Published online Jul 7, 2012. doi: 10.3748/wjg.v18.i25.3215
Revised: April 27, 2012
Accepted: May 26, 2012
Published online: July 7, 2012
AIM: To investigate the correlation of 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) with clinical features and the prediction of treatment response.
METHODS: A total of 83 hepatocellular carcinoma (HCC) patients undergoing 18F-FDG PET before transarterial chemolipiodolization with systemic chemo-infusion between October, 2006 and May, 2009 were retrospectively enrolled. The patients included 68 men and 15 women (mean age, 60 ± 10.7 years). The effect of 18F-FDG-monitored PET uptake on clinical features and on the evaluated treatment response was ascertained with modified Response Evaluation Criteria in Solid Tumors. The PET parameters of maximal standardized uptake value of the tumor (Tsuvmax), the ratio of the tumor maximal standardized uptake value (SUV) to the liver maximal SUV (Tsuvmax/Lsuvmax) and the ratio of tumor maximal SUV to the liver mean SUV (Tsuvmax/Lsuvmean) were tested as predictive factors.
RESULTS: Among the 3 SUV parameters, the Tsuvmax/Lsuvmean ratio (cutoff value of 1.90) was significantly associated with tumor burden including tumor size, tumor number, α-fetoprotein levels and tumor stage (P < 0.001, P = 0.008, P = 0.011, P < 0.001, respectively). The objective response rates in patients with a high SUV ratio (≥ 1.90) were significantly better than those with a low SUV ratio (< 1.90) (P = 0.020). The overall survival rates of patients exhibiting a low Tsuvmax/Lsuvmean ratio (< 1.90) and those with a high SUV ratio (≥ 1.90) was 38.2 and 10.3 mo, respectively (P < 0.01). However, the time to progression showed no significant difference between the groups (P = 0.15).
CONCLUSION: 18F-FDG PET can be an important predictor of HCC treatment. In particular, the Tsuvmax/Lsuvmean ratio (cutoff value of 1.90) can provide useful information in treatment prognosis for HCC patients treated with locoregional therapy.
- Citation: Song MJ, Bae SH, Yoo IR, Park CH, Jang JW, Chun HJ, Choi BG, Lee HG, Choi JY, Yoon SK. Predictive value of 18F-fluorodeoxyglucose PET/CT for transarterial chemolipiodolization of hepatocellular carcinoma. World J Gastroenterol 2012; 18(25): 3215-3222
- URL: https://www.wjgnet.com/1007-9327/full/v18/i25/3215.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i25.3215
Hepatocellular carcinoma (HCC) is the fifth most common malignancy, with an increasing incidence worldwide[1], and the third most common cause of cancer related death[2]. Surveillance programs have been implemented for cirrhotic patients. However, curative therapies such as resection or transplantation can be applied to fewer than 30% of HCC patients[3], because most are diagnosed at an intermediate-to-advanced stage of the disease. The prognosis of HCC patients remains poor, and life expectancy is difficult to predict because of variable factors that include tumor burden and liver reserve function[4]. Thus, it is important to assess the aggressive nature and metabolic change in HCC because this information is valuable in predicting the treatment response and in aiding in the selection of treatment modalities. One approach used to assess the biological activity of a tumor is positron emission tomography (PET).
18F-fluorodeoxyglucose (FDG) PET is an imaging modality that can gauge the glucose metabolism of tumors, which has been established as a useful diagnostic tool for evaluating extrahepatic metastasis[5]. However, 18F-FDG PET has limitations in its ability to detect primary HCC because of variable F-FDG uptake in HCC[6]. Nonetheless, PET monitored FDG uptake has the potential to be an additional tool for assessing biological behavior in HCC[7,8]. The difference in uptake between a tumor and the liver can be expressed as a standardized uptake value (SUV) ratio (T/Lsuv), which is associated with prognostic aspects of tumor aggressiveness such as differentiation grade, tumor size/number and tumor recurrence in liver transplantation[8]. It is feasible that FDG uptake, similar to tumor size/number and vascular invasion, may be of value in the prognosis of the treatment response in HCC[9,10]. However, the usefulness of 18F-FDG PET has not been investigated in the prediction of treatment response for HCC by transarterial chemolipiodolization (TACL) with systemic chemo-infusion.
The present study was undertaken to evaluate whether FDG uptake in HCC correlated with tumor characteristics and to determine, which PET/computed tomography (CT) parameter is especially important as a predictor of treatment response. Finally, the probability of prognostic ability of 18F-FDG PET for the prediction of treatment response in HCC was assessed.
A total of 83 HCC patients, who were aged > 18 years were retrospectively selected and analyzed. Patients included 68 men and 15 women (mean age, 60 ± 10.7 years). All patients had undergone 18F-FDG PET within 3 d before treatment between October, 2006 and May, 2009. The median duration of follow-up was 10.3 mo (range, 1.8-35.7 mo). The eligibility criteria were as follows: no previous transarterial chemo embolization, chemotherapy or radiotherapy; a confirmed diagnosis of HCC according to the American Association for the Study of Liver Disease criteria; an Eastern Cooperative Oncology Group performance status of 0 or 1; and preserved liver function (Child-Pugh class A or B). Patients with potentially resectable or ablative lesions who were high risk for surgery and radiofrequency ablation were also enrolled. Exclusion criteria included any extrahepatic metastasis, another primary tumor, advanced liver disease (bilirubin levels > 3 mg/dL, and a level of aspartate aminotransferase (AST) or alanine transaminase (ALT) > 5 × the upper limit of normal). The study was approved by the institutional Ethics Review Board and was in compliance with the Declaration of Helsinki.
The treatment regimen was a combination of intra-arterial epirubicin (50 mg/m2) and/or cisplatin (60 mg/m2) in a mixture of lipiodol (5-10 mL) without gelform embolization, and received an additional systemic infusion of 5-fluorouracil (200 mg/m2) after completing the transarterial chemolipiodolization[11,12]. The dose or treatment interval was modified whenever any treatment-related toxicity was encountered. Based on our previous report, the following formula for dosage modification was derived: administration dosage = D × body surface area (BSA) × M where D is initial dosage of each agent, BSA is body surface area, and M is the modification rate = (white blood cell count/4000) × [1-(age-45)/100] × [1-(Child-Pugh score-5)/10]. According to this formula, the administration dosage of each chemotherapeutic agent was modified. Only the Child-Pugh score was calculated using this formula if the patient’s white blood cell count was > 4000/mm3 or the patient’s age was < 45 years[13].
Follow-up imaging and laboratory tests including α-fetoprotein, albumin, bilirubin, AST, ALT and prothrombin time, were performed 4 wk after treatment. Repeat treatment was scheduled within 4 wk after follow-up imaging if there was residual viable tumor.
All CTs were performed with a 64-slice multidetector CT (Siemen Somatom Sensation 64, Munich, Germany). CT examination was performed using a 4-phase protocol including a non-enhanced acquisition. Arterial phase (delay 20-30 s), portal venous phase (delay 60 s) and delayed venous phase (delay 80 s) were obtained using 120 mL of contrast (Iopromide 300 mg I/L, Schering, Germany) at a rate of 4 mL/s. The images were acquired with a slice thickness of 5 mm.
Treatment response was evaluated at 1 mo after receiving 3 sessions of TACL by modified Response Evaluation Criteria in Solid Tumors[14]. In the modified criteria of the tumor response for HCC, complete response (CR) is the disappearance of any intratumoral arterial enhancement in all lesions; partial response (PR) is at least a 30% decrease in the sum of the diameters of viable (contrast enhancement in the arterial phase) lesions; progressive disease (PD) is an increase of at least 20% in the sum of the diameters of viable lesions; stable disease (SD) denotes any cases that do not qualify for either PR or PD[15]. Objective response (OR) included CR and PR.
For the analysis of treatment response, all images were retrospectively assessed based on consensus by two attending radiologists. The imaging parameters including tumor size and number, portal vein thrombosis and Barcelona Liver Clinic Cancer (BCLC) stage was also evaluated. According to OR, HCC patients were categorized into an objective response group and a non-response group. The clinical features including the Tsuvmax/Lsuvmean ratio were evaluated in both groups. Treatment response according to SUV ratio was analyzed based on stage.
All patients fasted for at least 6 h prior to the PET/CT study. 18FDG (370-555 MBq) was injected intravenously, and scanning began 60 min later. None of the patients had blood glucose levels > 130 mg/dL before the injection. No intravenous contrast agent was administered. Data were acquired using a combined PET/CT in-line system, Biograph Turepoint (Siemens Medical Solutions, Knoxville, TN). The acquisition time was 2-3 min per bed position during PET/CT scanning. Precontrast CT began at the orbitomeatal line and progressed to the proximal thigh (130 kVp, 80 mAs, and 5 mm slice thickness; 120 kVp, 50 mAs, and 5 mm slice thickness). The PET scan followed immediately over the same body region. The CT data were used for attenuation correction, and images were reconstructed using a standard ordered-subset expectation maximization algorithm. The axial spatial resolution was 4.5 mm or 6.5 mm at the center of the field of view.
To evaluate 18F-FDG uptake, the region of interest (ROI) was drawn for each tumor, and the normal liver and measured standardized uptake value in each ROI were determined. The ROI was drawn to encircle the highest activity of each tumor. For normal liver regions, two circular 1.5 cm-diameter ROIs were drawn in both lobes. All tumor and non-tumor regions were defined by correlation with diagnostic CT undergone within 3 d. The maximum SUV (SUVmax) was measured in each ROI, and mean SUV (SUVmean) was measured in each normal-liver ROI.
To evaluate the usefulness of 18F-FDG PET, calculated parameters included the following: the SUVmax of tumor (Tsuvmax), the ratio of tumor SUVmax to liver maximal SUV (Tsuvmax/Lsuvmax), and the ratio of tumor SUVmax to liver mean SUV (Tsuvmax/Lsuvmean). The predictive value of each factor for the treatment response was analyzed based on analysis of the area under the receiver-operating-characteristic curve. After determination of the most effective 18F-FDG PET predictive factor, this parameter was compared with other prognostic factors, including tumor size and number, portal vein thrombosis, serum α-fetoprotein (AFP) and BCLC stage. The significance of the prognostic value was analyzed with Mann-Whitney and Fisher’s exact tests in a univariate analysis and by logistic regression testing in a multivariate analysis. A value of P < 0.05 was considered significant (SPSS 16, IL, Chicago).
The patients included 68 men and 15 women. The average age was 60 ± 10.7 years and hepatitis B virus infected patients were 78%. 85.5% of the patients had Child-Pugh class A liver function. The mean values of tumor number and size were 2.2 ± 1.6 cm and 7.5 ± 5.0 cm, respectively. The BCLC stage of the patients consisted of stage A (n = 26, 31.3%), B (n = 20, 24%) and C (n = 37, 44.5%).
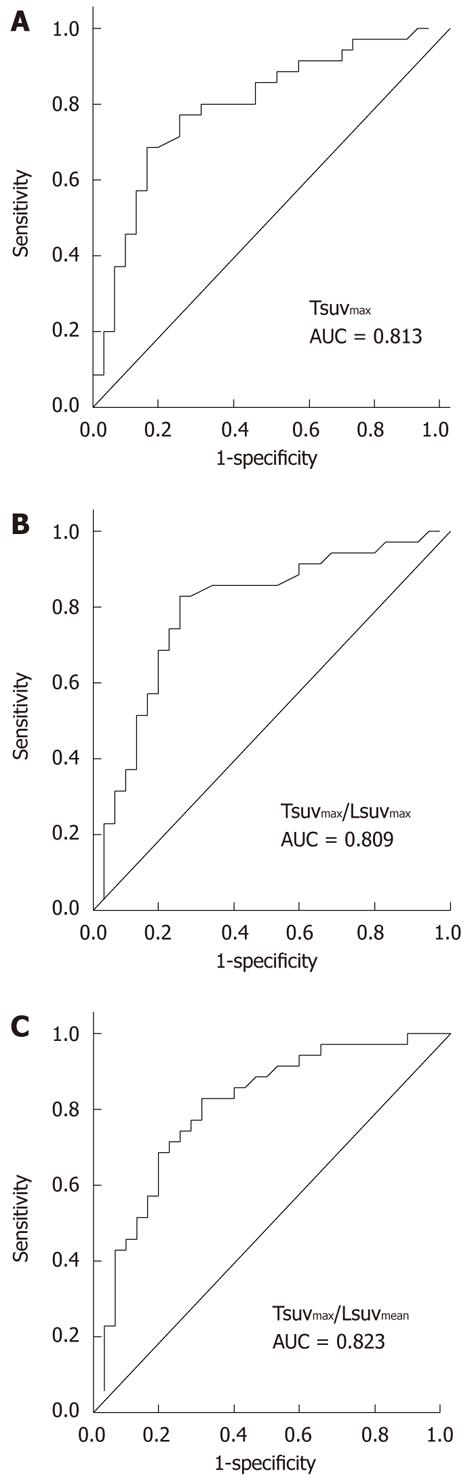
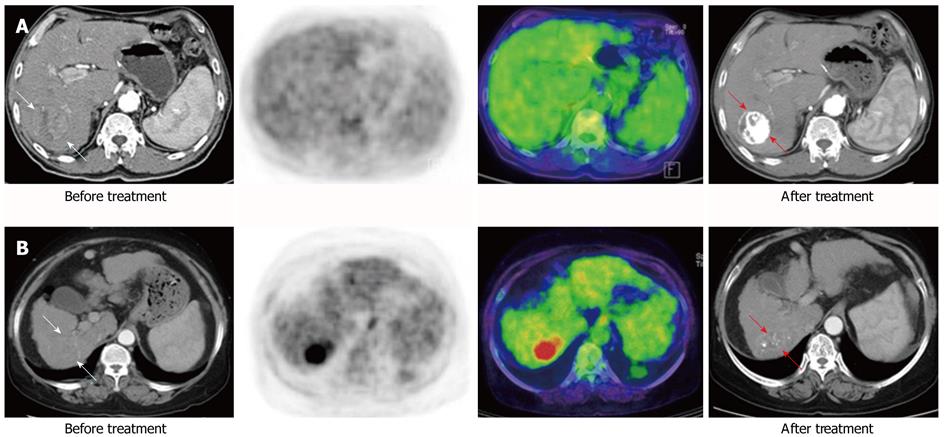
The median values of Tsuvmax, Tsuvmax/Lsuvmax and Tsuvmax/Lsuvmean were 4.03 (1.5-20.8), 1.36 (0.77-7.64) and 1.82 (0.96-10.79), respectively. The area under the curve of Tsuvmax/Lsuvmean was the highest on the receiver-operating-characteristic curve (Figure 1). The cutoff value of Tsuvmax, Tsuvmax/Lsuvmax and Tsuvmax/Lsuvmean was 4.0, 1.45 and 1.90, respectively. The cutoff level of Tsuvmax/Lsuvmean was used as the effective parameter of 18F-FDG PET in the prediction of an objective response to HCC treatment. The tumor characteristics of the 83 patients according to the cutoff value of the Tsuvmax/Lsuvmean ration are summarized in Table 1. Forty patients displayed a Tsuvmax/Lsuvmean≥ 1.90, and the other 43 patients showed a Tsuvmax/Lsuvmean < 1.90. Two examples of FDG uptake according to Tsuvmax/Lsuvmean ratio are shown in Figure 2. The number and size of the tumors, portal vein thrombosis, serum AFP, and BCLC stage in patients with Tsuvmax/Lsuvmean (≥ 1.90) indicated significantly more poor prognostic characteristics than in the other patient group.
