metabolism disturbances in an animal model of insulin resistance. Diabetes Res Clin Pract. 2007;77:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 98. | Tsuchiya H, Sakabe T, Akechi Y, Ikeda R, Nishio R, Terabayashi K, Matsumi Y, Hoshikawa Y, Kurimasa A, Shiota G. A close association of abnormal iron metabolism with steatosis in the mice fed a choline-deficient diet. Biol Pharm Bull. 2010;33:1101-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 99. | Aigner E, Theurl I, Theurl M, Lederer D, Haufe H, Dietze O, Strasser M, Datz C, Weiss G. Pathways underlying iron accumulation in human nonalcoholic fatty liver disease. Am J Clin Nutr. 2008;87:1374-1383. [PubMed] |
| 100. | Otogawa K, Kinoshita K, Fujii H, Sakabe M, Shiga R, Nakatani K, Ikeda K, Nakajima Y, Ikura Y, Ueda M. Erythrophagocytosis by liver macrophages (Kupffer cells) promotes oxidative stress, inflammation, and fibrosis in a rabbit model of steatohepatitis: implications for the pathogenesis of human nonalcoholic steatohepatitis. Am J Pathol. 2007;170:967-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 101. | Gentile CL, Pagliassotti MJ. The role of fatty acids in the development and progression of nonalcoholic fatty liver disease. J Nutr Biochem. 2008;19:567-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |