Published online Jul 28, 2012. doi: 10.3748/wjg.v18.i28.3673
Revised: March 27, 2012
Accepted: March 29, 2012
Published online: July 28, 2012
AIM: To investigate the clinicopathological features of intraductal neoplasm of the intrahepatic bile duct (INihB).
METHODS: Clinicopathological features of 24 cases of INihB, which were previously diagnosed as biliary papillomatosis or intraductal growth of intrahepatic biliary neoplasm, were reviewed. Mucin immunohistochemistry was performed for mucin (MUC)1, MUC2, MUC5AC and MUC6. Ki-67, P53 and β-catenin immunoreactivity were also examined. We categorized each tumor as adenoma (low grade), borderline (intermediate grade), and malignant (carcinoma in situ, high grade including tumors with microinvasion).
RESULTS: Among 24 cases of INihB, we identified 24 tumors. Twenty of 24 tumors (83%) were composed of a papillary structure; the same feature observed in intraductal papillary neoplasm of the bile duct (IPNB). In contrast, the remaining four tumors (17%) showed both tubular and papillary structures. In three of the four tumors (75%), macroscopic mucin secretion was limited but microscopic intracellular mucin was evident. Histologically, 16 tumors (67%) were malignant, three (12%) were borderline, and five (21%) were adenoma. Microinvasion was found in four cases (17%). Immunohistochemical analysis revealed that MUC1 was not expressed in the borderline/adenoma group but was expressed only in malignant lesions (P = 0.0095). Ki-67 labeling index (LI) was significantly higher in the malignant group than in the borderline/adenoma group (22.2 ± 15.5 vs 7.5 ± 6.3, P < 0.01). In the 16 malignant cases, expression of MUC5AC showed borderline significant association with high Ki-67 LI (P = 0.0622). Nuclear expression of β-catenin was observed in two (8%) of the 24 tumors, and these two tumors also showed MUC1 expression. P53 was negative in all tumors.
CONCLUSION: Some cases of INihB have a tubular structure, and are subcategorized as IPNB with tubular structure. MUC1 expression in INihB correlates positively with degree of malignancy.
- Citation: Naito Y, Kusano H, Nakashima O, Sadashima E, Hattori S, Taira T, Kawahara A, Okabe Y, Shimamatsu K, Taguchi J, Momosaki S, Irie K, Yamaguchi R, Yokomizo H, Nagamine M, Fukuda S, Sugiyama S, Nishida N, Higaki K, Yoshitomi M, Yasunaga M, Okuda K, Kinoshita H, Nakayama M, Yasumoto M, Akiba J, Kage M, Yano H. Intraductal neoplasm of the intrahepatic bile duct: Clinicopathological study of 24 cases. World J Gastroenterol 2012; 18(28): 3673-3680
- URL: https://www.wjgnet.com/1007-9327/full/v18/i28/3673.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i28.3673
Mucin-producing tumors arising from the bile duct have been reported previously[1-5], but as a disease category, consensus has not yet been reached. Recently, Shibahara et al[6] and Zen et al[7] have contributed to the development of the concept of papillary tumors in the bile duct that resemble intraductal papillary mucinous neoplasm of the pancreas (IPMN-P) and pancreatic intraepithelial neoplasia. These bile duct tumors show papillary proliferation in the bile duct with mucin secretion, and are considered as intraductal papillary neoplasm of the bile duct (IPN-B), that is, the biliary counterpart of IPMN-P[8,9]. Recent studies have indicated that IPN-B could be subcategorized according to the results of mucin immunohistochemistry[6,7], and the similarity of IPNB and IPMN-P has also been described.
In our current study, we conducted histological and immunohistochemical re-examination of 24 cases of intraductal neoplasm of the intrahepatic bile duct (INihB), which were previously reported as biliary papillomatosis or intraductal growth type of intrahepatic biliary neoplasm[10-13].
We defined INihB as a tumor that (1) was localized in the liver; (2) arose within the intrahepatic bile duct; (3) had a major lesion that was noninfiltrative and showed an intraductal proliferation pattern; and (4) clinicopathologically communicated with the surrounding bile duct.
We identified 24 cases of INihB from the medical record at Kurume University Hospital and affiliated institutions. These cases were previously diagnosed as intraductal intrahepatic biliary neoplasm or biliary papillomatosis[13]. Tissue sections of 4 μm thickness were prepared from paraffin-embedded tissue samples, and stained with hematoxylin and eosin (HE) in the usual manner. The slides were reviewed by the three pathologists (YN, HK and ON).
HE-stained sections were reviewed and each tumor was categorized into three groups according to the degree of malignancy by using the criteria in IPMN-P[8]: adenoma (low grade), borderline (intermediate grade), and malignant (carcinoma in situ and high grade). Tumors with microinvasion were categorized as malignant.
Paraffin-embedded, 4-μm-thick sections on a coated glass slides were stained by using the BenchMark XT (Ventata Automated Systems, Inc., Tucson, AZ, United States) with the following antibodies: mucin (MUC)1 (mouse monoclonal, Ma695, dilution 1:100; Novocastra, Newcastle, United Kingdom); MUC2 (mouse monoclonal, Ccp58, dilution 1:100; Novocastra); MUC5AC (mouse monoclonal, CLH2, dilution 1:100; Novocastra); MUC6 (mouse monoclonal, CLH5, dilution 1:50; Novocastra); p53 (mouse monoclonal, DO-7, dilution 1:200; Novocastra); β-catenin (mouse monoclonal, β-catenin-1, dilution 1:200; Dako, Glostrup, Denmark); and Ki-67 (mouse monoclonal, MIB-1, dilution 1:100; Dako). This automated system uses the streptavidin-biotin complex method with DAB as a chromogen (Ventana iVIEW DAB Detection Kit).
Regarding the MUC profile, we evaluated cytoplasmic and luminal surface staining by referring to the method of Shibahara et al[6], and the cells were considered positive when either one or both of the two components were stained. The percentage of positively stained neoplastic cells was also calculated and graded as follows: -, < 5% of the neoplastic cells were stained; +, ≥ 5% and < 20% were stained; 2+, ≥ 20% and < 50% were stained, and 3+, ≥ 50% were stained. These evaluations were conducted by three pathologists (YN, HK and ON). Positivity of p53 and β-catenin were defined by distinct and diffuse nuclear staining among the neoplastic cells. Ki-67 staining was counted on a minimum of 1000 tumor cells and Ki-67 labeling index (LI) was calculated as the percentage of positively stained cells to total cells.
Associations of Ki-67 LI and expression of MUC family (0, 1+ or 2+, 3+) with the degree of malignancy (adenoma, borderline or malignant), and association between Ki-67 LI and MUC profile were examined using the Kruskal-Wallis test and Fisher’s exact test. To apply Fisher’s exact test, we classified MUC profile into two categories of 0 or 1+ and 2+ or 3+. Association was considered significant when P < 0.05. All the statistical analyses were conducted by SAS version 9.12 (SAS Institute Inc., Cary, NC, United States) and R version 2.9.0.
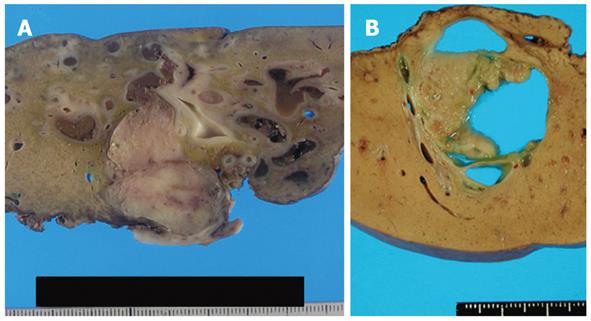
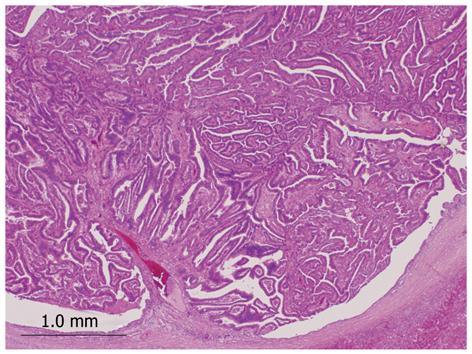
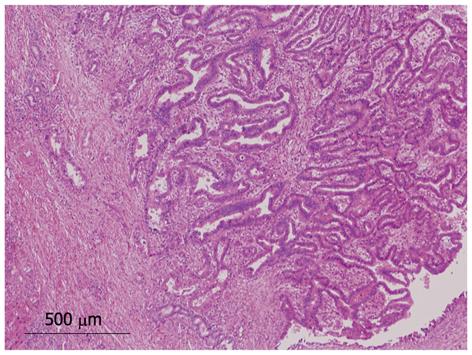
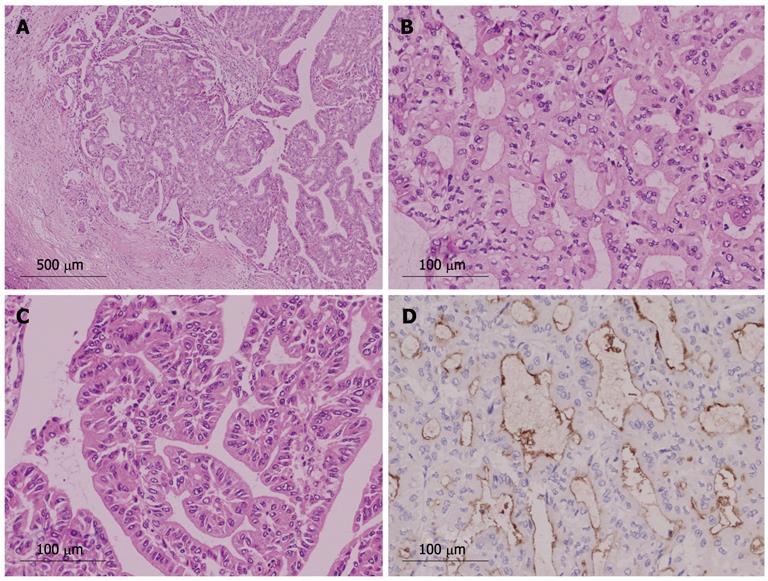
We identified 24 tumors in 24 patients. Clinical findings are summarized in Table 1. Median age at the initial diagnosis was 64 years (mean: 63.0 ± 8.1), and the male: female ratio was 1:1. Sixteen tumors (67%) were located in the left lobe. Nineteen tumors (79%) were cystic type associated with mucin hypersecretion, and five tumors (21%) were duct-ectatic type without mucin hypersecretion (Figure 1). Twenty tumors (83%) had papillary structures in the bile duct as IPNB (Figure 2). The remaining four tumors (17%) presented the following features: the tumor was localized and proliferated in the bile duct, and the tumor showed both tubular and papillary structures. In three of the four tumors (75%), macroscopic mucin secretion was limited but microscopic intracellular mucin was evident. Histologically, 16 tumors (67%) were malignant, three (12%) were borderline, and five (21%) were adenoma. Microinvasion was found in four tumors (21%) (Figure 3), and these were categorized as malignant. There was no ovarian-like stroma in any cases.
| Age (yr), median (mean ± SD) | 64.0 (63.0 ± 8.1) |
| Gender, n (%) | |
| Male | 12 (50) |
| Female | 12 (50) |
| Location, n (%) | |
| Left lobe | 16 (67) |
| Right lobe | 8 (33) |
| Tumor size (cm), median (mean ± SD) | 30 (42.2 ± 26.6) |
| Macroscopic findings of bile duct, n (%) | |
| Duct-ectatic | 5 (21) |
| Cystic | 19 (79) |
| Macroscopic mucin hypersecretion, n (%) | |
| Present | 19 (79) |
| Absent | 5 (21) |
| Histological structure pattern, n (%) | |
| Papillary | 20 (83) |
| Tubular and papillary | 4 (17) |
| Histological grade, n (%) | |
| Malignant | 16 (67) |
| Borderline | 3 (12) |
| Adenoma | 5 (21) |
| Microinvasion, n (%) | |
| Present | 4 (17) |
| Absent | 20 (83) |
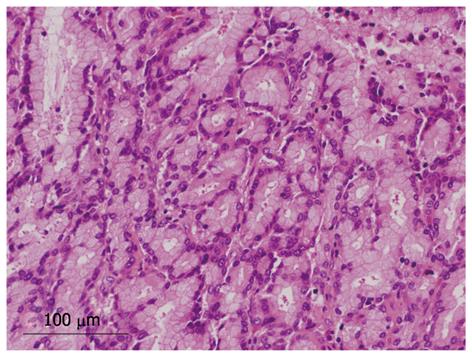
Four tumors (17%) were composed of both papillary and tubular structures (Table 2). Histological findings of four patients were similar to those of intraductal tubulopapillary neoplasm (ITPN-P)[14] or intraductal tubular neoplasm of the pancreas (ITN-P)[15]. One of these four cases was previously reported as a biliary papillomatosis by Taguchi et al[13], and the tumor spread from the intrahepatic to extrahepatic bile duct. Another two cases also showed extension of tumor with bile duct dilation. However, the remaining tumor was cystic type and macroscopic mucin hypersecretion was evident. Among tubular structures, a pyloric gland-like structure (Figure 4) was prominent. Three cases were not malignant, but one tumor showed cytological atypia, such as enlarged nuclei with pleomorphism, and was considered as malignant (Figure 5). Atypical cells lining the tubular structure were cuboidal or columnar with little or no cytoplasmic mucin. None of these four cases were associated with tumor invasion.
| Case 1 | Case 2 | Case 31 | Case 4 | |
| Age (yr) | 77 | 49 | 63 | 52 |
| Gender | Male | Female | Male | Male |
| Location | Left lobe | |||
| Tumor size (cm) | 10 | 2 | 6 | 2.6 |
| Mucin produced | Absent | Present | ||
| macroscopically | ||||
| Macroscopic findings | Duct-ectatic type | Cystic | ||
| Histopathological grade | Malignant | Borderline | Adenoma | Adenoma |
| Microinvasion | Absent | |||
| Mucin immunohistochemistry | ||||
| MUC1 | 3+ | - | - | 1+ |
| MUC2 | - | - | - | - |
| MUC5AC | - | 1+ | - | - |
| MUC6 | 3+ | 3+ | + | 3+ |
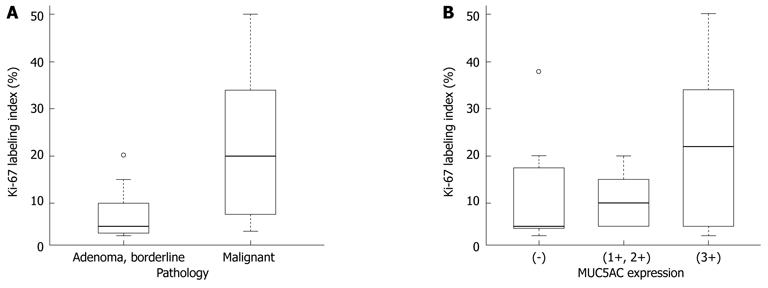
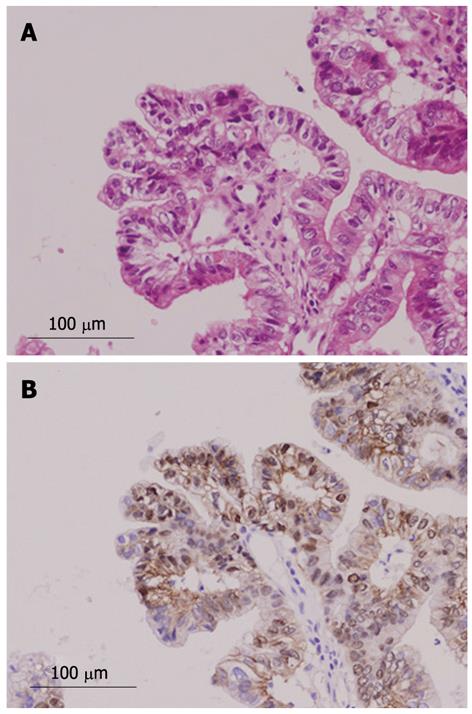
Immunohistochemical findings are shown in Table 3. MUC1 was not expressed in the borderline/adenoma group but was expressed in malignant lesions (P = 0.0095). No specific pattern of expression was observed for other MUC family members. The Ki-67 LI was significantly higher in the malignant group than in the borderline/adenoma group (22.2 ± 15.5 vs 7.5 ± 6.3, P < 0.01, Figure 6A). In the 16 malignant tumors, there was an association of borderline significance between expression of MUC5AC and higher Ki-67 LI (P = 0.0622, Figure 6B). Expression of β-catenin was found in two of the 24 tumors (8%, Figure 7), and these two tumors were also positive for MUC1. P53 was negative in all 24 tumors.
| Malignantn (16.67%) | Borderline n (3.12%)/adenoma n (5.21%) | |
| MUC11 | ||
| 3+ (38%) | 9 (56) | 0 |
| 2+ (0%) | 0 | 0 |
| 1+/- (62%) | 7 (44) | 8 (100) |
| MUC2 | ||
| 3+ (13%) | 1 (6) | 2 (25) |
| 2+ (4%) | 1 (6) | 0 |
| 1+/- (83%) | 14 (88) | 6 (75) |
| MUC5AC | ||
| 3+ (46%) | 7 (44) | 4 (50) |
| 2+ (12%) | 2 (12) | 1 (13) |
| 1+/- (42%) | 7 (44) | 3 (37) |
| MUC6 | ||
| 3+ (46%) | 4 (25) | 5 (63) |
| 2+ (12%) | 4 (25) | 1 (22) |
| 1+/- (44%) | 8 (50) | 2 (25) |
| Proliferation | ||
| Ki-67 (LI, %, mean ± SD)2 | 22.2 ± 15.5 | 7.5 ± 6.3 |
| Genetic status | ||
| TP53 overexpression | 0 | 0 |
| β-catenin nuclear expression (17%)3 | 2 (13) | 0 |
Many studies have been conducted on IPMN-P, and histomorphological criteria for diagnosis have been determined[8]. Regarding similar lesions in the bile duct, the definition of IPNB[7] and mucin-producing bile duct tumors[6] is proposed as a new conceptual framework of biliary intraductal neoplasms. These biliary lesions present the same histological features of IPMN-P, and Zen et al[16,17] divided them into four categories, namely, pancreaticobiliary type, intestinal type, gastric type, and oncocytic type. In contrast, Shibahara et al[6] subclassified the lesions into two distinct categories, namely, columnar type and cuboidal type. Columnar type resembles intestinal type, and cuboidal type resembles pancreaticobiliary type or intraductal oncocytic papillary neoplasm, and they hypothesized that these two categories could be the biliary counterparts of IPMN-P.
Among our 24 cases of INihB, 19 cases (79%) were the cystic type that was proposed by Shibahara et al[6], and this type is known as a biliary cystic tumor. Devaney et al[18] reported that ovarian-like stroma was observed in 85% of the adenoma and 28% of the adenocarcinoma tumors. However, none of our cases had ovarian-like stroma. The presence or absence of ovarian-like stroma is an important factor for the classification of IPNB. Recently, Zen et al[17] proposed that biliary cystic tumor could be a cystic variant of IPNB, having bile duct communication and absence of ovarian-like stroma. Based on our findings, the cystic type reported by Shibahara et al[6] and biliary cystic variant of IPNB reported by Zen et al[16] are the most common type among INihB, and they can be recognized by their characteristic macroscopic findings, that is, a cystic change with mucin hypersecretion, even though the tumors have ovarian-like stroma.
In our current study, 20 of 24 INihB tumors (83%) showed purely papillary proliferation that is a morphological feature of IPMN-P, and we diagnosed them as IPNB. In contrast, both papillary and tubular structures were present in the remaining four tumors (17%), and three of these four tumors were duct-ectatic type without macroscopic mucin secretion. These features are common findings in ITN-P or ITPN-P, which are rare diseases[15,19-24]. These tubulopapillary tumors can be distinctly subcategorized in INihB based on their characteristic macroscopic and histological findings. Intraductal neoplasms with a tubular structure have been reported in the pancreatic area. Albores-Saavedra et al[19] reported that tumors with a tubular structure included: (1) glands resembling pyloric glands; (2) glands lined by cells with no cytoplasmic mucin and with mild nuclear atypia; and (3) glands lined by pink oncocytic cells. Recently, Yamaguchi et al[14] proposed the concept of ITPN-P and defined nine diagnostic criteria. Similarly, some authors have described intraductal lesions in which IPNB with formation of the tubular structure is regarded as intraductal tubular neoplasm of the bile duct (ITNB)[25,26]. In our four cases, we observed morphological similarity with ITN-P or ITPN-P. In particular, Case 1 consisted mainly of a tubular structure and some papillary patterns, and the neoplastic cells were markedly atypical. In addition, immunohistochemical results were MUC1(+) and MUC5AC(-), which were different from ITN-P. Our case 1 was considered as an ITPN-B subcategory. As discussed above, IPNB contained IPNB with a tubular structure, although small in number, and there might be cases with similar characteristics to ITN-P or ITPN-P.
We also examined Ki-67 and p53 immunoreactivity in the 24 cases of INihB. Ki-67 expression differed significantly between malignant and borderline/adenoma (22.2 ± 15.5 vs 7.5 ± 6.3, P < 0.01), but P53 was negative in all cases. Based on these data, Ki-67 LI correlates with the degree of malignancy in INihB. Shibahara et al[6] reported significantly high Ki-67 expression and poorer prognosis in the columnar type than in the cuboidal type. In this study, we also investigated the relationship between MUC1/MUC5AC immunostaining and malignant potential. Some studies have reported that the pancreatobiliary type IPMN-P, which is positive for MUC1, is highly malignant[8]. Other studies have suggested that MUC1 contributes significantly to tumor growth and metastasis, and that downregulation of MUC1 protein expression decreases the metastatic potential of pancreatic adenocarcinoma[27]. The association between MUC1 and malignant potential has also been suggested in cholangiocarcinoma[28]. Although we did not find a significant association between MUC5AC expression and the degree of malignancy or cell proliferation potential, there was a borderline association between the higher expression of MUC5AC and the higher Ki-67 LI in the malignant group. Some authors also have reported that MUC5AC is more strongly expressed in advanced tumors[17,28]. Thus, we suggest that expression of MUC1 and MUC5AC is related, MUC1 more strongly so than MUC5AC, to the degree of malignancy in INihB.
Nuclear expression of β-catenin was found in 8% of INihB (2/24), and MUC1 was also positive in these cases. β-catenin is a mediator in the canonical Wnt signal transduction pathway[29] and activation of the Wnt pathway is associated with high proliferation and dedifferentiation in human biliary tumors[30]. Nuclear accumulation of β-catenin was reported in 15% of intrahepatic cholangiocarcinomas (Sugimachi et al[31]); 25% of IPN-B, 20% of IPN-B associated with intrahepatic cholangiocarcinoma, and 0% of biliary intraepithelial neoplasms (Itatsu et al[32]); and 25% of IPN-B (Abraham et al[33]). The Wnt signaling pathway is frequently activated in biliary neoplasms[32]. We believe when dealing with INihB one should consider performing immunohistochemical staining for MUC1 and β-catenin, in addition to histomorphological examination, to evaluate malignant potential of the tumor.
In conclusion, we propose the concept of INihB that includes IPNB and IPNB with tubular structure subcategories, that is, ITNB and intraductal tubulopapillary neoplasm of the bile duct. IPNB with tubular structure is rare, and (1) is mostly duct-ectatic without macroscopic mucin hypersecretion; and (2) histologically, has both papillary and tubular structures. In INihB, MUC1 expression correlates positively with the degree of malignancy and cell proliferation potential. MUC5AC expression also correlates with the degree of malignancy, although the relationship is less robust. Further pathological and molecular studies are necessary to clarify the characteristics of INihB.
The clinicopathological characteristics of intraductal neoplasm of the intrahepatic bile duct (INihB) remains unclear. In this article, the authors present the clinicopathological features of 24 cases of INihB.
Some studies have reported that the pancreatobiliary-type intraductal papillary mucinous neoplasm (IPMN-P), which is positive for mucin (MUC)1 expression, is highly malignant, and other studies have suggested that MUC1 contributes significantly to tumor growth and metastasis, and that downregulation of MUC1 protein expression decreases the metastatic potential of pancreatic adenocarcinoma. The association between MUC1 and malignant potential has also been suggested in cholangiocarcinoma. Some authors have reported that MUC5AC is more strongly expressed by advanced tumors. In this study, the authors demonstrated that MUC1 and MUC5AC expression may be related to the malignant potential of INihB.
Recent reports have highlighted the relationship between MUC profile and malignancy potential. In particular, the authors investigated the relationship between MUC1/MUC5AC immunohistochemical staining and Ki-67 labeling index.
This study offers a better understanding of clinicopathological characteristics of INihB and a potential strategy to predict tumor behavior for better patient care.
MUC1/MUC5AC are mucin proteins in the pancreatobiliary/gastric epithelium, respectively. It has been suggested that these proteins play an important role in tumor growth and metastasis in pancreatobiliary disease.
Immunohistochemical analysis revealed that Ki-67 expression in the 24 INihBs was significantly high in the malignant group. MUC staining showed that MUC1 was not expressed in the borderline/adenoma group but was expressed only in malignant lesions, and the Ki-67 labeling index was significantly higher in the malignant group than in the borderline/adenoma group. These results may represent a mechanism of MUC protein in tumor growth, that is, malignant potential of the tumor.
Peer reviewer: Dr. George Sgourakis, 2nd Surgical Department, Surgical Oncology Unit, Red Cross Hospital, 11 Mantzarou Str, 15451 Athens, Greece
S- Editor Gou SX L- Editor Kerr C E- Editor Zhang DN
| 1. | Ohtsubo K, Ohta H, Sakai J, Mouri H, Nakamura S, Ikeda T, Kifune K, Yoshikawa J, Harada K, Nakanuma Y. Mucin-producing biliary papillomatosis associated with gastrobiliary fistula. J Gastroenterol. 1999;34:141-144. [PubMed] |
| 2. | Lim JH, Kim YI, Park CK. Intraductal mucosal-spreading mucin-producing peripheral cholangiocarcinoma of the liver. Abdom Imaging. 2000;25:89-92. [PubMed] |
| 3. | Sakamoto E, Hayakawa N, Kamiya J, Kondo S, Nagino M, Kanai M, Miyachi M, Uesaka K, Nimura Y. Treatment strategy for mucin-producing intrahepatic cholangiocarcinoma: value of percutaneous transhepatic biliary drainage and cholangioscopy. World J Surg. 1999;23:1038-1043; discussion 1043-1044;. [PubMed] |
| 4. | Kokubo T, Itai Y, Ohtomo K, Itoh K, Kawauchi N, Minami M. Mucin-hypersecreting intrahepatic biliary neoplasms. Radiology. 1988;168:609-614. [PubMed] |
| 5. | Kim HJ, Kim MH, Lee SK, Yoo KS, Park ET, Lim BC, Park HJ, Myung SJ, Seo DW, Min YI. Mucin-hypersecreting bile duct tumor characterized by a striking homology with an intraductal papillary mucinous tumor (IPMT) of the pancreas. Endoscopy. 2000;32:389-393. [PubMed] |
| 6. | Shibahara H, Tamada S, Goto M, Oda K, Nagino M, Nagasaka T, Batra SK, Hollingsworth MA, Imai K, Nimura Y. Pathologic features of mucin-producing bile duct tumors: two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2004;28:327-338. [PubMed] |
| 7. | Zen Y, Sasaki M, Fujii T, Chen TC, Chen MF, Yeh TS, Jan YY, Huang SF, Nimura Y, Nakanuma Y. Different expression patterns of mucin core proteins and cytokeratins during intrahepatic cholangiocarcinogenesis from biliary intraepithelial neoplasia and intraductal papillary neoplasm of the bile duct--an immunohistochemical study of 110 cases of hepatolithiasis. J Hepatol. 2006;44:350-358. [PubMed] |
| 8. | Furukawa T, Klöppel G, Volkan Adsay N, Albores-Saavedra J, Fukushima N, Horii A, Hruban RH, Kato Y, Klimstra DS, Longnecker DS. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794-799. [PubMed] |
| 9. | Adsay NV, Adair CF, Heffess CS, Klimstra DS. Intraductal oncocytic papillary neoplasms of the pancreas. Am J Surg Pathol. 1996;20:980-994. [PubMed] |
| 10. | Isaji S, Kawarada Y, Taoka H, Tabata M, Suzuki H, Yokoi H. Clinicopathological features and outcome of hepatic resection for intrahepatic cholangiocarcinoma in Japan. J Hepatobiliary Pancreat Surg. 1999;6:108-116. [PubMed] |
| 11. | Helpap B. Malignant papillomatosis of the intrahepatic bile ducts. Acta Hepatogastroenterol (. Stuttg). 1977;24:419-425. [PubMed] |
| 12. | Kim YI, Yu ES, Kim ST. Intraductal variant of peripheral cholangiocarcinoma of the liver with Clonorchis sinensis infection. Cancer. 1989;63:1562-1566. [PubMed] |
| 13. | Taguchi J, Yasunaga M, Kojiro M, Arita T, Nakayama T, Simokobe T. Intrahepatic and extrahepatic biliary papillomatosis. Arch Pathol Lab Med. 1993;117:944-947. [PubMed] |
| 14. | Yamaguchi H, Shimizu M, Ban S, Koyama I, Hatori T, Fujita I, Yamamoto M, Kawamura S, Kobayashi M, Ishida K. Intraductal tubulopapillary neoplasms of the pancreas distinct from pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2009;33:1164-1172. [PubMed] |
| 15. | Nakayama Y, Inoue H, Hamada Y, Takeshita M, Iwasaki H, Maeshiro K, Iwanaga S, Tani H, Ryu S, Yasunami Y. Intraductal tubular adenoma of the pancreas, pyloric gland type: a clinicopathologic and immunohistochemical study of 6 cases. Am J Surg Pathol. 2005;29:607-616. [PubMed] |
| 16. | Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, Kurumaya H, Katayanagi K, Kawashima A, Masuda S. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333-1343. [PubMed] |
| 17. | Zen Y, Fujii T, Itatsu K, Nakamura K, Konishi F, Masuda S, Mitsui T, Asada Y, Miura S, Miyayama S. Biliary cystic tumors with bile duct communication: a cystic variant of intraductal papillary neoplasm of the bile duct. Mod Pathol. 2006;19:1243-1254. [PubMed] |
| 18. | Devaney K, Goodman ZD, Ishak KG. Hepatobiliary cystadenoma and cystadenocarcinoma. A light microscopic and immunohistochemical study of 70 patients. Am J Surg Pathol. 1994;18:1078-1091. [PubMed] |
| 19. | Albores-Saavedra J, Sheahan K, O'Riain C, Shukla D. Intraductal tubular adenoma, pyloric type, of the pancreas: additional observations on a new type of pancreatic neoplasm. Am J Surg Pathol. 2004;28:233-238. [PubMed] |
| 20. | Kato N, Akiyama S, Motoyama T. Pyloric gland-type tubular adenoma superimposed on intraductal papillary mucinous tumor of the pancreas. Pyloric gland adenoma of the pancreas. Virchows Arch. 2002;440:205-208. [PubMed] |
| 21. | Bakotic BW, Robinson MJ, Sturm PD, Hruban RH, Offerhaus GJ, Albores-Saavedra J. Pyloric gland adenoma of the main pancreatic duct. Am J Surg Pathol. 1999;23:227-231. [PubMed] |
| 22. | Oh DK, Kim SH, Choi SH, Jang KT. Intraductal tubular carcinoma of the pancreas: a case report with the imaging findings. Korean J Radiol. 2008;9:473-476. [PubMed] |
| 23. | Itatsu K, Sano T, Hiraoka N, Ojima H, Takahashi Y, Sakamoto Y, Shimada K, Kosuge T. Intraductal tubular carcinoma in an adenoma of the main pancreatic duct of the pancreas head. J Gastroenterol. 2006;41:702-705. [PubMed] |
| 24. | Shahinian HK, Sciadini MF, Springer DJ, Reynolds VH, Lennington WJ. Tubular adenoma of the main pancreatic duct. Arch Surg. 1992;127:1254-1255. [PubMed] |
| 25. | Sato Y, Osaka H, Harada K, Sasaki M, Nakanuma Y. Intraductal tubular neoplasm of the common bile duct. Pathol Int. 2010;60:516-519. [PubMed] |
| 26. | Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol. 2010;2:419-427. [PubMed] |
| 27. | Tsutsumida H, Swanson BJ, Singh PK, Caffrey TC, Kitajima S, Goto M, Yonezawa S, Hollingsworth MA. RNA interference suppression of MUC1 reduces the growth rate and metastatic phenotype of human pancreatic cancer cells. Clin Cancer Res. 2006;12:2976-2987. [PubMed] |
| 28. | Park SY, Roh SJ, Kim YN, Kim SZ, Park HS, Jang KY, Chung MJ, Kang MJ, Lee DG, Moon WS. Expression of MUC1, MUC2, MUC5AC and MUC6 in cholangiocarcinoma: prognostic impact. Oncol Rep. 2009;22:649-657. [PubMed] |
| 29. | Gumbiner BM. Signal transduction of beta-catenin. Curr Opin Cell Biol. 1995;7:634-640. [PubMed] |
| 30. | Kiesslich T, Alinger B, Wolkersdörfer GW, Ocker M, Neureiter D, Berr F. Active Wnt signalling is associated with low differentiation and high proliferation in human biliary tract cancer in vitro and in vivo and is sensitive to pharmacological inhibition. Int J Oncol. 2010;36:49-58. [PubMed] |
| 31. | Sugimachi K, Taguchi K, Aishima S, Tanaka S, Shimada M, Kajiyama K, Sugimachi K, Tsuneyoshi M. Altered expression of beta-catenin without genetic mutation in intrahepatic cholangiocarcinoma. Mod Pathol. 2001;14:900-905. [PubMed] |
| 32. | Itatsu K, Zen Y, Ohira S, Ishikawa A, Sato Y, Harada K, Ikeda H, Sasaki M, Nimura Y, Nakanuma Y. Immunohistochemical analysis of the progression of flat and papillary preneoplastic lesions in intrahepatic cholangiocarcinogenesis in hepatolithiasis. Liver Int. 2007;27:1174-1184. [PubMed] |
| 33. | Abraham SC, Lee JH, Hruban RH, Argani P, Furth EE, Wu TT. Molecular and immunohistochemical analysis of intraductal papillary neoplasms of the biliary tract. Hum Pathol. 2003;34:902-910. [PubMed] |