| Tsuvmax/Lsuvmean | Tsuvmax/Lsuvmean | P value | |
| < 1.90 (n = 43) | ≥1.90 (n = 40) | ||
| Mean age ± SD (yr) | 60 ± 11.7 | 59.9 ± 9.7 | 0.276 |
| Sex (male:female) | 34:9 | 34:6 | 0.574 |
| Etiology | 0.802 | ||
| HBV/HCV/alcohol/others | 35/4/1/3 | 34/5/0/1 | |
| Tumor number | 1.8 ± 1.3 | 2.7 ± 1.8 | 0.008 |
| Single/multiple | 0.008 | ||
| Tumor size (cm) | 5.2 ± 3.2 | 10 ± 5.5 | 0.000 |
| < 3 cm | 14 | 1 | 0.000 |
| 3-5 cm | 11 | 8 | |
| > 5 cm | 18 | 31 | |
| Portal vein thrombosis | 0.000 | ||
| Absent/present | 39/4 | 18/22 | |
| Child-Pugh class | 0.758 | ||
| A/B | 36/7 | 35/5 | |
| Serum AFP (ng/dL) | 2928.5 ± 11573.6 | 35275.2 ± 103428 | 0.011 |
| < 400/> 400 | 30/13 | 18/22 | 0.028 |
| BCLC stage | 0.000 | ||
| A/B/C | 21/13/9 | 7/28/2005 |
CR was observed in 29 (34.9%) of the 83 patients; PR, SD and PD was observed in 16 (19.3%), 20 (24.1%) and 18 (21.7%) patients, respectively. Objective response rates were different above (77.7%) and below (23.6%) the 1.90 cutoff value of Tsuvmax/Lsuvmean (P < 0.001).
According to treatment response, HCC patients were categorized into an objective response group and a non-response group. The clinical features associated with objective response are shown in Table 2. Tumor size, portal vein thrombosis, BCLC stage and Tsuvmax/Lsuvmean ratio showed significant differences and were also determined as prognostic factors in univariate analysis. However, in the multivariate analysis, Tsuvmax/Lsuvmean was the only significant factor for objective response (Table 3).
| Objective response group(n = 45) | Non-objective response group(n = 38) | P value | |
| Mean age ± SD (yr) | 60.8 ± 11.5 | 59.0 ± 9.7 | 0.372 |
| Sex (male:female) | 37:8 | 31:7 | 0.940 |
| Etiology | 0.555 | ||
| HBV/HCV/alcohol/others | 36/5/1/3 | 33/4/0/1 | |
| Tumor number | 0.077 | ||
| Single/multiple | 25/20 | 13/25 | |
| Tumor size (cm) | 4.4 ± 2.3 | 11.2 ± 4.9 | 0.000 |
| Portal vein thrombosis | 0.000 | ||
| Absent/present | 42/3 | 15/23 | |
| Child-Pugh class | 0.756 | ||
| A/B | 38/7 | 33/5 | |
| Serum AFP | 3795 ± 12054 | 35951 ± 106137 | 0.054 |
| BCLC stage | 0.000 | ||
| A/B/C | 26/12/7 | 0/8/30 | |
| Tsuvmax/Lsuvmea | 0.000 | ||
| 1.90 </≥ 1.90 | 34/11 | 9/29 |
| P value | ||
| Factors | Univariate | Multivariate |
| Tumor number single/multiple | 0.077 | 0.357 |
| Tumor size (cm) | 0.000 | 0.530 |
| Portal vein thrombosis | 0.000 | 0.386 |
| Absent/present | ||
| BCLC stage | 0.000 | 0.408 |
| A/B/C | ||
| Tsuvmax/Lsuvmean | 0.000 | 0.020 |
| 1.90 </≥ 1.90 | ||
The treatment response according to the Tsuvmax/Lsuvmean ratio was analyzed based on BCLC stage (Table 4). Treatment response showed a significant difference on BCLC stage B and C (P = 0.048 and P < 0.001, respectively). These data implicated the Tsuvmax/Lsuvmean ratio as being associated with HCC in more than the intermediate stage.
| Treatment response | |||||||
| BCLC stage | Tsuvmax/Lsuvmean | CR | PR | SD | PD | Total | P value |
| A | < 1.90 | 18 | 3 | 0 | 0 | 21 | 1.0 |
| (n = 26) | ≥ 1.90 | 4 | 1 | 0 | 0 | 5 | |
| B | < 1.90 | 5 | 3 | 2 | 3 | 13 | 0.048 |
| (n = 20) | ≥ 1.90 | 0 | 4 | 3 | 0 | 7 | |
| C | < 1.90 | 2 | 4 | 4 | 0 | 10 | 0.000 |
| (n = 37) | ≥ 1.90 | 0 | 1 | 11 | 15 | 27 | |
| Total | 29 | 16 | 20 | 18 | 83 | ||
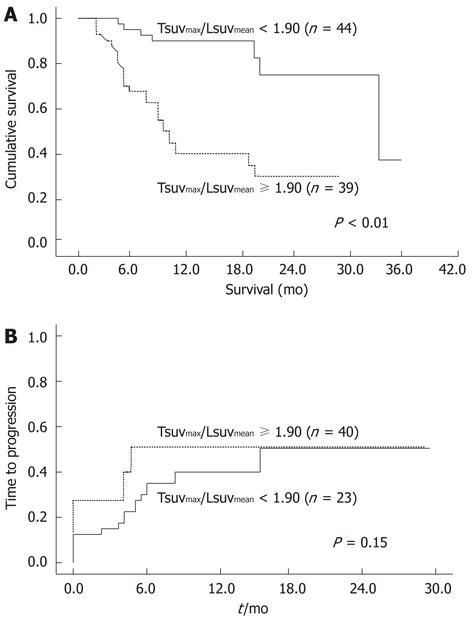
During follow-up, 31 of the 83 patients (37.3%) died. The median survival time was 416 d (range, 55-1221 d). The overall survival rates of patients exhibiting a low Tsuvmax/Lsuvmean ratio (< 1.90) and those with a high SUV ratio (≥ 1.90) was 38.2 mo and 10.3 mo, respectively. The survival curve is shown in Figure 3. The cumulative survival rates at 6 mo, 12 mo, and 24 mo were 91%, 88%, and 75% in patients with low SUV ratio and 63%, 42%, and 33% in those with high SUV ratio. The patients exhibiting a low SUV ratio (< 1.90) survived significantly longer than those with a high SUV ratio (≥ 1.90). In addition, tumor size (P = 0.006), number (P < 0.001), portal vein thrombosis (P < 0.001), AFP (P = 0.016) and BCLC stage (P < 0.001) were related to overall survival rates. In the multivariate analysis, the tumor-to-liver ratio of SUV significantly increased survival rate (Table 5). These results suggest that this 18F-FDG PET parameter was associated with survival in HCC patients.
| Factors | P value | Exp (B) | 95% CI |
| Age | |||
| < 60 yr/≥ 60 yr | 0.135 | 0.814 | 0.832-3.955 |
| Tumor size | |||
| < 3 cm | 0.520 | ||
| 3-5 cm | 0.456 | 2.420 | 0.269-20.861 |
| > 5 cm | 0.519 | 0.689 | 0.237-2.320 |
| Tumor number | |||
| Single/multiple | 0.079 | 0.413 | 0.154-1.107 |
| Portal vein thrombosis | |||
| Absent/present | 0.348 | 0.636 | 0.247-1.638 |
| BCLC stage | |||
| A | 0.032 | ||
| B | 0.034 | 0.082 | 0.008-0.824 |
| C | 0.053 | 0.314 | 0.097-1.017 |
| Tsuvmax/Lsuvmean | |||
| 1.90 </≥ 1.90 | 0.036 | 0.337 | 0.122-0.932 |
Progression of HCC after objective response was observed in 13 of 39 patients (32.5%) with a low Tsuvmax/Lsuvmean ratio (< 1.90) and 13 of 24 patients (56.5%) with a high SUV ratio (≥ 1.90). Although progression of low ratio patients was slower than that of high ratio patients, no significant difference was found between both groups (Figure 3B, P = 0.15).
18F-FDG PET is an imaging modality that can be used to assess glucose metabolism of tumors. PET detects high 18F-FDG uptake in rapidly growing tumors in which the rate of glycolysis increases[8,16]. PET CT has been widely utilized for detection of extrahepatic metastasis from HCC[5,17]. Recent quantitative studies of glucose utilization in liver tumors have shown that PET uptake is useful for tumor characterization and assessment of therapeutic response[18-21]. 18F-FDG uptake in HCC depends on the difference in the activity of glucose-6-phosphatase, which is responsible for the conversion of FDG-G-phosphate to FDG[20]. Increased uptake of 18F-FDG is associated with poorly differentiated HCC and poor outcome indicating that 18F-FDG uptake in HCC is closely related to tumor progression and prognosis[7].
The present study demonstrates that 18F-FDG PET is a feasible tool for assessing biological behavior in HCC. The increase of FDG uptake in HCC was significantly associated with tumor burdens such as size, number of tumors and portal vein thrombosis. Because these tumor burdens are regarded as predictive factors of the aggressiveness of HCC, glucose metabolism on 18F-FDG PET may be a factor that is related to the aggressive character of tumors. FDG uptake is increased in more advanced HCC stages. The results suggest that PET might be useful as a modality for assessing biologic activity in HCC.
This study focused on the predictive value of FDG PET uptake for evaluating the treatment response in HCC patients. Transarterial chemoembolization is the main treatment that is indicated for unresectable HCC in intermediate stage based on the BCLC guideline[22,23], but there are limited data on managing these patients[24-27]. Still, the homogeneity of the treatment modality was important for more reliable analysis. We previously reported promising results with combination treatment using TACL and systemic chemo-infusion therapy for advanced HCC with portal vein invasion[12]. This combination therapy could be applied for treating advanced HCC. At this point, the present study determined the predictive factors for treatment response of the combination therapy in deciding on a management strategy for HCC. Tumor burdens such as tumor size and the reserved liver function have been previously reported to be predictive factors for treatment response[24]. However, these factors are unable to exactly predict the degree of tumor malignancy, and there is a need for considering PET CT in assessing the biological activity of HCC as an additional predictive factor.
18F-FDG uptake showed the potential to predict the TACL with systemic chemo-infusion treatment response for HCC, with a cutoff Tsuvmax/Lsuvmean value of 1.90. Objective response rates were significantly different above (77.7%) and below (23.6%) the cutoff value (P < 0.001). According to stage, the prediction of treatment response after three cycles of TACL was significantly better in the B and C stage than in the A stage (P = 0.048, P = 0.000, respectively). These facts demonstrate the predictive value for 18F-FDG uptake in HCC.
Presently, the SUV ratio correlated with treatment response suggesting that this ratio may be a useful index of HCC aggressiveness[28-30]. Previous reports have demonstrated comparative SUV ratio between tumors and non-tumors is a more useful parameter than the SUV of tumors[28,31,32]. Because 18F-FDG uptake is affected by underlying liver cirrhosis, the ratio reflects more the underlying variation of glucose metabolism in the liver than SUV of tumor itself[33,34]. This SUV ratio correlates with tumor volume doubling time and the differentiation of HCC[5]. The tumor-to-non tumor ratio of SUV may be an effective parameter of progression or aggressiveness in HCC.
Furthermore, our principal finding is that the SUV ratio in FDG PET diagnosis before treatment is an independent predictor of survival in unresectable HCC patients. This finding is compatible with previous studies performed in other treatments such as LT and liver resection[28,35]. In the univariate analysis, tumor size, portal vein thrombosis and BCLC stage were determined to be as significant as the Tsuvmax/Lsuvmean ratio. The increase in FDG uptake ratio in HCC was significantly associated with the aggressive character of the tumor burden such as size, number of tumors and portal vein thrombosis. However, in the multivariate analysis, Tsuvmax/Lsuvmean was a significant prognostic factor with BCLC stage. This result suggests the ratio of 18F-FDG uptake might provide additional information to staging system.
The BCLC staging system has been used to predict outcome and inform decision about treatment strategy in HCC[36]. Although this system includes variables such as tumor burden and liver function reserve, according to Child-Pugh class and performance status, this stage does not adequately consider the biological activity of HCC. FDG-PET permits the evaluation of glucose metabolism in HCC and the detection of extrahepatic metastasis[37]. The determination of the SUV ratio might contribute to the clinical management of HCC patients and compensate for the drawbacks of this staging system for the prediction of treatment response and prognosis in HCC.
This study has some limitations inherent to a retrospective study. First, the number of HCC cases for each stage was relatively small, although PET/CT was performed to detect extrahepatic metastasis and to evaluate the treatment response. Second, sorafenib is considered to be the standard treatment in advanced HCC. Sorafenib was not applicable in the advanced HCC cases in our study. However, we showed beneficial results with combination treatment using TACL and systemic chemo-infusion therapy for advanced HCC and the homogeneity of treatment would rather allow a more reliable analysis. We intended to analyze the correlation of tumor characteristics and treatment response according to 18F-FDG uptake. Prospective studies are needed to confirm these results in the future.
In summary, this study shows that 18F-FDG PET is a significant predictor of treatment response with TACL and systemic chemo-infusion therapy in HCC. The Tsuvmax/Lsuvmean, can be a significant way of distinguishing, overall survival. Therefore, 18F-FDG PET could provide effective information on the prognosis of the treatment response in the evaluation of HCC cases.
18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) is an imaging modality that can assess the glucose metabolism of tumors. PET monitoring of FDG uptake may be an additional tool to assess biological behavior of hepatocellular carcinoma (HCC). The present study evaluated the correlation of 18F-FDG PET with clinical features and prediction of treatment response.
This study showed that the standardized uptake value ratio of FDG uptake could be used to predict the treatment response and overall survival of HCC patients treated with transarterial chemo embolization (TACE).
18F-FDG PET is a significant predictor of treatment response with transarterial chemolipiodolization (TACL) and systemic chemo-infusion therapy in HCC. The Tsuvmax/Lsuvmean (cutoff value of 1.90) was significantly associated with overall survival of HCC patients.
The study results suggest that the FDG PET is a potential modality that could be used in predicting treatment prognosis for HCC patients treated with locoregional therapy.
TACL refers to transarterial treatment with chemotherapeutic agents and lipiodol without embolic materials such as gelatin or polyvinyl alcohol particles. Systemic chemo-infusion is a type of systemic chemotherapy that is administrated after TACE.
In this study, the authors have studied the role of FDG PET in the evaluation of treatment response in patient undergoing the TACL procedure for treatment of HCC. Based on a retrospective review of 83 patients, the authors find that FDG PET can be used to predict the treatment response to HCC. This study is interesting and well performed, and the authors need to be lauded for their efforts. The findings of this study will definitely contribute to the scientific literature and improve our understanding of the biological behavior of HCC.
Peer reviewers: Dr. Nagy Naguib Naeem Naguib, Institute for Diagnostic, Johann Wolfgang Goethe University Hospital, Theodor Stern Kai 7 Haus 23, 60590 Frankfurt, Germany; Dr. Avinash Kambadakone R, Department of Radiology, Massachusetts General Hospital, 55 Fruit Street, White 270, Boston, MA 02114, United States
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. [PubMed] |
| 2. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2651] [Cited by in RCA: 2599] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 3. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] |
| 4. | Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int. 2009;29:502-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 275] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 5. | Sugiyama M, Sakahara H, Torizuka T, Kanno T, Nakamura F, Futatsubashi M, Nakamura S. 18F-FDG PET in the detection of extrahepatic metastases from hepatocellular carcinoma. J Gastroenterol. 2004;39:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Trojan J, Schroeder O, Raedle J, Baum RP, Herrmann G, Jacobi V, Zeuzem S. Fluorine-18 FDG positron emission tomography for imaging of hepatocellular carcinoma. Am J Gastroenterol. 1999;94:3314-3319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 182] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Lee JD, Yun M, Lee JM, Choi Y, Choi YH, Kim JS, Kim SJ, Kim KS, Yang WI, Park YN. Analysis of gene expression profiles of hepatocellular carcinomas with regard to 18F-fluorodeoxyglucose uptake pattern on positron emission tomography. Eur J Nucl Med Mol Imaging. 2004;31:1621-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Yang SH, Suh KS, Lee HW, Cho EH, Cho JY, Cho YB, Yi NJ, Lee KU. The role of (18)F-FDG-PET imaging for the selection of liver transplantation candidates among hepatocellular carcinoma patients. Liver Transpl. 2006;12:1655-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Cascales Campos P, Ramirez P, Gonzalez R, Febrero B, Pons JA, Miras M, Sanchez Bueno F, Robles R, Parrilla P. Value of 18-FDG-positron emission tomography/computed tomography before and after transarterial chemoembolization in patients with hepatocellular carcinoma undergoing liver transplantation: initial results. Transplant Proc. 2011;43:2213-2215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Kim HO, Kim JS, Shin YM, Ryu JS, Lee YS, Lee SG. Evaluation of metabolic characteristics and viability of lipiodolized hepatocellular carcinomas using 18F-FDG PET/CT. J Nucl Med. 2010;51:1849-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Kirchhoff TD, Rudolph KL, Layer G, Chavan A, Greten TF, Rosenthal H, Kubicka S, Galanski M, Manns MP, Schild H. Chemoocclusion vs chemoperfusion for treatment of advanced hepatocellular carcinoma: a randomised trial. Eur J Surg Oncol. 2006;32:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Jang JW, Bae SH, Choi JY, Oh HJ, Kim MS, Lee SY, Kim CW, Chang UI, Nam SW, Cha SB. A combination therapy with transarterial chemo-lipiodolization and systemic chemo-infusion for large extensive hepatocellular carcinoma invading portal vein in comparison with conservative management. Cancer Chemother Pharmacol. 2007;59:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Jang JW, Park YM, Bae SH, Choi JY, Yoon SK, Chang UI, Nam SW, Kim BS. Therapeutic efficacy of multimodal combination therapy using transcatheter arterial infusion of epirubicin and cisplatin, systemic infusion of 5-fluorouracil, and additional percutaneous ethanol injection for unresectable hepatocellular carcinoma. Cancer Chemother Pharmacol. 2004;54:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3303] [Article Influence: 220.2] [Reference Citation Analysis (36)] |
| 15. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21630] [Article Influence: 1351.9] [Reference Citation Analysis (1)] |
| 16. | Sweeney MJ, Ashmore J, Morris HP, Weber G. Comparative biochemistry hepatomas. IV. isotope studies of glucose and fructose metabolism in liver tumors of different growth rates. Cancer Res. 1963;23:995-1002. [PubMed] |
| 17. | Burk D, Woods M, Hunter J. On the significance of glucolysis for cancer growth, with special reference to Morris rat hepatomas. J Natl Cancer Inst. 1967;38:839-863. [PubMed] |
| 18. | Mocherla B, Kim J, Roayaie S, Kim S, Machac J, Kostakoglu L. FDG PET/CT imaging to rule out extrahepatic metastases before liver transplantation. Clin Nucl Med. 2007;32:947-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Okazumi S, Isono K, Enomoto K, Kikuchi T, Ozaki M, Yamamoto H, Hayashi H, Asano T, Ryu M. Evaluation of liver tumors using fluorine-18-fluorodeoxyglucose PET: characterization of tumor and assessment of effect of treatment. J Nucl Med. 1992;33:333-339. [PubMed] |
| 20. | Iwata Y, Shiomi S, Sasaki N, Jomura H, Nishiguchi S, Seki S, Kawabe J, Ochi H. Clinical usefulness of positron emission tomography with fluorine-18-fluorodeoxyglucose in the diagnosis of liver tumors. Ann Nucl Med. 2000;14:121-126. [PubMed] |
| 21. | Messa C, Choi Y, Hoh CK, Jacobs EL, Glaspy JA, Rege S, Nitzsche E, Huang SC, Phelps ME, Hawkins RA. Quantification of glucose utilization in liver metastases: parametric imaging of FDG uptake with PET. J Comput Assist Tomogr. 1992;16:684-689. [PubMed] |
| 22. | Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1325] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 23. | Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012;262:43-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 24. | Shen H, Agarwal D, Qi R, Chalasani N, Liangpunsakul S, Lumeng L, Yoo H, Kwo P. Predictors of outcome in patients with unresectable hepatocellular carcinoma receiving transcatheter arterial chemoembolization. Aliment Pharmacol Ther. 2007;26:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Bruix J, Llovet JM, Castells A, Montañá X, Brú C, Ayuso MC, Vilana R, Rodés J. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology. 1998;27:1578-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 391] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 26. | Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, Lencioni R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 409] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 27. | Forner A, Llovet JM, Bruix J. Chemoembolization for intermediate HCC: is there proof of survival benefit? J Hepatol. 2012;56:984-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Seo S, Hatano E, Higashi T, Hara T, Tada M, Tamaki N, Iwaisako K, Ikai I, Uemoto S. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res. 2007;13:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 29. | Sun L, Guan YS, Pan WM, Chen GB, Luo ZM, Wu H. Positron emission tomography/computer tomography in guidance of extrahepatic hepatocellular carcinoma metastasis management. World J Gastroenterol. 2007;13:5413-5415. [PubMed] |
| 30. | Kim BK, Kang WJ, Kim JK, Seong J, Park JY, Kim DY, Ahn SH, Lee DY, Lee KH, Lee JD. (18) F-fluorodeoxyglucose uptake on positron emission tomography as a prognostic predictor in locally advanced hepatocellular carcinoma. Cancer. 2011;Equb ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Yamamoto Y, Nishiyama Y, Kameyama R, Okano K, Kashiwagi H, Deguchi A, Kaji M, Ohkawa M. Detection of hepatocellular carcinoma using 11C-choline PET: comparison with 18F-FDG PET. J Nucl Med. 2008;49:1245-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 32. | Sun L, Wu H, Pan WM, Guan YS. Positron emission tomography/computed tomography with (18)F-fluorodeoxyglucose identifies tumor growth or thrombosis in the portal vein with hepatocellular carcinoma. World J Gastroenterol. 2007;13:4529-4532. [PubMed] |
| 33. | Megyesi C, Samols E, Marks V. Glucose tolerance and diabetes in chronic liver disease. Lancet. 1967;2:1051-1056. [PubMed] |
| 34. | Petrides AS, DeFronzo RA. Glucose metabolism in cirrhosis: a review with some perspectives for the future. Diabetes Metab Rev. 1989;5:691-709. [PubMed] |
| 35. | Kornberg A, Küpper B, Thrum K, Katenkamp K, Steenbeck J, Sappler A, Habrecht O, Gottschild D. Increased 18F-FDG uptake of hepatocellular carcinoma on positron emission tomography independently predicts tumor recurrence in liver transplant patients. Transplant Proc. 2009;41:2561-2563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2876] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 37. | Lin CY, Chen JH, Liang JA, Lin CC, Jeng LB, Kao CH. 18F-FDG PET or PET/CT for detecting extrahepatic metastases or recurrent hepatocellular carcinoma: A systematic review and meta-analysis. Eur J Radiol. 2011;Equb ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |