Published online Jul 28, 2012. doi: 10.3748/wjg.v18.i28.3681
Revised: March 28, 2012
Accepted: April 12, 2012
Published online: July 28, 2012
AIM: To further investigate the role of human B7 homolog 1 (B7-H1) in the mechanism of persistent hepatitis B virus (HBV) infection.
METHODS: Peripheral and intra-hepatic B7-H1 expression were compared by flow cytometry and immunochemical staining between two 2 distinct groups, one being chronic HBV tolerance patients (CHB-T) and the other being acute hepatitis B patients (AHB). B7-H1 mRNA expression level was also compared by real time polymerase chain reaction between CHB-T and AHB patients. The location of intra-hepatic B7-H1 and CD40 expression were analyzed by immunofluorescence. The levels of B7-H1 and CD40 expression on cultured myeloid dendritic cells (mDCs) with or without hepatitis B surface antigen (HBsAg) treatment were analyzed dynamically by flow cytometry. Intracellular interferon-γ (IFN-γ) staining and the stimulatory capacity of mDC of cultured mDC with or without HBsAg treatment were also compared by flow cytometry.
RESULTS: Peripheral B7-H1 expression on mDCs was increased significantly in AHB compared to CHB-T patients (P < 0.05). In the liver tissues from CHB-T patients, B7-H1 positive cells were almost absent despite a persistently elevated serum HBsAg load. In contrast, there were indeed increased B7-H1-positive cells in situ in the liver tissue from AHB. In vitro analysis showed the parallel upregulation of B7-H1 and CD40 on CD11c+ mDCs after the onset of stimulation. Addition of recombinant hepatitis B surface antigen (rHBsAg) significantly decreased CD40 expression (P < 0.05 at 16 h, 20 h and 24 h time points). B7-H1 expression was also inhibited by rHBsAg, and the inhibition rate of CD40 was greater than that of B7-H1. This preferential inhibition of CD40 expression on mDCs by rHBsAg resulted in the dysfunction of mDCs and T cells in the mixed leucocyte reaction (MLR) system. With rHBsAg pretreatment, in a carboxyfluorescein diacetate succinimidyl ester (CFSE) labeled MLR system at a ratio of 1:5 responder cell-stimulator cell (R/S), the CFSEdim percentage of T cells decreased from 85.1% to 25.4% and decreased from 30.3% to 12.0% at 1:10 R/S. IFN-γ production by CD8+ T cells, in the MLR system, was reduced significantly by HBsAg pretreatment. At ratios of 1:5 R/S, the percentage of IFN-γ and CD8 dual positive T cells decreased from 55.2% ± 5.3% to 15.1% ± 3.1% (P < 0.001), and decreased from 35.0% ± 5.1% to 7.3% ± 2.7% at ratios of 1:10 R/S (P < 0.001).
CONCLUSION: B7-H1 is not a signature of immune dysfunction, but an inflammation marker. HBsAg regulate immune response by tipping the balance between B7-H1 and CD40.
- Citation: Zhang WJ, Xie HY, Duan X, Wan YL, Peng CH, Shi SH, Su R, Zheng ZH, Pan LL, Zhou L, Zheng SS. Study of human B7 homolog 1 expression in patients with hepatitis B virus infection. World J Gastroenterol 2012; 18(28): 3681-3695
- URL: https://www.wjgnet.com/1007-9327/full/v18/i28/3681.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i28.3681
Patients with self-limited acute hepatitis B (AHB) can develop appropriate virus-specific immune responses, however these immune responses are insufficient to eliminate the virus in chronic hepatitis B patients and eventually lead to chronic hepatitis B virus (HBV) tolerance (CHB-T). To date, the mechanisms underlying this defect of the HBV specific immune response have not been fully elucidated. T-cell exhaustion, lack of CD4+ T-cell help, induction of T-cell tolerance and viral variation may contribute to HBV persistence[1-8]. However, it is also possible that the antigen presenting cells (APCs) of hepatitis B patients fail to prime an appropriate T-cell response[9-13].
Dendritic cells (DCs) are professional APCs that are specialized for the initiation and regulation of T-cell immunity. The efficiency of DCs in activating T cells is determined by many factors. DCs express high levels of positive co-stimulatory molecules, [B7.1 (CD80), B7.2 (CD86), and CD40] which interact with receptors on T cells to mediate T cell activation[14]. On the other hand, DCs also express negative molecules such as human B7 homolog 1 (B7-H1) and human B7 homolog DC (B7DC), which bind to programmed death 1 (PD1) to deliver a co-inhibitory signal to T cells, thus leading to T-cells tolerance[14]. Based on these studies, the balance between positive and negative co-stimulatory molecules is viewed as a key factor for determining the outcome of HBV infection[15,16].
Previous studies have indicated that the B7-H1/PD-1 pathway plays a key role in myeloid dendritic cells (mDCs) dysfunction and T-cell exhaustion when these cells are exposed to high HBV or hepatitis C virus (HCV) antigen loads. In addition, blockade of the PD-1/B7-H1 interaction can restore the allostimulatory ability of mDCs and the function of HBV and HCV-specific CD8+ T cells with increased proliferation, cytotoxicity and cytokine production[2,17-19]. Some studies have reported that B7-H1 and PD1, which are expressed in circulating mDCs and HBV specific T cells respectively, were significantly upregulated in chronic hepatitis B patients. The specificity of this upregulation to HBV or HCV infection was determined by the lack of a significant increase in B7-H1 and PD1 in cytomegalovirus, Epstein-Barr virus and influenza A infected patients[20-22]. Based on these studies, relatively high levels of B7-H1/PD-1 expression were viewed as the signature of impairments in the HBV- and HCV- specific immune response. Both HBV and HCV might exploit the B7-H1/PD1 pathway to facilitate persistent infection.
On the other hand, recent studies revealed that B7-H1 and PD1 expression are significantly upregulated in the early phase of AHB infection, and successful viral clearance is correlated with a decrease in PD1 expression[12,23]. These results are in line with reports showing that B7-H1 and PD1 expression is upregulated in liver nonparenchymal cells during the acute phase of inflammation to limit an over-vigorous inflammatory response[24].
Our clinical observation revealed that chronic HBV infection was composed of 2 different components. On one hand, the CHB-T condition is characterized by active HBV-DNA replication, but shows no serum alanine aminotransferase (ALT) upregulation. The other component is a fluctuating inflammation condition that shows repeated inflammation flare-ups with fluctuating levels of serum ALT and HBV-DNA.
To examine the role of B7-H1 in the mechanism of HBV tolerance, we compared B7-H1 expression between AHB and CHB-T patients.
The results showed that peripheral and intra-hepatic expression of B7-H1 was more significant in AHB than that in CHB-T patients. In the liver tissue of CHB-T subjects, B7-H1 was almost absent despite a persistently elevated hepatitis B surface antigen (HBsAg) load. In vitro analysis showed that CD40 and B7-H1 were upregulated synchronously after the onset of stimulation, and CD40 was preferentially inhibited by recombinant hepatitis B surface antigen (rHBsAg), which impaired the allostimulatory capacity of mDCs and interferon-γ (IFN-γ) production by CD8+ T cells in the mixed leucocyte reaction (MLR) system.
Our findings showed that the analysis of B7-H1 expression alone is not sufficient to elucidate the mechanism of HBV immune tolerance. B7-H1 is an inflammatory marker, but not an absolute indicator of HBV-specific immune tolerance. The preferential inhibition of CD40 expression by rHBsAg can be considered as part of a mechanism by which HBV impairs the immune response and results in persistent infection in CHB-T patients.
This study examined 27 adults with HBV infection (18 men, 9 women) from August 2008 to August 2010 that were hospitalized in our unit. None of the patients were treated with steroids before sampling. Concurrence of HCV and human immunodeficiency virus infections were excluded from enrolled individuals. The study protocol was approved by the Ethics Committee of our unit, and informed consent was obtained from each subject. The patients were assigned to 2 distinct groups based on plasma HBV DNA loads and ALT levels. The first group (n = 12) was formed by CHB-T with HBV-DNA replication and HBs-Ag production, but normal ALT levels (normal range: 10-40 U/L). The other group (n = 15) was composed of AHB patients with active HBV replication and significantly elevated serum ALT levels. For comparison, 5 uninfected healthy controls (HC) and 3 patients with autoimmune hepatitis (AIH) were enrolled as controls. The clinical data of these patients at the time of the first physician consultation are summarized in Table 1.
| Male/female | ALT | HBsAg | HBV-DNA load | |
| AHB (n = 15) | 10/5 | 296 ± 110 | 404 ± 98 ng/mL | (2 - 7) × 104 (n = 4), (3 - 6) × 105 (n = 5), (1 - 6) × 106 (n = 4), (1 - 2) × 107 (n = 2) |
| CHB-T (n = 12) | 8/4 | Normal (10-40 μ/mL) | 363 ± 58 ng/mL | (3 - 7) × 105 (n = 6), (1 - 5) × 106 (n = 5), (1.1) × 107 (n = 1) |
| AIH (n = 3) | 0/3 | 301 ± 79.3 | Negative | Negative |
| HC (n = 5) | 3/2 | Normal (10-40 μ/mL) | Negative | Negative |
B7-H1 expression on circulating mDCs was measured using 1 mL of fresh heparinized peripheral blood. Cells were lysed with fluorescence-activated cell sorter (FACS) lysing solution (BD Pharmingen) to remove red blood cells and then incubated with antibodies against B7-H1-Phycoerythrin (PE) and CD11c-fluorescein isothiocyanate (FITC) for 20 min at room temperature. After washing twice with phosphate buffered saline (PBS), the cells were analyzed by flow cytometry on a FACSCalibur (BD Biosciences).
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood of healthy donors by Ficoll density gradient centrifugation. PBMCs were then plated (5 × 106 cells/well) into a 6-well plate and incubated at 37 °C for 2 h. T-cell-enriched and T-cell-depleted fractions were prepared by adherence to plastic in complete RPMI 1640 medium. The immature dendritic cells (iDCs) were prepared from the T-cell-depleted fraction by culturing cells in the presence of granulocyte macrophage colony-stimulating factor (50 ng/mL) and interleukin 4 (IL-4) (50 U/mL) for 5 d.
iDCs were prepared from PBMC and incubated in a 24-well plate in RPMI 1640 medium containing 10% FCS and treated with or without rHBsAg-adr (1 μg/mL). (r)HBsAg adr was purified from transfected Chinese Hamster Ovary (CHO) cells. Cell lysates of CHO without HBsAg transfection served as a negative control. After 5 d, these cells were stimulated by 20 μg poly (I:C), and then collected at 4 h, 8 h, 16 h, 20 h, and 24 h after stimulation. The cells were incubated with anti-B7-H1-PE and CD40-FITC antibodies for 20 min at room temperature. After washing three times with PBS, the expression of B7-H1 and CD40 was analyzed by flowjo7.6.
Total RNA from liver tissues was isolated using RNeasy kits (Qiagen). Reverse transcription of RNA was performed using a SuperScript One-Cycle cDNA kit (Invitrogen). The cDNA served as a template in real-time polymerase chain reaction (PCR). The human B7-H1 primers for RT-PCR were used as follow: 5'-TTTACTGTCACGGTTCCC-3' (sense) and 5'-TGTTCTTATCCTCCATTTCC-3' (antisense); human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers, 5'-CTGCCCCCTCTGCTGATG-3' (sense) and 5'-TCCACGATACCAAAGTTGTCATG-3' (antisense). All reactions were performed in triplicate. The B7-H1 mRNA expression of different specimens was normalized to GAPDH. Relative mRNA levels are presented as unit values of 2-ΔΔCt, where Ct is the threshold cycle value defined as the fractional cycle number at which the target fluorescent signal passes a fixed threshold above baseline.
Acetone-fixed liver tissue cryosections (7 μm) were incubated with anti B7-H1 antibodies (Abcam) at 4 °C overnight. After DAB peroxidase staining, positive cells (brown color) were counted in 3 different fields by 2 independent observers. To determine B7-H1, CD40, CD68, macrophage inflammatory protein (MIP3) α, secondary lymphoid tissue chemokine (SLC), CD11c, chemokine (C-C) receptor (CCR) 7 and HBsAg expression, immunofluorescence double staining was performed. Briefly, liver tissues were incubated for 12 h at 4 °C with diluted primary Abs followed by diluted secondary Abs for 1 h at room temperature. The following primary antibodies were used for CD11c, CD40, B7-H1, CD68, SLC, MIP3α, CCR7 and HBs-Ag: mouse antihuman CD40, CD68 and CD11c (diluted 1:100; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, United States), and-, goat antihuman B7-H1, MIP3α, SLC, CCR7 (diluted 1:100; Santa Cruz). As secondary antibodies, a rabbit anti-goat IgG PE-conjugated antibody (diluted 1:100; Santa Cruz), a rat anti-mouse IgG FITC-conjugated antibody, and a rat anti-mouse IgG rhodamine-conjugated antibody were used.
Purified mDCs with or without HBsAg treatment were matured for 24 h in a 96-well flat-bottom culture plate (at 1 × 105 cells/200 μL) in culture medium containing poly (I:C) (20 μg /mL; Sigma). On the following day, mDCs were treated with mitomycin C and were added at different concentrations (1/5 and 1/10) into purified T cells from a normal healthy volunteer (1 × 105 cells/200 μL) and labeled with carboxyfluorescein diacetate succinimidyl ester [CFSE (1 mmol/mL); Molecular Probes, Eugene, OR] for 5 d. T-cells proliferation was assessed by the loss of CFSE analyzed by flow cytometry. Flow cytometric data were analyzed by CellQuest (Becton Dickinson). All measurements were performed in triplicate.
Next, IFN-γ produced during these MLRs was measured. T cells were collected after 5 d of mixed culture, phorbol myristate acetate (1 μg/mL), ionomycin (0.1 mg/mL; T cell receptor-bypassing reagents) and 0.1 mg/mL monamycin (Sigma-Aldrich) were added into a mixed culture system 4 h before analysis. T cells were then washed in PBS, stained with anti-CD8 (FITC) mAb and then permeabilized and fixed according to the manufacturer’s instructions. After a further wash in PBS, cells were stained with anti-IFN-γ-PE mAb at room temperature for 20 min. After 2 additional washes, cells were fixed and acquired immediately on flow cytometry on a FACSCalibur (BD Biosciences).
All experimental conditions were pair analyzed against their controls. Data on co-stimulatory molecules, and cytokine and B7-H1 mRNA expression were analyzed by the Student’s t test. The correlation between ALT levels and B7-H1 expression obtained by FACS was assessed by Pearson’s correlation analysis. Data corresponding to the expression of co-stimulatory molecules and cytokine production are expressed as mean ± SE. A P value of < 0.05 was considered statistically significant.
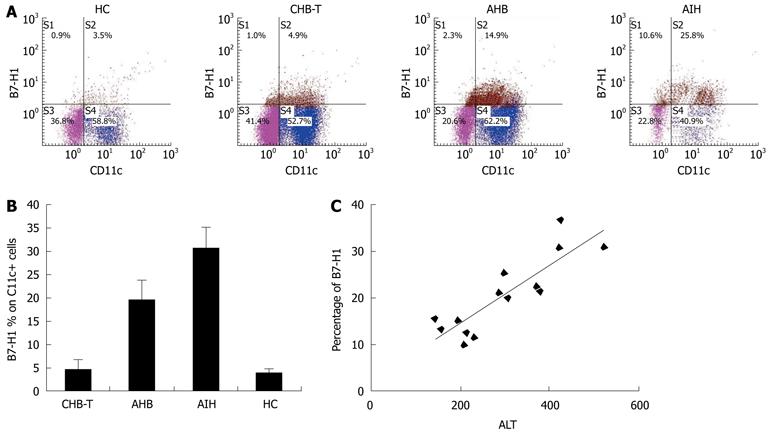
The levels of B7-H1 expression were detected on circulating CD11c + DCs from AHB, CHB-T, and AIH patients, and HC (Figure 1A). B7-H1 expression on circulating CD11c + DCs from enrolled patients was generally increased, in particular in AHB patients, who expressed higher levels of B7-H1 than CHB-T individuals (P < 0.05, Figure 1B). In CHB-T patients, B7-H1 expression also increased, but was not significantly different from that in HC. AIH patients exhibited the highest levels of B7-H1 expression on CD11c + DCs among these groups (all P < 0.05, Figure 1B). Correlation analysis revealed that there was a significant, positive correlation between B7-H1 expression on circulating CD11c + DCs and serum ALT levels in AHB patients (r = 0.809, Figure 1C).
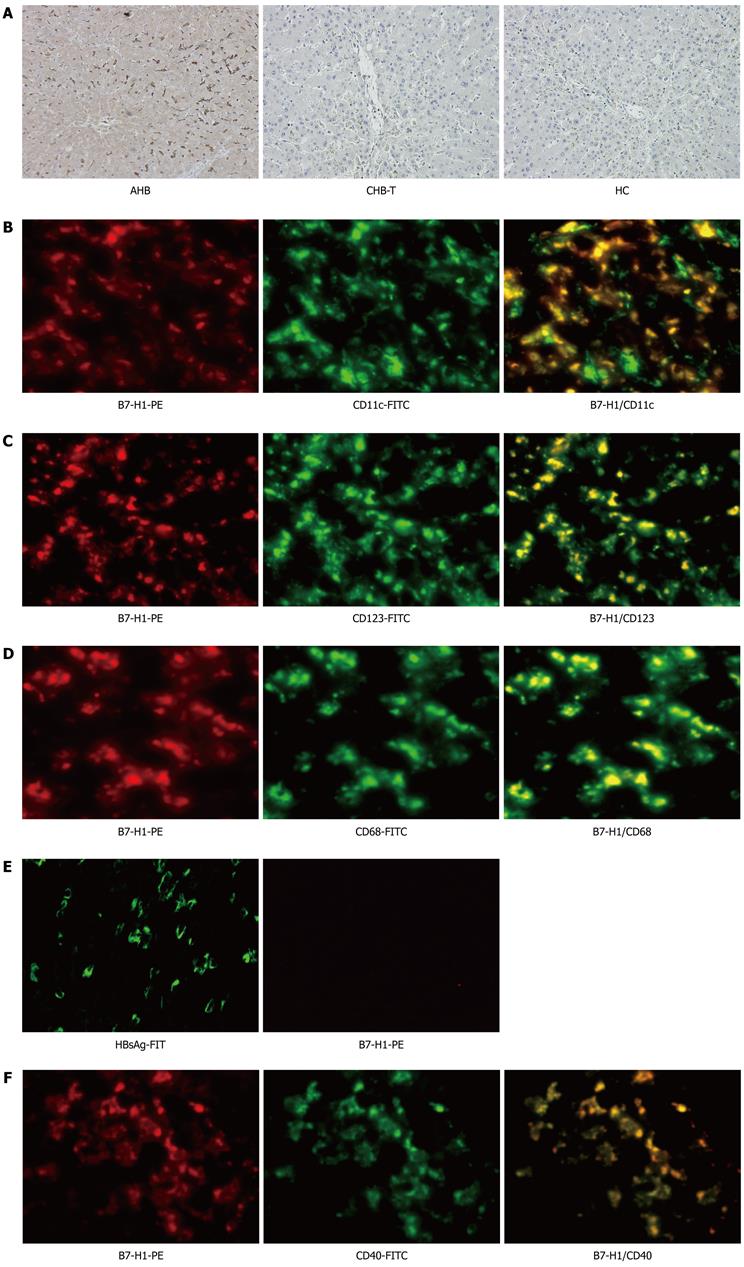
Immunohistochemical staining performed to analyze intrahepatic B7-H1 expression showed an increased number of B7-H1-positive cells in situ in the livers of AHB patients. In contrast, B7-H1-positive cells were almost completely absent in the livers of CHB-T subjects and healthy donors (Figure 2A). Immunofluorescence double staining revealed that almost all CD11c + DCs, CD123 + DCs and Kupffer cells expressed B7-H1 molecules in the liver tissue from AHB patients (Figure 2B-D), while in liver tissue from CHB-T patients, B7-H1 positive cells were almost completely absent despite extensive HBsAg expression (Figure 2E). These results were in line with data obtained with circulating CD11c + DCs. In addition, in the liver tissue from AHB patients, B7-H1 was always co-expressed with the positive co-stimulatory molecule CD40 (Figure 2F).
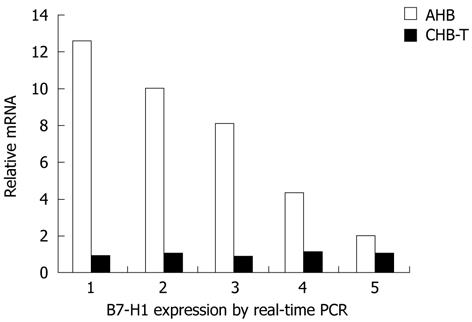
B7-H1 mRNA expression levels in liver tissues were further compared between AHB and CHB-T patients by real-time PCR. As show in Figure 3, B7-H1 mRNA expression levels were significantly higher in AHB than in CHB-T liver tissue. The highest relative B7-H1 mRNA expression level in AHB liver tissue showed a 12-fold increase over the CHB-T level. The lowest B7-H1 mRNA level showed a 2-fold increase in AHB compared to CHB-T liver tissue (Figure 3).
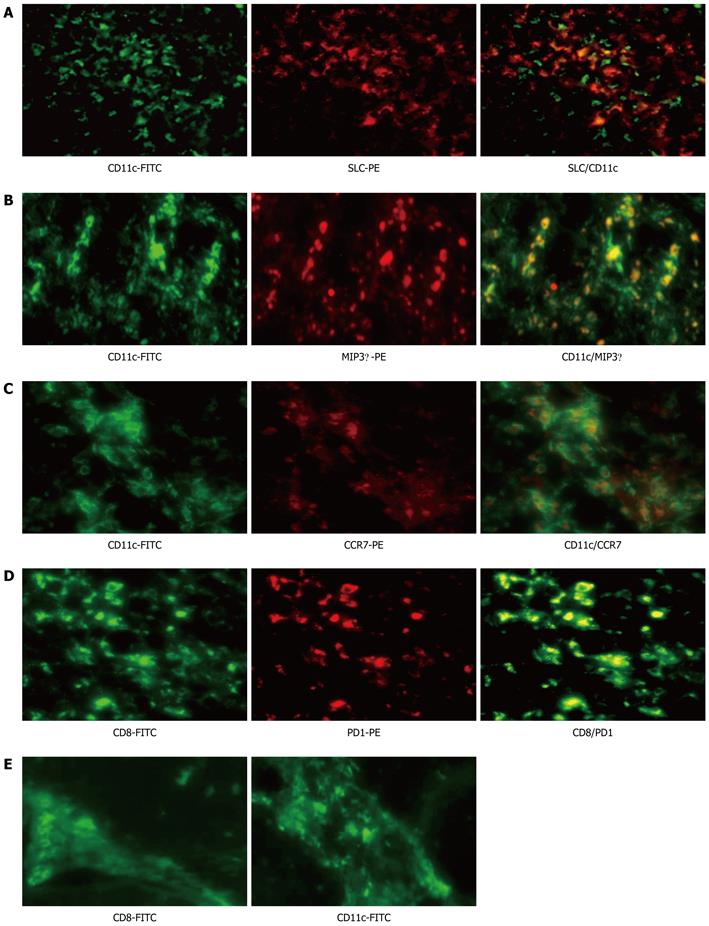
Peripheral and intrahepatic B7-H1 expression in AHB patients was far more significant than that of CHB-T. These results suggest that B7-H1 is a marker of AHB. To further confirm this conclusion, serially sectioned liver tissues from AHB patients were examined for the markers of inflammatory response, including DCs, lymphocytes infiltration and chemokines expression by immunofluorescence double staining. Liver tissue with significant B7-H1 expression showed significant infiltration of CD11c+ and CD8+-positive cells. Chemokines such as MIP3α and SLC were also expressed extensively by CD11c + DCs (Figure 4A and B). Mature mDCs expressing CCR7 and activated CD8+ lymphocytes expressing PD1 were recruited to the liver lobe (Figure 4D). In contrast, in liver tissue from CHB-T patients with significant HBsAg but no B7-H1 expression, CD11c+ and CD8+ infiltration cells were predominantly localized in fibrous septa rather than in the sinusoidal area (Figure 4E). These results suggest that B7-H1 was preferentially expressed at the site of AHB.
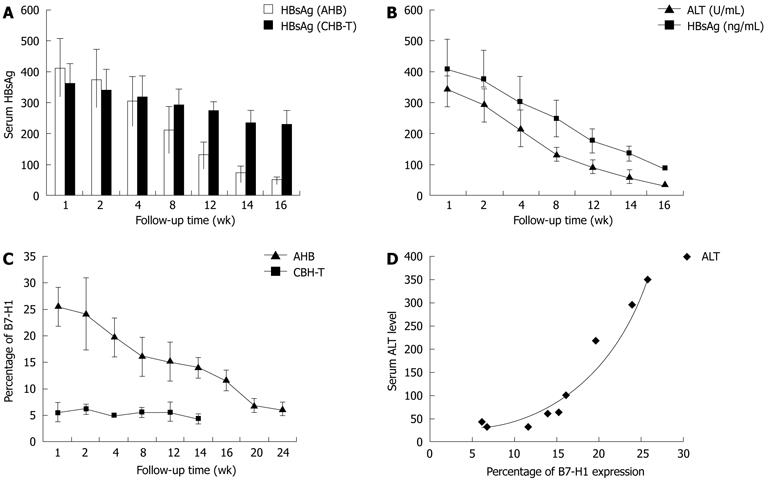
The correlation between B7-H1 expression and the degree of inflammation in hepatitis was analyzed by detecting B7-H1 expression on circulating mDCs during a follow-up period ranging from 1 mo to 6 mo. In AHB patients, the suppression of HBV replication after anti-viral treatment was accompanied by a decrease in serum HBsAg and ALT levels, and followed by a gradual decrease in B7-H1 expression on circulating mDCs (Figure 5A and B). Two or three mo after the ALT level returned to normal range, the percentage of B7-H1 positive cells decreased within CD11c positive cells from 21.3 ± 5.6 to 6.1 ± 1.4 (Figure 4C). B7-H1 expression levels, as assessed by mean fluorescence intensity (MFI), were also reduced (data not shown). The level of B7-H1 expression increased during the period of acute hepatitis and decreased when inflammation was reduced; supporting the conclusion that B7-H1 is an inflammatory marker. Notably, B7-H1 expression levels remained relatively high (13.2 ± 3.3) when ALT returned to normal range at 16 wk (Figure 5C). Two or three months after the return of serum ALT level to normal, B7-H1expression further decreased to 6.1 ± 1.4, approaching healthy control levels (Figure 5B and C). Longitudinal correlation analysis revealed a positive correlation between B7-H1 expression and serum ALT levels in AHB patients (Figure 5D). In CHB-T patients, although serum HBV-DNA and HBS-Ag load remained high, peripheral B7-H1 expression levels did not increased significantly (Figure 5C).
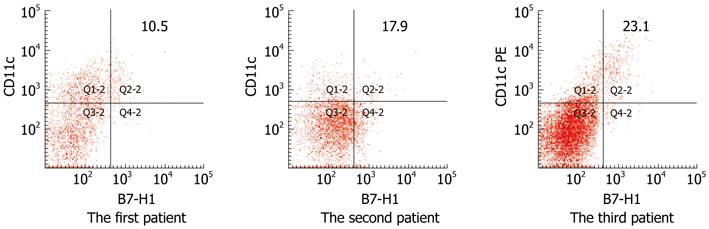
As mentioned above, chronic HBV infection had two different components, namely the CHB-T condition and the chronic active hepatitis status, which was characterized by repeated inflammation flare ups. Detection of peripheral B7-H1 expression in 3 chronic active hepatitis B patients during the inflammatory flare up phase with increasing serum ALT and HBV-DNA level showed that B7-H1 expression on circulating CD11c positive cells increased significantly in all three patients (Figure 6).
The above data showed that the intra and extra-hepatic HBs-Ag load remained persistently high in CHB-T subjects, while B7-H1 and CD40 expression were nearly absent. To further explore the relationship between HBsAg load and B7-H1 and CD40 expression, the effects of HBsAg towards B7-H1 and CD40 expression were analyzed in vitro. Population of mDCs with and without HBsAg pretreatment in PBMCs was determined by flow cytometry. The forward scatter (FSC) and side scatter (SSC) of mDCs with rHBsAg pretreatment were not altered significantly compared to those without rHBsAg pretreatment (Figure 7A). This result suggests that the vitality of mDCs with or without HBsAg pretreatment is similar.
The kinetics of CD40 and B7-H1 expression on poly (I:C)-stimulated mDCs with or without rHBs-Ag pretreatment were analyzed in vitro. The expression of B7-H1 and CD40 on mDCs was upregulated synchronously in response to poly (I:C) stimulation. The expression of CD40 was detected on the surface of mDCs at 1h and gradually increased to peak level at 24 h after the onset of stimulation. During the same period, B7-H1 also increased gradually, reaching the highest expression level after 24 h of stimulation. In addition, when rHBs-Ag was added during the period of DC maturation at a final concentration of 1 μg/mL, the up-regulation of CD40 and B7-H1 was inhibited simultaneously (Figure 7B-D). The rate of CD40 and B7-H1 inhibition was calculated using the following equation: inhibition rate = 1 - (MFI from mDCs with HBsAg pretreatment/MFI from mDCs without HBsAg pretreatment]. It is noteworthy that the inhibition rate of CD40 was greater than that of B7-H1 (Figure 7E).
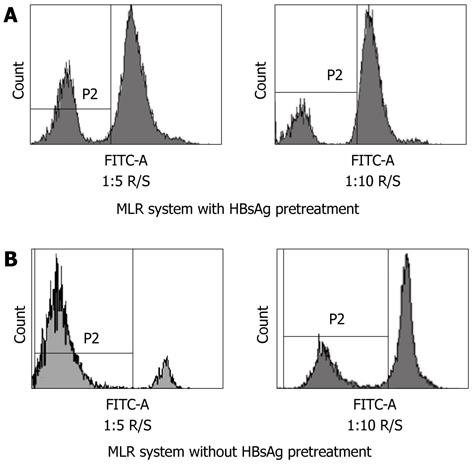
Since the inhibition rate of CD40 was greater than that of B7-H1, we further investigated whether the T-cell stimulatory capacity of mDCs pretreated by HBs-Ag was impaired. The mDC was stimulated by poly (I:C) for 20 h with or without HBs-Ag pretreatment, and co-cultured at 1:5 and 1:10 ratios with HLA-mismatched allogeneic T cells labeled with cytoplasmic dye CFSE. After 5 d of incubation, T-cell proliferation was assessed by the dilution of CFSE. In the allo-MLR system pretreated by HBsAg, with 1:5 and 1:10 responder cell-stimulator cell (R/S) ratios, approximately 25.4% or 12.0% of the responder T cells were CFSEdim proliferating blasts, respectively (Figure 8A). In the allo-MLR system without HBsAg pretreatment, the percentage of CFSEdim proliferating T cells was 85.1% in the 1:5 R/S ratio and 30.3% in the 1:10 R/S ratio, respectively (Figure 8B). The results showed that mDCs pretreated by HBsAg were less efficient at inducing T-cell proliferation at ratios of 1:5 and 1:10 compared with mDCs without HBsAg pretreatment.
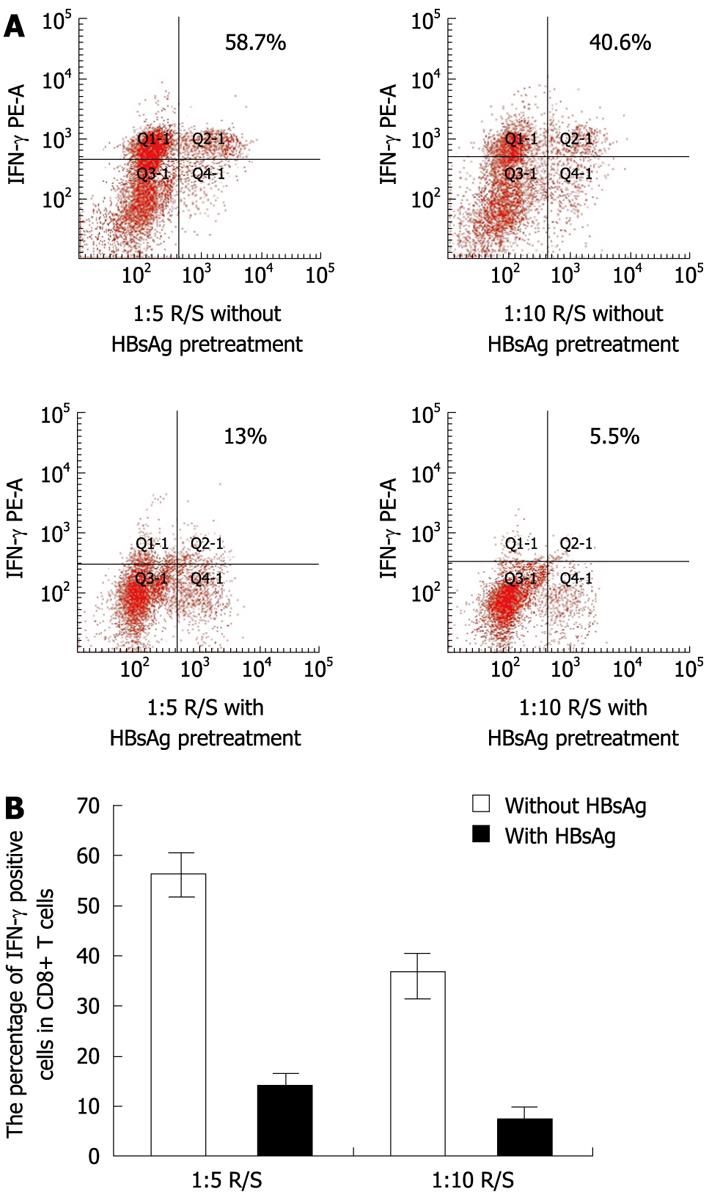
In correlation with T-cell proliferation, the intracellular IFN-γ produced in these MLRs was measured after 5 d of co-culture. In the MLRs system at ratios of 1:5 and 1:10 R/S, without HBsAg pretreatment, the percentage of IFN-γ positive cells in CD8+ T cells was greater than 55.2% ± 5.3% and 35.0% ± 5.1% respectively. In the MLRs system, with HBsAg pretreatment, the percentage of IFN-γ positive cells in CD8+ T cells decreased to 15.1% ± 3.1% at a 1:5 R/S ratio and 7.3% ± 2.7% at a 1:10 R/S ratio, respectively (Figure 9A and B). These results showed that IFN-γ production by CD8+ T cells was reduced significantly in the HBsAg pretreated MLRs system.
Recent studies have shown that functional defects in DCs may play a pivotal role in viral persistence during chronic HBV infection. However, the molecular mechanism by which the impaired mDCs induce HBV-specific T cell immune tolerance remains elusive. Previous studies implicated increased B7-H1/PD-1 signaling in DC malfunction and viral-specific T-cell exhaustion in persistent HBV or HCV infections, which were associated with disease progression. In vitro blockade of B7-H1 signaling not only enhanced the mDC mediated allostimulatory capacity, but also up-regulated IL-12 production[17-22]. Based on these data, B7-H1 was viewed as a signature of impairments in HBV-specific immune response.
The present data indicate that B7-H1 is not a signature of HBV immune malfunction, but rather an inflammatory marker of acute hepatitis. Firstly, AIH patients, who did not possess viral etiology, exhibited the highest levels of B7-H1 expression on mDCs among the groups studied. Secondly, B7-H1 expression at protein and mRNA levels was more significant in AHB patients, who were characterized by high levels of serum ALT, than in CHB-T subjects. Thirdly, in AHB patients, despite significant upregulation of intra-hepatic B7-H1 expression, the inflammatory response in liver tissues was vigorous, while in the liver tissues from CHB-T subjects, markers of inflammation were not observed in liver lobe. Only a few inflammatory cells were located in fibrous septa, and B7-H1 molecules were mostly absent despite extensive HBsAg expression. Finally, longitudinal analysis revealed that the level of B7-H1 expression increased during the period of acute hepatitis and decreased when the inflammation was reduced.
Based on our clinical observations, chronic hepatitis B was divided into 2 different components, namely the immune tolerant HBV condition (characterized by active HBV-DNA replication, but with no serum ALT level upregulation) and the chronic active hepatitis condition (which show repeated inflammation flare ups).
In the present study, B7-H1 expression was also analyzed on circulating mDCs in chronic active hepatitis subjects during the hepatitis flare-up period, and the results showed that B7-H1 expression on circulating mDCs was up-regulated to the same extent as in AHB subjects. These data further demonstrate that B7-H1 is a marker of hepatitis.
The current longitudinal analysis revealed that B7-H1 expression on circulating mDCs remained relatively high when serum ALT returned to normal range on week 16, but decreased to near normal level after serum ALT had remained within normal range for 2 mo or 3 mo, indicating a lag phase between the decrease of serum ALT and the return of B7-H1 expression to normal levels. If the blood sample was acquired within the lag phase, B7-H1 expression was higher than in HC.
In the liver tissue of AHB patients, the majority of B7-H1-positive cells co-expressed CD40, indicating that B7-H1 is always expressed on activated APCs. These results are in line with previous findings showing that B7-H1 was significantly increased by proinflammatory cytokines, including IFN-γ and tumor necrosis factor-α (TNF-α)[12,23]. The present results also show the presence of markers of inflammation such as infiltrating inflammatory cells and elevated chemokine expression in the liver tissue from AHB patients, with significant B7-H1 expression. On the other hand, in the liver lobes of CHB-T subjects, both B7-H1 molecules and other inflammatory characters were almost absent. Correlation analysis revealed a positive correlation between B7-H1 expression and liver damage, reflected by the levels of serum ALT, both cross-sectionally and longitudinally. Based on these data, we concluded that B7-H1 expression can be induced by inflammatory microenvironment and that the level of B7-H1 expression can serve as a marker of the degree of inflammation.
In vitro analysis showed that the expression of B7-H1 and CD40 on mDCs was upregulated synchronously in response to poly (I:C) stimulation. This result suggested that a delicate balance between the positive and negative co-stimulate molecules may exist in activated mDCs. The B7-H1/ PD1 interaction may be involved in weakening the positive stimulatory signal, limiting the immune response to avoid an extensive inflammatory response. This concept is in line with several studies supporting the conclusion that the B7-H1/PD-1 pathway may assist the liver in protecting itself from immune-mediated destruction[24-28]. Zhang et al[23] reported that the delayed expression of B7-H1 or PD1 lead to fulminant hepatitis. In conclusion, the up-regulation of B7-H1 may contribute to maintaining immune response under the upper limit to avoid severe immune mediated liver damage during acute HBV infection. In CHB-T patients, immune responses were not elicited and B7-H1 expression was not elevated. These phenomena are consistent with the rule of immune regulation, which states “no activation, no inhibition”.
Previous data revealed that the HBV antigen has immunoregulatory abilities. Studies have reported that exposure to higher level of HBsAg load may lead to mDC dysfunction[29,30]. Op den Brouw et al[10] and Loirat et al[31] reported that both HBsAg and HCsAg have the ability to suppress CD40 or HLA-DR expression and thus contribute to HBV or HCV persistence. Loirat et al[31] reported that recombinant HBsAg interacts with monocytes through the lipopolysaccharide (LPS) receptor CD14, resulting in diminished LPS-induced monocyte activation. HBsAg was shown to reduce LPS-induced TNF-α production through interference with the activation of extracellular regulated protein kinases-1,2 and c-Jun NH2-terminal kinase-1,2[32-34]. These reports suggest that HBsAg and HBsAg have the ability to weaken the immune response.
The results of the present study revealed that HBsAg load was maintained at a high level in liver tissue as well as in peripheral blood from CHB-T subjects with no significant B7-H1 upregulation. Based on these previous data and the present results, we speculated that B7-H1 expression may also be inhibited by HBsAg, followed by CD40 suppression. To confirm this, the kinetic B7-H1 and CD40 expression in isolated mDCs stimulated by poly (I:C) with or without HBsAg pretreatment were analyzed. The results showed that the upregulation of CD40 and B7-H1 were significantly inhibited by HBsAg. Interestingly, HBsAg displayed a preferential inhibitory effect towards CD40 upregulation. The inhibition rate of CD40 was significantly greater than that of B7-H1, suggesting that HBsAg has the ability to regulate the balance between the CD40 and B7-H1.
Several previous studies have shown the activation of DCs after HBsAg impulsion, but either uric acid or cytokines were added in these investigations to active DCs[35,36]. Horiike et al[36] reported that an alum adjuvant was needed in the HBsAg vaccine to active DCs during vaccine therapy. Proof of the low immunogenicity of HBsAg was shown in the research by Reignat et al[37] and Webster et al[38] which demonstrated that HBsAg specific CD8+ T cells are characterized by an HBV-tolerant phenotype.
Whether the preferential inhibition of CD40 resulted in the impairment of T cell proliferation by mDCs remains unclear. To elucidate this aspect, we compared the T cell stimulatory capacity of mDCs with or without HBsAg pretreatment by MLR. The results showed that T cell proliferation at 1:5 and 1:10 (R/S) ratio was significantly decreased in the HBsAg pretreated MLR system. In addition to T cells proliferation, we examined whether antiviral ability of T cells was affected. Because IFN-γ plays a key role in the control virus infection, the intracellular content of IFN-γ produced during these MLRs was analyzed by flow cytometry. We found that in the HBsAg-pretreated MLR system, IFN-γ production by CD8+ T cells decreased significantly. Only 15% of CD8+ T cells were IFN-γ positive, while more than 50% were IFN-γ positive in the MLR without HBsAg pretreatment. These results suggested that HBsAg has the ability to regulate the immune response by regulating the balance between CD40 and B7-H1, leading to a reduction in T-cell proliferation and IFN-γ production.
Our results indicated that B7-H1 is an inflammatory marker. It is induced by the inflammatory microenviroment and can serve as a marker of the degree of inflammation. The increase in B7-H1 expression correlated with the expression of positive co-stimulatory molecules to weaken the activation signal for T cells and generate a balance between co-inhibitory and co-stimulatory signal. HBsAg has the ability to tip this balance through preferential inhibition of CD40 expression, leading to a reduction in T cell proliferation and IFN-γ production. In CHB-T subjects, maintenance of high HBsAg load may impair HBV immunity through the regulation of the balance between CD40 and B7-H1 expression.
Based on our results, we speculated that the outcome of hepatitis B depends on 2 factors. The first is the level of HBV antigen load and HBV replication. The second is the upper limit of the HBV specific immune response set by co-inhibitory molecules such as B7-H1. If the HBV antigen and HBV-DNA are eliminated successfully by the immune response under this upper limit, the hepatitis B patient should recover. The higher the upper limit, the better the chance of HBV elimination.
Patients with self-limited acute hepatitis B (AHB) can develop appropriate virus-specific immune responses, however these immune responses are insufficient to eliminate the virus in chronic hepatitis B patients and eventually lead to chronic hepatitis B virus (HBV) tolerance. Previous studies have shown that the programmed death 1 and human B7 homology 1 (PD1/B7-H1) interaction impairs HBV-specific immune response and facilitates HBV specific immune tolerance. HBV persistent infection was always attributed to the up-regulation of PD1 and B7-H1 expression. To date, the mechanisms underlying this defect of the HBV specific immune response have not been fully elucidated.
Studies have indicated that the B7-H1/PD1 pathway plays a key role in myeloid dendritic cells (mDCs) dysfunction and T-cell exhaustion when these cells are exposed to high HBV or hepatitis C virus (HCV) antigen loads. The blockade of the PD1/B7-H1 interaction can restore the allostimulatory ability of mDCs and the function of HBV and HCV-specific CD8+ T cells with increased proliferation, cytotoxicity and cytokine production. Some studies have reported that the B7-H1 and PD1 expression were significantly upregulated in chronic hepatitis B patients. The specificity of this upregulation to HBV or HCV infection was determined by the lack of a significant increase in B7-H1 and PD1 in cytomegalovirus, Epstein-Barr virus and influenza A-infected patients. Based on these studies, relatively high levels of B7-H1/PD1 expression were viewed as the signature of impairments in the HBV- and HCV- specific immune response. Both HBV and HCV might exploit the B7-H1/PD1 pathway to facilitate persistent infection. On the other hand, recent studies revealed that B7-H1 and PD1 expression is significantly upregulated in the acute phase of hepatitis B. The upregulation of B7-H1 and PD1 expression protects tissue from severe immune mediated liver damage during AHB. These results are in line with reports showing that B7-H1 and PD1 expression is upregulated in liver nonparenchymal cells during the acute phase of inflammation to limit an over-vigorous inflammatory response.
In this article, the authors reached the conclusion that co-inhibitory molecules, such as B7-H1, are always upregulated during AHB. This phenomenon suggests that co-inhibitory molecules set the upper-limit to immune response to avoid severe liver damage by an over-vigorous immune response. If HBV antigen and HBV-DNA are successfully eliminated by the immune response under this upper limit, the hepatitis B patient should recover. The higher the upper limit, the better the chance of HBV elimination.
Previous studies attributed malfunction of HBV specific immune response to higher expression levels of PD1 and B7-H1. Based on our data, the authors reached the conclusion that B7-H1 is always upregulated during AHB. Increasing co-inhibitory signals, such as PD1/B7-H1, set the upper-limit to the immune response. If HBV were eliminated under the upper limit of immune response, the hepatitis B patient should recover. This study provides new insight into a co-inhibitory signal pathway function during the clinical course of AHB.
Due to simultaneous expression of CD40 and B7-H1, the authors supposed that an upper threshold of the immune response set by co-inhibitory molecules may exist. HBV antigens should be eliminated efficiently before the immune response reached the upper threshold level, otherwise acute HBV infection may turn into a chronic infectious state. The design of this study is reasonable; the results and conclusion of this paper are reliable; and the notion about an “upper threshold of HBV specific immune response” is somewhat attractive.
Peer reviewer: Thomas Bock, Professor, Robert Koch-Institute, Nordufer 20, 13353 Berlin, Germany
S- Editor Gou SX L- Editor Rutherford A E- Editor Zheng XM
| 1. | Yao ZQ, King E, Prayther D, Yin D, Moorman J. T cell dysfunction by hepatitis C virus core protein involves PD-1/PDL-1 signaling. Viral Immunol. 2007;20:276-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Evans A, Riva A, Cooksley H, Phillips S, Puranik S, Nathwani A, Brett S, Chokshi S, Naoumov NV. Programmed death 1 expression during antiviral treatment of chronic hepatitis B: Impact of hepatitis B e-antigen seroconversion. Hepatology. 2008;48:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398-11403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 453] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 4. | Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, Missale G, Ferrari C. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 231] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Lazarevic I, Cupic M, Delic D, Svirtlih NS, Simonovic J, Jovanovic T. Prevalence of hepatitis B virus MHR mutations and their correlation with genotypes and antiviral therapy in chronically infected patients in Serbia. J Med Virol. 2010;82:1160-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Cui XJ, Cho YK, Song HJ, Choi EK, Kim HU, Song BC. Molecular characteristics and functional analysis of full-length hepatitis B virus quasispecies from a patient with chronic hepatitis B virus infection. Virus Res. 2010;150:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Urbani S, Boni C, Amadei B, Fisicaro P, Cerioni S, Valli MA, Missale G, Ferrari C. Acute phase HBV-specific T cell responses associated with HBV persistence after HBV/HCV coinfection. Hepatology. 2005;41:826-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Kondo Y, Ueno Y, Kobayashi K, Kakazu E, Shiina M, Inoue J, Tamai K, Wakui Y, Tanaka Y, Ninomiya M. Hepatitis B virus replication could enhance regulatory T cell activity by producing soluble heat shock protein 60 from hepatocytes. J Infect Dis. 2010;202:202-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Fan Y, Jiang WZ, Wen JJ, Hao WL, Du JN, Liu X, Qian M. B7-DC-silenced dendritic cells induce stronger anti-HBV immunity in transgenic mice. Arch Virol. 2009;154:1813-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Op den Brouw ML, Binda RS, van Roosmalen MH, Protzer U, Janssen HL, van der Molen RG, Woltman AM. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: a possible immune escape mechanism of hepatitis B virus. Immunology. 2009;126:280-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (2)] |
| 11. | Duan XZ, Zhuang H, Wang M, Li HW, Liu JC, Wang FS. Decreased numbers and impaired function of circulating dendritic cell subsets in patients with chronic hepatitis B infection (R2). J Gastroenterol Hepatol. 2005;20:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Chen L, Zhang Z, Chen W, Zhang Z, Li Y, Shi M, Zhang J, Chen L, Wang S, Wang FS. B7-H1 up-regulation on myeloid dendritic cells significantly suppresses T cell immune function in patients with chronic hepatitis B. J Immunol. 2007;178:6634-6641. [PubMed] |
| 13. | Geng L, Jiang G, Fang Y, Dong S, Xie H, Chen Y, Shen M, Zheng S. B7-H1 expression is upregulated in peripheral blood CD14+ monocytes of patients with chronic hepatitis B virus infection, which correlates with higher serum IL-10 levels. J Viral Hepat. 2006;13:725-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607-1618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 476] [Cited by in RCA: 461] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 15. | Selenko-Gebauer N, Majdic O, Szekeres A, Höfler G, Guthann E, Korthäuer U, Zlabinger G, Steinberger P, Pickl WF, Stockinger H. B7-H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J Immunol. 2003;170:3637-3644. [PubMed] |
| 16. | Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 181] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | Rutebemberwa A, Ray SC, Astemborski J, Levine J, Liu L, Dowd KA, Clute S, Wang C, Korman A, Sette A. High-programmed death-1 levels on hepatitis C virus-specific T cells during acute infection are associated with viral persistence and require preservation of cognate antigen during chronic infection. J Immunol. 2008;181:8215-8225. [PubMed] |
| 19. | Jeong HY, Lee YJ, Seo SK, Lee SW, Park SJ, Lee JN, Sohn HS, Yao S, Chen L, Choi I. Blocking of monocyte-associated B7-H1 (CD274) enhances HCV-specific T cell immunity in chronic hepatitis C infection. J Leukoc Biol. 2008;83:755-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215-4225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 737] [Article Influence: 40.9] [Reference Citation Analysis (1)] |
| 21. | Kasprowicz V, Schulze Zur Wiesch J, Kuntzen T, Nolan BE, Longworth S, Berical A, Blum J, McMahon C, Reyor LL, Elias N. High level of PD-1 expression on hepatitis C virus (HCV)-specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J Virol. 2008;82:3154-3160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 22. | Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249-9258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 308] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 23. | Zhang Z, Zhang JY, Wherry EJ, Jin B, Xu B, Zou ZS, Zhang SY, Li BS, Wang HF, Wu H. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology. 2008;134:1938-149, 1938-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198:39-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 308] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 25. | Mühlbauer M, Fleck M, Schütz C, Weiss T, Froh M, Blank C, Schölmerich J, Hellerbrand C. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol. 2006;45:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 26. | Karrar A, Broomé U, Uzunel M, Qureshi AR, Sumitran-Holgersson S. Human liver sinusoidal endothelial cells induce apoptosis in activated T cells: a role in tolerance induction. Gut. 2007;56:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ, Lu L, Qian S. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40:1312-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 249] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 28. | Bertoletti A, Gehring AJ. The immune response during hepatitis B virus infection. J Gen Virol. 2006;87:1439-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 293] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 29. | Kassel R, Cruise MW, Iezzoni JC, Taylor NA, Pruett TL, Hahn YS. Chronically inflamed livers up-regulate expression of inhibitory B7 family members. Hepatology. 2009;50:1625-1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Saito K, Ait-Goughoulte M, Truscott SM, Meyer K, Blazevic A, Abate G, Ray RB, Hoft DF, Ray R. Hepatitis C virus inhibits cell surface expression of HLA-DR, prevents dendritic cell maturation, and induces interleukin-10 production. J Virol. 2008;82:3320-3328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Loirat D, Mancini-Bourgine M, Abastado JP, Michel ML. HBsAg/HLA-A2 transgenic mice: a model for T cell tolerance to hepatitis B surface antigen in chronic hepatitis B virus infection. Int Immunol. 2003;15:1125-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Vanlandschoot P, Van Houtte F, Roobrouck A, Farhoudi A, Leroux-Roels G. Hepatitis B virus surface antigen suppresses the activation of monocytes through interaction with a serum protein and a monocyte-specific receptor. J Gen Virol. 2002;83:1281-1289. [PubMed] |
| 33. | Vanlandschoot P, Roobrouck A, Van Houtte F, Leroux-Roels G. Recombinant HBsAg, an apoptotic-like lipoprotein, interferes with the LPS-induced activation of ERK-1/2 and JNK-1/2 in monocytes. Biochem Biophys Res Commun. 2002;297:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Shimizu Y, Guidotti LG, Fowler P, Chisari FV. Dendritic cell immunization breaks cytotoxic T lymphocyte tolerance in hepatitis B virus transgenic mice. J Immunol. 1998;161:4520-4529. [PubMed] |
| 35. | Ma XJ, Tian DY, Xu D, Yang DF, Zhu HF, Liang ZH, Zhang ZG. Uric acid enhances T cell immune responses to hepatitis B surface antigen-pulsed-dendritic cells in mice. World J Gastroenterol. 2007;13:1060-1066. [PubMed] |
| 36. | Horiike N, Md Fazle Akbar S, Ninomiya T, Abe M, Michitaka K, Onji M. Activation and maturation of antigen-presenting dendritic cells during vaccine therapy in patients with chronic hepatitis due to hepatitis B virus. Hepatol Res. 2002;23:38-47. [PubMed] |
| 37. | Reignat S, Webster GJ, Brown D, Ogg GS, King A, Seneviratne SL, Dusheiko G, Williams R, Maini MK, Bertoletti A. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J Exp Med. 2002;195:1089-1101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 186] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 38. | Webster GJ, Reignat S, Brown D, Ogg GS, Jones L, Seneviratne SL, Williams R, Dusheiko G, Bertoletti A. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J Virol. 2004;78:5707-5719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 330] [Article Influence: 15.7] [Reference Citation Analysis (1)] |