Published online May 28, 2012. doi: 10.3748/wjg.v18.i20.2554
Revised: September 28, 2011
Accepted: February 16, 2012
Published online: May 28, 2012
AIM: To determine if serum inter-cellular adhesion molecule 1 (ICAM-1) is an early marker of the diagnosis and prediction of severe acute pancreatitis (SAP) within 24 h of onset of pain, and to compare the sensitivity, specificity and prognostic value of this test with those of acute physiology and chronic health evaluation (APACHE) II score and interleukin-6 (IL-6).
METHODS: Patients with acute pancreatitis (AP) were divided into two groups according to the Ranson’s criteria: mild acute pancreatitis (MAP) group and SAP group. Serum ICAM-1, APACHE IIand IL-6 levels were detected in all the patients. The sensitivity, specificity and prognostic value of the ICAM-1, APACHE IIscore and IL-6 were evaluated.
RESULTS: The ICAM-1 level in 36 patients with SAP within 24 h of onset of pain was increased and was significantly higher than that in the 50 patients with MAP and the 15 healthy volunteers (P < 0.01). The ICAM-1 level (25 ng/mL) was chosen as the optimum cutoff to distinguish SAP from MAP, and the sensitivity, specificity, positive predictive value, negative predictive value (NPV), positive likelihood ratio and negative likelihood ratio were 61.11%, 71.42%, 0.6111, 0.7142, 2.1382 and 0.5445, respectively. The area under the curve demonstrated that the prognostic accuracy of ICAM-1 (0.712) was similar to the APACHE-IIscoring system (0.770) and superior to IL-6 (0.508) in distinguishing SAP from MAP.
CONCLUSION: ICAM-1 test is a simple, rapid and reliable method in clinical practice. It is an early marker of diagnosis and prediction of SAP within the first 24 h after onset of pain or on admission. As it has a relatively low NPV and does not allow it to be a stand-alone test for the diagnosis of AP, other conventional diagnostic tests are required.
- Citation: Zhu HH, Jiang LL. Serum inter-cellular adhesion molecule 1 is an early marker of diagnosis and prediction of severe acute pancreatitis. World J Gastroenterol 2012; 18(20): 2554-2560
- URL: https://www.wjgnet.com/1007-9327/full/v18/i20/2554.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i20.2554
Most cases of acute pancreatitis (AP) are mild and self limiting, and recover spontaneously, but approximately 20% of attacks turn to severe acute pancreatitis (SAP) with a life-threatening morbidity and a mortality rate of 20%-30%. Hence, early diagnosis and prediction of the severity of AP are of particular significance[1]. Early prediction of the severity of AP is still difficult in clinical practice. Ranson’s score can only be evaluated 48 h after admission. The acute physiology and chronic health evaluation II (APACHE II) score can be used within a few hours after admission, but its complex and cumbersome performance limits its clinical use[2]. Several laboratory markers have been developed over the past decade for the early diagnosis and prediction of SAP. Since there is no correlation found between the degree of structural damage to pancreas and clinical manifestation of the disease, no ideal predictive system or biochemical marker has been available. Several reports showed that serum intercellular adhesion molecule-1 (ICAM-1) levels were elevated during the course of AP and correlated with the severity of the disease and patient outcome. ICAM-1 can be detected as an early marker in the diagnosis of lung injury[3,4]. However, the details of the clinical use of this test for early diagnosis and prediction of SAP remain obscure, especially within the first 24 h in the patients after admission. The aim of this prospective study was to evaluate the use of the ICAM-1 in early diagnosis and prediction of SAP.
All patients with AP were included in the primary analysis according to the guidelines of diagnosis and treatment of AP established by Branch of Gastroenterology, Chinese Medical Association in 2003[5]. The diagnosis of AP was based on the following features: (1) Prolonged abdominal pain characteristic of AP; (2) Elevated serum amylase and/or lipase levels by at least 3-folds that of normal range; and (3) Characteristic findings of AP on abdominal ultrasonography and/or computed tomography (CT) scan. Patients who were admitted within the first 24 h of the onset of abdominal pain were not included in the study. Patients with an accompanying disease that might influence the outcome data were excluded, such as postoperative, post-traumatic, post-endoscopic retrograde cholangiopancreatographic pancreatitis. Other causes of acute abdominal pain were ruled out. Eighty-six patients with AP included in this pilot study were analyzed in a prospective 1-year investigation performed at a single institution.
The study was approved by the Committee of Research Ethics of our hospital, and informed consent was obtained from all the patients and healthy volunteers before enrollment. Demographics (gender, age, occupation, course and characteristics of symptom) and the cause of the pancreatitis (cholelithiasis, alcohol abuse, hyperlipidemia and others) were recorded. Routine clinical observation, laboratory test and treatment were performed. The APACHE-II score was determined within the first 24 h and 48 h of the onset of pain after admission[6]. Ultrasonography was performed every other day in all the patients and/or spiral CT with intravenous contrast was performed in some patients within 48-72 h after admission to assess the extent of inflammation and the degree of pancreas necrosis according to Balthazar’s classification. After the examinations, patients’ data were reviewed to determine the eligibility for inclusion into the study.
Ranson’s score was recorded in the first 24 h and 48 h after admission. Since Ranson et al[7] in 1974 identified 11 prognostic factors, considerable researches have been undertaken to find the ideal predictor(s) that allow rapid and correct assessment of the severity of AP to suit different clinical and regional settings. SAP was categorized based on the clinical and laboratory data using Ranson’s score. Cases meeting less than three positive criteria were classified as mild acute pancreatitis (MAP) and those meeting three or more positive criteria were classified as SAP.
To check the time kinetics of rise in plasma ICAM-1 and interleukin-6 (IL-6) during AP, its levels were quantified in two time-points. The potential of ICAM-1 and IL-6 to predict SAP within the first 24 h and 48 h after the onset of symptoms or on admission was examined. A 5-mL sample of peripheral venous blood was collected twice from the 85 patients with AP. Blood samples obtained from the 15 healthy volunteers served as controls. The plasma was separated by centrifugation at 3000 r/min for 10 min at 4 °C and stored at -20 °C until assayed. Plasma ICAM-1 and IL-6 were quantified with commercially available enzyme-linked immunosorbent assay (ELISA) kits (Sen-Xiong Technology Limited Company, Shanghai, China). ELISA plates were read at 450 nm and data was collected. Measurement was performed according to the instructions of the manufacturer.
After the examinations, data from each patient were reviewed to ascertain the eligibility for inclusion into the study. The data of gender, etiology of SAP and MAP, and the categorical variables were compared using χ2 test. Analysis of variance was performed in the continuous variables of age, ICAM-1, IL-6 levels and other relative laboratory tests for SAP and MAP using Student’s t test. The differences were considered statistically significant if P≤ 0.05. In order to differentiate SAP from MAP within the first 24 h of the onset of symptoms, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and positive likelihood ratio (PLR) and negative likelihood ratio (NLR) at different serum levels of ICAM-1 compared with APACHE II and IL-6 levels were calculated, respectively. The level of higher PLR and lower NLR was chosen as the optimum cutoff point. A receiver operating characteristic (ROC) curves were constructed to determine the reference cutoff point for ICAM-1, APACHE II and IL-6 levels that could distinguish between SAP and MAP. An index of the goodness of the test of area under the curve (AUC) was used to measure the ability of distinguishing SAP from MAP. A perfect test had an area of 1.0, while a non-discriminating test had an area of 0.5. All statistical analyses were performed using SPSS v8.00 statistical analysis software (SPSS Inc., Cary, NC).
The prospective study population consisted of 86 consecutive patients with AP (48 men, 38 women with a mean age of 50.7 years; range, 17-79 years) and 15 healthy volunteers (10 men, 5 women with a mean age of 48.7 years; range, 28-58 years). Forty-nine (56.9%) patients were diagnosed as having MAP and 36 (41.8%) as having SAP according to the Ranson’s criteria. The clinical characteristics of the patients with AP are summarized in Table 1. Gallstones were the most common cause of both SAP and MAP (n = 19, 52.7% and n = 20, 40.8%, respectively). There was no difference in the sex, race and etiology of the disease between the SAP and MAP patients. The mean serum amylase and glucose levels were significantly higher in SAP than in MAP (P < 0.05). There was no significant difference in the serum level of total bilirubin between the two groups at the first 24 h after the onset of pain.
| SAP, n = 36 | MAP, n = 50 | P value | |
| Age (mean ± SE, yr) | 53.19 ± 14.9 | 49.4 ± 10.9 | > 0.05 |
| Gender (male/female) | 18/18 | 30/20 | > 0.05 |
| Length of hospital stay (d) | 18.3 ± 1.6 | 7.3 ± 1.4 | < 0.001 |
| Etiology, n (%) | |||
| Gall stones | 19 (52.7) | 20 (40.8) | < 0.05 |
| Alcohol | 4 (11.1) | 8 (16.3) | |
| Idiopathic | 13 (36.1) | 21 (42.8) | |
| Test at first 24 h | |||
| Blood amylase (U/L) | 456.8 ± 175.7 | 389 ± 125.7 | < 0.05 |
| Total bilirubin (mmol/mL) | 39.10 ± 22.42 | 29.15 ± 25.88 | > 0.05 |
| Blood glucose (mmol/mL) | 9.61 ± 4.77 | 6.87 ± 2.63 | < 0.05 |
| Blood calculus | 2.41 ± 0.33 | 2.63 ± 2.16 | > 0.05 |
| Test at 48-72 h | |||
| Pancreatic necrosis | 16 | 2 | < 0.01 |
| Ranson’s score | 5.3 ± 0.5 | 1.5 ± 0.24 | < 0.001 |
Mean scores of APACHE-II, serum ICAM-1 and IL-1 levels in patients with SAP and MAP
The mean scores of APACHE-II in the SAP patients within the first 24 h after the onset of pain were also significantly higher than in the MAP patients (P < 0.001). The mean serum level of ICAM-1 in the SAP patients within the first 24 h after the onset of pain was significantly higher than in the MAP patients (P < 0.001) and the healthy controls (P < 0.001). Significantly higher levels of IL-6 were found in the SAP patients as compared with the MAP patients at the 24 h and the healthy controls (P < 0.001). The APACHE-II scores within the first 24 h after admission to hospital were significantly higher in SAP than in MAP patients (P < 0.001) (Table 2). The mean serum level of ICAM-1 in SAP patients at 48-72 h after admission was obviously higher than in SAP patients within the first 24 h after admission (P < 0.001). The level of IL-6 declined in the AP patients at 48-72 h after admission, but it was still obviously higher in the SAP patients than in the MAP patients (P < 0.001). The mean scores of APACHE-II in SAP and MAP at the 48-72 h after the onset of pain were slightly increased, but the scores in SAP were significantly higher than that in MAP in the first 24 h (Table 3).
| SAP, n = 36 | MAP, n = 50 | Control | t | P value | |
| APACHE-II | 14.47 ± 5.81 | 7.57 ± 1.44 | 7.9132 | < 0.001 | |
| ICAM-1 (ng/mL) | 29.68 ± 8.04 | 16.77 ± 4.37 | 8.12 ± 2.33 | 9.5028 | < 0.001 |
| IL-6 (ng/mL) | 68.76 ± 28.62 | 35.95 ± 11.56 | 14.46 ± 3.53 | 7.2700 | < 0.001 |
| Type | < 24 h | 48-72 h | t | P value | |
| APACHE-II | SAP | 14.47 ± 5.81 | 16.6 ± 2.03 | 2.0776 | < 0.05 |
| MAP | 7.57 ± 1.44 | 9.51 ± 1.74 | 6.0216 | < 0.001 | |
| ICAM-1 (ng/mL) | SAP | 29.68 ± 8.04 | 44.76 ± 12.08 | 6.2353 | < 0.001 |
| MAP | 16.77 ± 4.37 | 24.10 ± 5.88 | 7.0038 | < 0.001 | |
| IL-6 (ng/mL) | SAP | 68.76 ± 28.62 | 42.19 ± 12.77 | 5.0868 | < 0.001 |
| MAP | 35.95 ± 11.56 | 21.76 ± 8.65 | 6.8798 | < 0.001 |
The sensitivity, specificity, PPV, NPV, PLR and NLR at different serum ICAM-1 levels (10, 15, 25, 30, 35, 40 and 45 ng/mL) were determined respectively. The ICAM-1 level (25 ng/mL) with higher PLR and lower NLR was chosen as the optimum cutoff to distinguish SAP from MAP within first 24 h of the onset of symptoms, and the sensitivity, specificity, PPV, NPV, PLR and NLR were 61.11%, 71.42%, 0.6111, 0.7142, 2.1382 and 0.5445, respectively. The APACHE-II scores (< 4, 4-8, 9-12, 13-16, 17-20 and > 20) were also calculated. The sensitivity, specificity, PPV, NPV, PLR and NLR at the optimum cutoffs of APACHE-II score > 8 were 77.77%, 55.10%, 0.5600, 0.7714, 1.7320 and 0.2223, respectively. The different serum IL-6 levels (20, 30, 40, 50, 60, 70 and 80 ng/mL) were respectively calculated using the same method. The sensitivity, specificity, PPV, NPV, PLR and NLR at the optimum cutoffs of IL-6 50 ng/mL were 36.11%, 63.26%, 0.6169, 0.5740, 1.5869 and 0.9180, respectively (Table 4).
| Cutoffs | Sensitivity(%) | Specificity(%) | PPV | NPV | PLR | NLR | |
| APACHE-II | > 8 | 77.77 | 55.10 | 0.5600 | 0.7714 | 1.7320 | 0.2223 |
| ICAM-1 | 25 ng/mL | 61.11 | 71.42 | 0.6111 | 0.7142 | 2.1382 | 0.5445 |
| IL-6 | 50 ng/mL | 36.11 | 63.26 | 0.6190 | 0.5740 | 0.8826 | 1.1680 |
ROC curves and AUC of ICAM-1, APACHE-II and IL-6 in distinguishing SAP from MAP
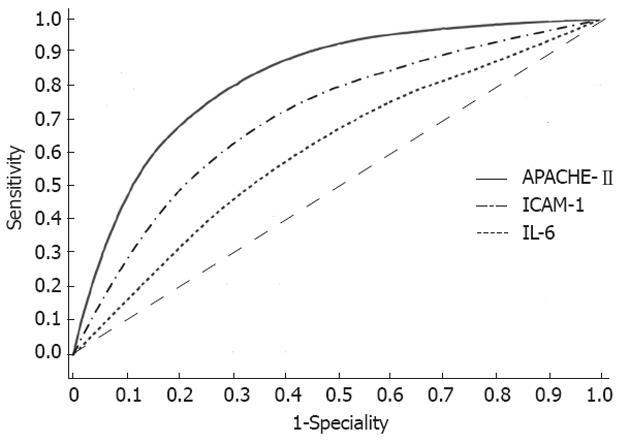
The optimum cutoffs of APACHE-II score > 8, serum ICAM-1 (25 ng/mL) and IL-6 (50 ng/mL) which were used to distinguish SAP from MAP within the first 24 h of the onset of symptoms were analyzed by constructing a ROC curve. The AUC of ICAM-1, APACHE-II and IL-6 in predicting SAP was 0.712, 0.770 and 0.508, respectively (Figure 1). The AUC demonstrated that the prognostic accuracy of ICAM-1 was similar to the APACHE-II scoring system. The AUC of serum ICAM-1 level was obviously superior to that of IL-6. The serum IL-6 level was not capable of distinguishing SAP from MAP within the first 24 h of the onset of symptoms.
In China, the exact incidence of AP is still unknown, but it is increasing gradually in recent years. AP is a life-threatening illness with an annual incidence of 30-50 attacks per 100 000 inhabitants[8]. It is an inflammatory process that presents different severity degrees, ranging from a mild self-limiting disease with interstitial edema in the pancreas, to a severe disease with extensive necrosis[9]. It progresses to a severe illness with a prolonged course in about 15%-20% of the patients. These severely ill patients may develop organ failure and/or local complications such as pancreatic necrosis. Approximately, 75% of the cases are mild with a mortality below 1%. Eighty percent of the patients could recover completely, while 20% had their disease worsened according to the Atlanta criteria. The mortality increases up to 20% if the disease progresses into a severe necrotizing form[10] and the mortality can be as high as 30%-40%[11]. Current management strategies for patients with SAP include early admission to intensive care units, vigorous intravenous resuscitation, and urgent endoscopic retrograde cholangiopancreatography when cholangitis or biliary obstruction is present, antibiotic prophylaxis in patients with pancreatic necrosis, and close patient monitoring.
Thus, the management of AP is still challenging mainly due to the delay in admission to hospital after the onset of symptoms and the difficulty in discriminating MAP from SAP, especially within the first 24 h. There is an urgent need for an early and accurate prediction of SAP to ensure timely interventions in a specialized care setting[12]. The early recognition and diagnosis are an important goal in the optimal management of SAP. Severity assessment is indispensable to the selection of proper initial treatment in the management of AP.
Several prognostic scoring systems are being used in predicting SAP: Ranson’s, Glasgow’s, APACHE-II, the bedside index for severity of AP (BISAP), and computed tomography severity index (CTSI). Papachristou et al[13] reported that the AUC for BISAP, Ranson’s, APACHE-II and CTSI in predicting SAP are 0.81, 0.94, 0.78 and 0.84, respectively in 185 patients with AP and found that all these scoring systems had a high accuracy in predicting SAP in the first 24 h after admission. The components of the BISAP score are clinically relevant and easy to obtain. Lujano-Nicolás et al[14] evaluated the severity of AP according to the Ranson’s and APACHE-II scores on admission in 28 patients with AP and correlated these scales with the local pancreatic complications according to the Balthazar classification. They found that these scales have discrepancies when compared with tomographic Balthazar and these scales were not correlated well with the tomographic Balthazar degrees. The Ranson’s prognostic signs and the Glasgow’s score can only be applied 48 h after admission. The APACHE-II score has the invaluable advantage of being useful within a few hours after admission, and it can be assessed serially. However, it is cumbersome, which limits its use in clinical practice. The current gold standard for staging AP combines the clinical criteria with CT, but because of the high cost, exposure to ionizing radiation, and lack of sensitivity and specificity in the early stage of the disease, it has limited availability.
Numerous efforts have been made in recent years to identify objective markers that can predict the severity of AP on admission. Various biochemical tests, such as C-reactive protein (CRP), tumor necrosis factor, IL-2 and IL 6, have been developed over the past decade for early diagnosis and prediction of severity of AP[15-17]. However, except for CRP, none of them can accurately predict the disease severity within 24 h of onset, and the outcome in triage to the intensive care unit has not been reported[18]. As there is no correlation between the degree of structural damage to pancreas and clinical manifestation of the disease, there has been no ideal predictive system or biochemical marker for this disease. Little is known about clinical predictors of early readmission for AP. The ideal predictors of the severity of AP are described as being simple, quick, highly sensitive, highly specific, safe, reproducible, and cheap. An immediate test with a high specificity, AUC and low NLR is required. Unfortunately, this ideal predictor is still not available[19].
Adhesion molecules are involved in the inflammatory response during AP. ICAM-1, a single-chain transmembrane glycoprotein with a molecular weight of 80-110 kDa, consists of five Ig-like domains, a hydrophobic transmembrane domain and a short cytoplasmic C-terminal domain. Its ligand includes lymphocyte function-associated antigen-1 and macrophage antigen-1. Under physiological conditions, ICAM-1 is expressed at a low level in endothelial cells and epithelial cells or constitutively on the surface of alveolar cells, providing the underlying molecular basis for cell recognition, activation, proliferation, differentiation and motility, thereby helping stabilize the internal environment of the body. ICAM-1 also plays a key role in pathological events, such as inflammatory reactions, including acute renal failure and acute pancreatitis[20].
One of the most common complications of AP is acute lung injury, during which ICAM-1 plays an important role by participating in leukocyte adhesion and activation as well as by inducing the “cascade effect” of inflammatory mediators, pulmonary microcirculation dysfunction and even acute respiratory distress syndrome, multiple organ failure or death. The upregulation of ICAM-1 expression in the lung during acute lung injury is one of main pathogeneses; the early detection of the ICAM-1 expression level may contribute to the prevention and treatment of acute lung injury[3,4]. Sun et al[20] investigated the ICAM-1 in mediating the development of AP from a local disease to a systemic illness in rats and found that upregulation of ICAM-1 and subsequent leukocyte infiltration appear to be significant events of pancreatic and pulmonary injuries. Intracapillary leucocyte accumulation represents a novel protective and potentially lifesaving mechanism of hemostasis in acute pancreatitis. This process depends on the expression of lymphocyte function-associated antigen 1 and ICAM-1 and precedes the classical steps of the leucocyte recruitment cascade[21]. Chooklin et al[22] measured the serum levels of pro-inflammatory and anti-inflammatory cytokines in 51 AP patients who were diagnosed with pancreatitis-associated lung injury with and without the development of organ dysfunction and found that in the pathogenesis of respiratory complications in AP cytokines, chemokines and adhesion molecules, in particular ICAM-1, play major roles. High ICAM-1 concentration was found in plasma during AP, which was not reduced by Dx treatment. Dexamethasone down-regulates ICAM-1 expression, but it does not completely prevent leukocyte recruitment during sodium taurocholate-induced AP[23]. Pancreatic ICAM-1 expression was increased in single-nucleotide polymorphism as compared with the controls. After calcitonin gene-related peptide application, pancreatic ICAM-1 expression was attenuated[24]. Graft pancreatitis is induced by ischemia/reperfusion injury in which neutrophil infiltration is believed to be a crucial early event. The data suggested that ICAM-1 was already up-regulated during cold ischemia, possibly representing the mechanism of early neutrophil infiltration observed in human pancreatic ischemia/reperfusion injury[25].
Irrespective of the underlying etiology, the immune response is almost identical in severe cases of AP. While the triggering factors of AP are still poorly understood, cytokines are considered as important mediators in the pathophysiology of SAP. Perejaslov et al[26] reported that peak levels of sICAM-1 on admission in AP 87 patients had a subsequent decrease and these mediators are correlated with the disease severity, development of multiple organ dysfunction syndrome and necrosis and may be used as prognostic markers. Several reports showed that serum ICAM-1 levels are elevated during the course of AP and correlate with the severity of the disease and patient outcome. ICAM-1 can be detected as an early marker for the diagnosis of lung injury[20]. Although serum ICAM-1 level was increased in AP patients and closely related to the development of SAP in many clinical studies, the details of the use of ICAM-1 for early diagnosis and prediction of SAP remain obscure, especially in the first 24 h after the admission. The aim of our prospective study was to evaluate the use of the ICAM-1 in early diagnosis and prediction of SAP, and to compare the sensitivity, specificity and prognostic value of this test with those of APACHE-II score and IL-6.
Among the 86 patients with AP in this study, 49 patients were diagnosed as having MAP and 36 as having SAP according to the Ranson’s criteria. The mean serum level of ICAM-1 in the SAP patients within the first 24 h of the onset of pain was significantly higher than in MAP patients and the healthy controls. The mean serum level of ICAM-1 in the SAP patients at 48-72 h after admission was obviously higher than in the SAP group. The result showed that ICAM-1 is a simple, rapid and reliable method for use within the first 24 h after admission. It has a higher specificity and a sensitivity for early diagnosis and prediction of SAP. The AUC value demonstrated that the prognostic accuracy of ICAM-1 is similar to the APACHE-II scoring system and obviously superior to IL-6 in distinguishing SAP from MAP. Previous reports showed the usefulness of serum IL-6 level in determining the severity of acute pancreatitis[18], but our data indicated that the serum IL-6 level could not distinguish SAP from MAP within the first 24 h. The exact reason for the difference is still unknown. Although the APACHE-II scoring system has a high prognostic accuracy, it is too complex and cumbersome, which limits its clinical use. However, ICAM-1 has a relatively low NPV and does not allow it to be a stand-alone tool for diagnosis of acute pancreatitis; and the use of other conventional diagnostic tools remains a requirement.
In conclusion, we found that serum ICAM-1 levels rose within the first 24 h after the onset of AP and that early measurement of serum ICAM-1 levels could distinguish SAP from MAP. It has a higher sensitivity, specificity and NPV. It could be used as a test in screening patients with AP and predicting the outcome in patients with SAP. The predictive accuracy of ICAM-1 is similar to APACHE-II scoring system and obviously superior to IL-6. But its low NPV does not allow it to be a stand-alone tool for the diagnosis and prediction of AP. To identify a novel predictor of severity and outcome of AP is needed so as to improve the predictive accuracy.
Most cases of acute pancreatitis (AP) are mild and self limiting, and recover spontaneously, but approximately 20% of attacks turn to severe acute pancreatitis (SAP) with a life-threatening morbidity and a mortality rate of 20%-30%. There is no established biomarker for early diagnosis and prediction of severe pancreatitis. Early diagnosis and prediction of the severity of AP is of particular significance.
Serum intercellular adhesion molecule-1 (ICAM-1) levels are elevated during the course of AP and correlate with the severity of the disease. ICAM-1 can be detected as an early marker for the diagnosis of lung injury. The details of the clinical use of ICAM-1 for early diagnosis and prediction of severity in AP remain obscure, especially in the first 24 h after the admission. This study evaluated the use of the ICAM-1 in early diagnosis and prediction of SAP, and compared the sensitivity, specificity and prognostic value of this test with those of acute physiology and chronic health evaluation (APACHE) II score and interleukin-6 (IL-6).
Serum ICAM-1 is elevated within the first 24 h after the onset of symptoms. Elevated ICAM-1 levels are associated with the severity of AP with a higher sensitivity, specificity and negative predictive value for early diagnosis and prediction of severity in AP. The predictive accuracy of ICAM-1 is similar to the APACHE-II scoring system and obviously superior to IL-6.
Serum ICAM-1 is an early marker for the diagnosis and prediction of SAP, and the test is simple, rapid and reliable that can be used in clinic practice. The results obtained are important for both early diagnosis and treatment of pancreatitis.
ICAM-1 is a single-chain transmembrane glycoprotein. Under physiological conditions, it is expressed at a low level in endothelial cells providing the underlying molecular basis for cell recognition, activation, proliferation, differentiation and motility. ICAM-1 also plays a key role in pathological events, such as inflammatory reactions, including acute renal failure and acute pancreatitis
The manuscript reports that ICAM-1 is a useful marker for early diagnosis and a potential predictor of severe acute pancreatitis. These data are important for early treatment for pancreatitis patients.
Peer reviewer: Toshiyuki Ishiwata, Associate Professor, Department of Pathology, Integrative Oncological Pathology, Nippon Medical School, 1-1-5 Sendagi, Bunkyo-ku, Tokyo 113-8602, Japan
S- Editor Gou SX L- Editor Ma JY E- Editor Li JY
| 1. | Kamer E, Unalp HR, Derici H, Tansug T, Onal MA. Early diagnosis and prediction of severity in acute pancreatitis using the urine trypsinogen-2 dipstick test: a prospective study. World J Gastroenterol. 2007;13:6208-6212. [PubMed] |
| 2. | Lempinen M, Stenman UH, Finne P, Puolakkainen P, Haapiainen R, Kemppainen E. Trypsinogen-2 and trypsinogen activation peptide (TAP) in urine of patients with acute pancreatitis. J Surg Res. 2003;111:267-273. [PubMed] |
| 3. | Zhang X, Wu D, Jiang X. Icam-1 and acute pancreatitis complicated by acute lung injury. JOP. 2009;10:8-14. [PubMed] |
| 4. | Zhao X, Andersson R, Wang X, Dib M, Wang X. Acute pancreatitis-associated lung injury: pathophysiological mechanisms and potential future therapies. Scand J Gastroenterol. 2002;37:1351-1358. [PubMed] |
| 5. | Branch of digestive diseases of Chinese Medical Association. Guidelines for diagnosis and treatment of acute pancreatitis(draft). Zhonghua Neike Zazhi. 2004;43:236-238. |
| 6. | Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818-829. [PubMed] |
| 7. | Ranson JH, Rifkind KM, Roses DF, Fink SD, Eng K, Spencer FC. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet. 1974;139:69-81. [PubMed] |
| 8. | McKay CJ, Imrie CW. The continuing challenge of early mortality in acute pancreatitis. Br J Surg. 2004;91:1243-1244. [PubMed] |
| 9. | Targarona Modena J, Barreda Cevasco L, Arroyo Basto C, Orellana Vicuña A, Portanova Ramírez M. Total enteral nutrition as prophylactic therapy for pancreatic necrosis infection in severe acute pancreatitis. Pancreatology. 2006;6:58-64. [PubMed] |
| 10. | Slavin J, Ghaneh P, Sutton R, Hartley M, Rowlands P, Garvey C, Hughes M, Neoptolemos J. Management of necrotizing pancreatitis. World J Gastroenterol. 2001;7:476-481. [PubMed] |
| 11. | Spanier BWM, Bruno MJ, Mathus-Vliegen EMH. Enteral nutrition and acute pancreatitis: a review. Gastroenterol Res Practice. 2010;2011:1155-1153. |
| 12. | Bollen TL, Singh VK, Maurer R, Repas K, van Es HW, Banks PA, Mortele KJ. A comparative evaluation of radiologic and clinical scoring systems in the early prediction of severity in acute pancreatitis. Am J Gastroenterol. 2012;107:612-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 13. | Papachristou GI, Muddana V, Yadav D, O'Connell M, Sanders MK, Slivka A, Whitcomb DC. Comparison of BISAP, Ranson's, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol. 2010;105:435-441; quiz 442. [PubMed] |
| 14. | Lujano-Nicolás LA, Pérez-Hernández JL, Durán-Pérez EG, Serralde-Zúñiga AE. Corelation among clinical, biochemical and tomographic criteria in order to evaluate the severity in acute pancreatitis. Rev Esp Enferm Dig. 2010;102:376-380. [PubMed] |
| 15. | Schütte K, Malfertheiner P. Markers for predicting severity and progression of acute pancreatitis. Best Pract Res Clin Gastroenterol. 2008;22:75-90. [PubMed] |
| 16. | Digalakis MK, Katsoulis IE, Biliri K, Themeli-Digalaki K. Serum profiles of C-reactive protein, interleukin-8, and tumor necrosis factor-alpha in patients with acute pancreatitis. HPB Surg. 2009;2009:878490. [PubMed] |
| 17. | Rau B, Steinbach G, Baumgart K, Gansauge F, Grünert A, Beger HG. The clinical value of procalcitonin in the prediction of infected necrosis in acute pancreatitis. Intensive Care Med. 2000;26 Suppl 2:S159-S164. [PubMed] |
| 18. | Nathens AB, Curtis JR, Beale RJ, Cook DJ, Moreno RP, Romand JA, Skerrett SJ, Stapleton RD, Ware LB, Waldmann CS. Management of the critically ill patient with severe acute pancreatitis. Crit Care Med. 2004;32:2524-2536. [PubMed] |
| 19. | Brisinda G, Vanella S, Crocco A, Mazzari A, Tomaiuolo P, Santullo F, Grossi U, Crucitti A. Severe acute pancreatitis: advances and insights in assessment of severity and management. Eur J Gastroenterol Hepatol. 2011;23:541-551. [PubMed] |
| 20. | Sun W, Watanabe Y, Wang ZQ. Expression and significance of ICAM-1 and its counter receptors LFA-1 and Mac-1 in experimental acute pancreatitis of rats. World J Gastroenterol. 2006;12:5005-5009. [PubMed] |
| 21. | Ryschich E, Kerkadze V, Deduchovas O, Salnikova O, Parseliunas A, Märten A, Hartwig W, Sperandio M, Schmidt J. Intracapillary leucocyte accumulation as a novel antihaemorrhagic mechanism in acute pancreatitis in mice. Gut. 2009;58:1508-1516. [PubMed] |
| 22. | Chooklin S. Pathogenic aspects of pulmonary complications in acute pancreatitis patients. Hepatobiliary Pancreat Dis Int. 2009;8:186-192. [PubMed] |
| 23. | Ramudo L, Yubero S, Manso MA, Sanchez-Recio J, Weruaga E, De Dios I. Effects of dexamethasone on intercellular adhesion molecule 1 expression and inflammatory response in necrotizing acute pancreatitis in rats. Pancreas. 2010;39:1057-1063. [PubMed] |
| 24. | Schneider L, Hartwig W, Flemming T, Hackert T, Fortunato F, Heck M, Gebhard MM, Nawroth PP, Bierhaus A, Buchler MW. Protective effects and anti-inflammatory pathways of exogenous calcitonin gene-related peptide in severe necrotizing pancreatitis. Pancreatology. 2009;9:662-669. [PubMed] |
| 25. | Wiessner R, Eisold S, Linnebacher M, Bünger C, Nizze H, Wacke R, Benz S, Schareck W, Klar E. Up-regulation of ICAM-1 during cold ischemia triggers early neutrophil infiltration in human pancreas allograft reperfusion. Transplant Proc. 2009;41:3622-3627. [PubMed] |
| 26. | Perejaslov A, Chooklin S, Bihalskyy I. Implication of interleukin 18 and intercellular adhesion molecule (ICAM)-1 in acute pancreatitis. Hepatogastroenterology. 2008;55:1806-1813. [PubMed] |