Published online May 28, 2012. doi: 10.3748/wjg.v18.i20.2526
Revised: February 6, 2012
Accepted: February 16, 2012
Published online: May 28, 2012
AIM: To determine the utility of endoscopic ultrasound-guided biliary drainage (EUS-BD) with a fully covered self-expandable metal stent for managing malignant biliary stricture.
METHODS: We collected data from 13 patients who presented with malignant biliary obstruction and underwent EUS-BD with a nitinol fully covered self-expandable metal stent when endoscopic retrograde cholangiopancreatography (ERCP) fails. EUS-guided choledochoduodenostomy (EUS-CD) and EUS-guided hepaticogastrostomy (EUS-HG) was performed in 9 patients and 4 patients, respectively.
RESULTS: The technical and functional success rate was 92.3% (12/13) and 91.7% (11/12), respectively. Using an intrahepatic approach (EUS-HG, n = 4), there was mild peritonitis (n = 1) and migration of the metal stent to the stomach (n = 1). With an extrahepatic approach (EUS-CD, n = 10), there was pneumoperitoneum (n = 2), migration (n = 2), and mild peritonitis (n = 1). All patients were managed conservatively with antibiotics. During follow-up (range, 1-12 mo), there was re-intervention (4/13 cases, 30.7%) necessitated by stent migration (n = 2) and stent occlusion (n = 2).
CONCLUSION: EUS-BD with a nitinol fully covered self-expandable metal stent may be a feasible and effective treatment option in patients with malignant biliary obstruction when ERCP fails.
- Citation: Kim TH, Kim SH, Oh HJ, Sohn YW, Lee SO. Endoscopic ultrasound-guided biliary drainage with placement of a fully covered metal stent for malignant biliary obstruction. World J Gastroenterol 2012; 18(20): 2526-2532
- URL: https://www.wjgnet.com/1007-9327/full/v18/i20/2526.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i20.2526
Endoscopic retrograde cholangiopancreatography (ERCP) is a well-established technique for providing biliary decompression in patients with bile duct obstruction. The success rate for effective biliary decompression with ERCP ranges from 90% to 95%[1]. However, there are patients in whom ERCP fails because of unsuccessful biliary cannulation, or inaccessible papilla due to duodenal stenosis caused by tumor invasion. In these cases, percutaneous biliary drainage (PTBD) is required. However, PTBD can lead to significant complications, including biliary peritonitis, hemobilia, pneumothorax, hematoma, liver abscesses, and patient discomfort related to the catheter[2].
Therefore, endoscopic ultrasound-guided biliary drainage (EUS-BD) using plastic stents has been introduced as an alternative to PTBD in cases of biliary obstruction when ERCP fails[3-6]. However, plastic stent malfunction due to stent clogging after EUS-BD is not uncommon[3,7,8]. Fully covered self-expandable metal stent (FCSEMS) with a large bore diameter may have advantages over plastic stents. Recently, EUS-BD with a FCSEMS was introduced in a few cases of malignant biliary obstruction after a failed ERCP[9]. The current study was conducted to determine the feasibility, outcomes, and risks of EUS-guided hepaticogastrostomy (EUS-HG) and EUS-guided choledochoduodenostomy (EUS-CD) with an FCSEMS as a biliary diversion technique in patients with malignant biliary obstruction for whom interventional ERCP was unsuccessful.
We collected data on all patients who presented with obstructive jaundice and who underwent EUS-BD after a failed ERCP during a 20-mo period from February 2009 to September 2010. Failed ERCP was defined as the inability to relieve jaundice or failed biliary cannulation. A total of 2209 ERCPs at Wonkwang University Hospital (n = 780) and Jeonbuk National University Hospital (n = 1429) were performed during the study period, and 366 required biliary decompression. Of the 22 (6%) patients who underwent alternative methods for biliary decompression as a result of failed ERCP, 13 had EUS-BD. Study eligibility was determined as follows: (1) Initial biliary cannulation or bile duct decompression by ERCP failed because of accompanying duodenal obstruction, periampullary tumor infiltration, and difficult cannulation (n = 11); (2) A high-grade left-sided hilar stricture as a result of segmental tumor progression with an occluded biliary metal stent was unable to be crossed by a guidewire (n = 2); (3) The patient refused PTBD (n = 13).
ERCP was performed by 3 experienced endoscopists (Kim TH, Kim SH and Lee SO). Each endoscopist performs 400 to 450 ERCPs annually. EUS-BD was performed by 2 experienced endoscopists (Kim TH and Kim SH) who perform more than 250 EUS procedures for pancreaticobiliary diseases annually.
In this study, stent occlusion was classified as predominantly tumor ingrowth (seen on fluoroscopy as a narrowing within the stent), tumor overgrowth (seen radiographically or endoscopically as a new narrowing at the proximal or distal margin of the stent), or sludge/debris (demonstrated as echoendoscopic findings or multiple radiographic filling defects that disappeared after extraction with a biliary balloon along with endoscopic visualization of the extracted sludge)[10]. The Institutional Review Boards at Wonkwang University Hospital and Jeonbuk National University Hospital, South Korea approved this study. All patients provided written informed consent for participation in this study.
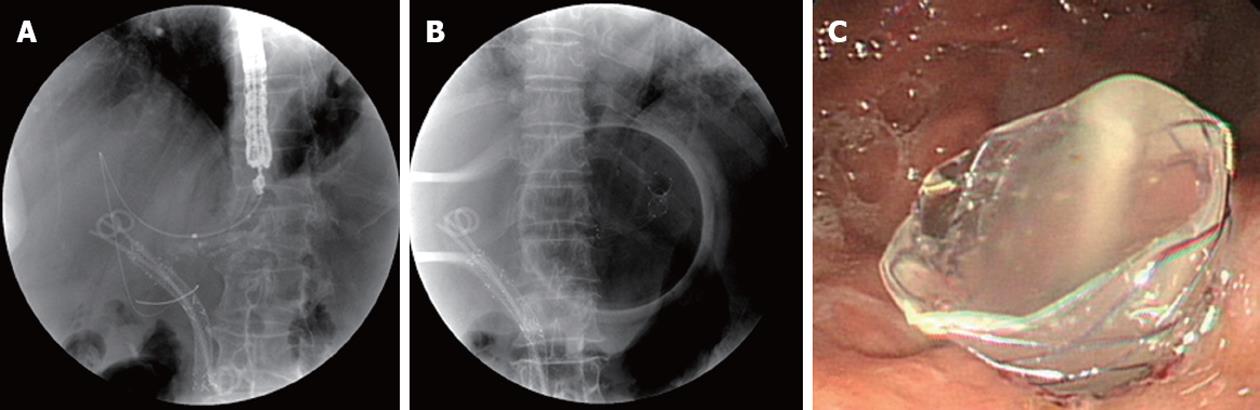
Antibiotics were permitted in all cases for 3 d to 5 d before and after the intervention. ERCP was initially attempted in each patient by using a therapeutic duodenoscope (TJF-240; Olympus Optical Co, Tokyo, Japan). When the ERCP was unsuccessful, an EUS was performed using a GF-UCT 240 linear-array echoendoscope (Olympus Corp., Tokyo, Japan) in a second session (on the same or next day). These patients underwent an EUS-HG or EUS-CD. Using the transgastric approach, the echoendoscope was placed in the cardia or the lesser curvature of the stomach to view the dilated segment 3 of the liver (Figure 1). Using the transduodenal approach, the echoendoscope was placed in the duodenal bulb to image the extrahepatic bile duct (Figure 2). Color Doppler ultrasonography was used to identify the regional vasculature, and a bile duct puncture was performed with a 19-gauge needle (EUSN-19-T; Cook Endoscopy, Winston-Salem, NC, United States). To confirm successful biliary access, contrast medium was injected under fluoroscopy to demonstrate biliary opacification. A 0.035-inch guidewire was introduced through the EUS needle and advanced in an antegrade or retrograde fashion. Afterwards, 6F and 7F tapered, biliary, bougie catheters (catheter tip, 4F; Cook Endoscopy) were inserted and removed, over the guidewire to dilate the tract. If there was resistance when advancing the 6F bougie catheter, a needle-knife (Microtome; Boston Scientific, Natick, MA, United States) with a 7F shaft diameter or tapered MTW catheter (WTW, Endoscopie, Wesel, Germany) was inserted over the guidewire to dilate the tract. To accomplish this, the tip of a needle-knife (Microtome) with a pure current was gently inserted over the guidewire into the biliary system. The needle was then withdrawn, and the needle-knife was pushed in to dilate the tract. A commercially available, fully silicon-covered metal stent with an 8F deployment system and an olive tip (BONASTENT, a nitinol stent with a 10-mm diameter and 6-cm length; Standard Sci Tech Inc., Seoul, South Korea) was placed under echoendoscopic and fluoroscopic view.
Technical success was defined as the deployment of the metal stent across the stomach or duodenum, along with the flow of contrast medium and/or bile through the stent. Functional success was defined as a reduction in bilirubin to less than 50% of the pretreatment value within 2 wk[11]. An early complication was defined as any stent-related complication within 30 d, including complications of bile leakage, pneumoperitoneum, bleeding, and stent migration. A late complication was defined as any stent-related complication, such as stent migration and stent occlusion, occurring 30 d after stent placement. Biliary reintervention was defined as any type of endoscopic, percutaneous, or surgical procedure that was required to improve biliary drainage after placement of the stent. Stent occlusion was defined as the recurrence of jaundice and cholestasis and/or evidence of a dilated biliary system on ultrasound (US) or computed tomography (CT) with a direct view of the upper endoscope, which in all cases would require biliary intervention. Procedure time was defined as the time between puncture of the biliary tract with a 19-gauge needle and placement of an FCSEMS.
Follow-up continued from stent insertion until the death of the patient or to the end of the study. Biochemical parameters and simple abdominal films were assessed at 2 d, 1 wk and 1 mo after stent placement and every 2 mo thereafter. Patient follow-up was based on outpatient examination findings. Imaging (US or CT and simple abdominal films) was routinely checked every 3 mo.
Procedure time (intrahepatic and extrahepatic approaches) and liver function test before and after the EUS-BD using a metal stent were compared using a 2-sample Wilcoxon signed-rank test. Statistical analysis was carried out using SPSS 12.0 (SPSS, Chicago, IL, United States), and a 2-tailed P value of < 0.05 was considered statistically significant. Findings are expressed as the median and range.
Patient characteristics, procedural data, and follow-up results are presented in Table 1. A total of 13 patients (9 men and 4 women) with malignant biliary obstruction underwent EUS-BD with a fully covered metal stent after a failed ERCP. The etiology of biliary obstruction was pancreatic cancer in 5 patients, distal common bile duct cancer in 6 patients, Klatskin’s tumor in 1 patient, and peripheral cholangiocarcinoma in 1 patient.
| No. of patient | Age/sex | Diagnosis | Reason for failed ERCP | Biliary drainage route | Device for puncture/dilatation | Diameter length of stent (mm/cm) | Technical success | Functional success | Complication | Reintervention | Duration (mo) of 1st stent placement | Status |
| 1 | 60/M | CBD cancer | Duodenal stenosis due to duodenal ulcer | Transduodenal | 19G FN/cystostome | 10/6 | Success | Success | No | No | Whipple’s operation | Alive |
| 2 | 83/F | CBD cancer | Failed to access the bile duct | Transduodenal | 19G FN/cystostome | 10/6 | Success | Success | Obstruction | Stent reinsertion | 6 | Dead |
| 3 | 76/F | CBD cancer | Failed to access the bile duct | Transduodenal | 19G FN/cystostome | 10/4 | Success | Success | Migration | Stent reinsertion | 2 | Dead |
| 4 | 68/F | CBD cancer | Duodenal obstruction due to mass | Transduodenal | 19G FN/cystostome | 10/6 | Success | Success | No | No | 7 | Alive |
| 5 | 66/M | Pancreatic cancer | Periampullary tumor infiltration | Transduodenal | 19G FN/tapered ERCP cannula and cystostome | 10/4 | Success | Success | Pneumoperitoneum/peritonitis | No | 3 | Dead |
| 6 | 68/M | Pancreatic cancer | Periampullary tumor infiltration | Transduodenal | 19G FN/bougie dilatator | 10/6 | Success | Success | No | No | 1 | Dead |
| 7 | 67/F | Pancreatic cancer | Periampullary tumor infiltration | Transduodenal | 19G FN/NK and bougie dilatator | 10/6 | Success | Success | No | No | 3 | Alive |
| 8 | 59/M | Pancreatic cancer | Periampullary tumor infiltration | Transduodenal | 19G FN/tapered ERCP cannula and cystostome | 10/6 | Success | Success | No | No | 4 | Alive |
| 9 | 80/M | Pancreatic cancer | Periampullary tumor infiltration | Transduodenal | 19G FN/tapered ERCP cannula and cystostome | 10/6 | Success | Success | No | No | 3 | Alive |
| 10 | 81/M | Klatskin’s tumor | Stricture could not be crossed (hilar) | Transgastric | 19G FN/cystostome | 10/6 | Success | Fail | Migration/pneumoperitoneum/peritonitis | PTBD | 5 | Dead |
| 11 | 55/M | Intrahepatic cholangiocarcinoma | Stricture could not be crossed (hilar) | Transgastric | 19G FN/cystostome | 10/6 | Success | Success | No | No | 3 | Dead |
| 12 | 62/M | Pancreatic cancer | Stricture could not be crossed (distal CBD) | Transgastric | 19G FN | 10/6 | Failed | Dead | ||||
| 13 | 70/M | CBD cancer | Stricture could not be crossed (distal CBD) | Transgastric | 19G FN/bougie dilatator | 10/6 | Success | Success | Migration | Stent reinsertion | 2 | Alive |
Reasons for failed ERCP were duodenal stenosis due to a previous duodenal ulcer (n = 1), tumor obstruction of the duodenum (n = 1), periampullary tumor infiltration (n = 5), failure to access the common bile duct (n = 2), high-grade stricture of the distal common bile duct with an occluded biliary plastic stent (n = 2), and high-grade left-sided hilar stricture from segmental tumor progression with an occluded biliary metal stent (n = 2). Patients with obstruction of the duodenum received a concomitant duodenal stent at time of the procedure.
Technical success was 92.3% (12/13); there was 1 failure of guidewire insertion after puncture through the transgastric approach, because the diameter of intrahepatic bile duct was so small and had sharp angulation. During EUS-BD, no wire passage for rendezvous was attempted in any of the patients. For biliary access, a cystostome only was used in 6 of the 12 patients (4 extrahepatic approach and 2 intrahepatic approach), a tapered MTW catheter and a cystostome together were used 3 of 12 patients, and just bougie dilatators were used in 3 patients, respectively. Median procedure time was 19.5 min (range, 14-35 min). Nine patients were treated using an extrahepatic approach (all transduodenal). Four patients were treated using an intrahepatic approach (all transgastric). The median diameter of the left intrahepatic bile duct as determined by EUS was 7.3 mm (range, 5.3-9.4 mm).
Functional success was 91.7% (11/12). After the placement of a EUS-BD, the median bilirubin level decreased significantly from 11.6 mg/dL to 1.7 mg/dL (P = 0.001). The median alkaline phosphatase level also decreased significantly from 1629.0 IU/L to 113.0 IU/L (P = 0.001). Migration of a metal stent to stomach occurred in 1 patient treated with the transgastric approach.
With the intrahepatic approach (n = 4), there was stent migration to the stomach with mild peritonitis (n = 1). With the extrahepatic approach (n = 9), there was pneumoperitoneum with mild peritonitis (n = 1). All patients with peritonitis were managed conservatively with antibiotics. Bleeding and cholangitis were not observed in any of the enrolled patients after the procedure. In addition, there was no significant pain after the procedure. Two patients with duodenal obstruction also received duodenal metal stents.
One patient with distal common bile duct (CBD) cancer underwent curative resection (Whipple’s operation), and this procedure did not disturb the operative procedure. None of the patients, except the patients that underwent the operation, were lost during the follow-up period (median, 5 mo; range, 1-12 mo). With regard to re-intervention, there was re-intervention in 4 of the 13 cases (30.7%), which was necessitated by stent migration (n = 3) and stent occlusion (n = 1). Stent migration occurred 2 d later (EUS-HG) and 2 mo later (EUS-CD and EUS-HG), respectively. In the patients with stent migration, the stents passed spontaneously without lodgment in the bowel. A second FCSEMS was placed through the previous choledochoduodenostomy site in 1 patient. In 2 patients with stent migration, PTBD was inserted into the obstructed left hepatic duct due to his severe general weakness. In 1 patient with stent occlusion by tumor ingrowth into the metal stent (choledochoduodenostomy), an additional FCSEMS was placed through the previous choledochoduodenostomy site.
EUS-BD may be an alternative method in some patients when endoscopic biliary drainage is unsuccessful because of failed biliary cannulation or tumor infiltration, which limits the endoscopic approach to the major papilla. At present, there are 2 approaches for EUS-BD, the transgastric route and the transduodenal route. A plastic or metal stent can be placed by this method. However, most papers reported EUS-BD with a plastic stent[4,5,7,8,12-14]. We thought that FCMEMS with a large bore diameter may have advantages over the plastic stent. There are a few papers describing EUS-BD with a metal stent[6,9,15] (Table 2), however, there are no reports regarding preoperative drainage of biliary obstruction by EUS-BD. The current study demonstrated that transduodenal EUS-BD using a nitinol FCSEMS may be safe and feasible after a failed ERCP. The reasons for failed ERCP were duodenal stenosis by a duodenal ulcer, periampullary tumor invasion, and difficult biliary cannulation. In one patient with distal CBD cancer who had duodenal stenosis due to a previous duodenal ulcer, initially EUS-BD was performed to resolve cholangitis and bile drainage, and Whipple’s operation was performed. But EUS-guided metal insertion in this patient did not disturb the operation procedure.
| Study | No. of EUS-BD-metal biliary stents | Technical success % | Clinical success % | Type of metal stent | Procedure-related complications (No. cases) |
| Will et al[6] | 6 | 100 | 83.3 | Partially covered (3), uncovered (3) | Cholangitis (1) |
| Artifon et al[27] | 1 | 100 | 100 | Partially covered | None |
| Bories et al[3] | 11 | 91 | 100 | Partially covered metal stent (3) | Biloma (1), cholangitis (1) |
| Park et al[9] | 9 | 100 | 100 | Fully covered | Pneumoperitoneum (2) |
| Park et al[28] | 5 | 100 | 100 | Fully covered | None |
| Siddiqui et al[29] | 8 | 100 | 100 | Fully covered | Duodenal perforation (1) |
| This study | 13 | 92.3 | 91.7 | Fully covered | Mild peritonitis (2) |
A significant number of self-expandable metal stent (SEMS) and plastic stents placed to palliate malignant obstructions will be occluded. Repeat transpapillary stent placement may be unsuccessful for an occluded biliary metal or plastic stent after the placement of a hilar metal stent or combined duodenal and biliary metal stent placement. In such circumstances, PTBD may be uncomfortable for patients and has a 10%-30% complication rate, with complications such as cholangitis, bile leak, peritonitis, and stent occlusion[16]. EUS-HG with a FCSEMS may be recommended in such circumstances. In our 2 patients with a hilar metal stent, dilation of the left main duct caused by segmental tumor progression was shown to be aggravated. In another 2 patients with periampullary cancer drained by a pigtail plastic stent, this stent was occluded by tumor ingrowth and bile plug. Because these patients had jaundice and cholangitis, EUS-HG with a FCSEMS was performed. Some experts have recommended the intrahepatic approach because it seems to be safer[7,15]. In the intrahepatic approach, one-step placement of a partially covered wall stent may have been limited in earlier studies because there was 1 bile peritonitis and 1 cholangitis. However, we found the transduodenal or extrahepatic approach to be safer and more effective and it is probably less technically difficult compared with the intrahepatic approach. The advantage is that the duodenum is very close to the extrahepatic bile duct and the duodenal wall is thin without major vascular structures.
There were complications with EUS-BD, including stent migration, pneumoperito-neum, and cholangitis[17,18]. In this study, during follow-up (median, 3 mo), there was re-intervention (4/12, 33.3%) necessitated by stent migration (n = 3) and stent occlusion (n = 1). The 3 stent migrations occurred 2 d later (EUS-HG), 2 mo later (EUS-CD), and 2 mo later (EUS-CD). The stent migration rate (3/12, 25.0%) was higher than that reported (7%) in another paper[9]. Although a flare at both ends and minimal shortening of this newly designed nitinol stent may contribute to the prevention of stent migration[19], a slippery covered metal stent with low axial force may lead to migration. In addition, marked CBD dilatation may have affected FCSEMS floating resulting in the minimization of the anchoring effect of the proximal flared end of the FCSEMS.
Studies with uncovered SEMS reported stent occlusion rates ranging between 18% and 46%[20,21] with the main cause of obstruction being tumor ingrowth. Although partially or fully covered SEMS were designed to prevent this complication, these stents can not completely protect tumor ingrowth in the biliary metal stent[22]. Occasional authors have reported no tumor ingrowth with covered SEMSs. However, these series often include few patients and frequently have a relatively high prevalence of tumor overgrowth or sludge formation as a cause of stent failure[23-25]. Our study shows tumor overgrowth was observed in one case with distal CBD cancer.
During the EUS-BD procedure, the leakage of bile into the peritoneum with peritonitis can occur because the gap between the stent and the fistula is likely to occur. The shortening of metal stent can lead to failure of correct stent placement, and stent migration and dislocation after placement. Experience with partially coverd SEMS is limited and associated with a higher complication rates due to stent shortening[3,6]. To prevent bile leakage, we placed a FCSEMS that is made of nitinol. This stent, with both ends flared, was designed to prevent distal or proximal migration. The nitinol stent has less shortening compared with a partially covered Wall stent, and a fully covered metal stent may prevent bile leakage[19]. Therefore, due to the characteristics of this stent, it may prevent bile peritonitis. Because the diameter of the working channel of the linear array echoendoscope is only 3.7 mm, it is possible to quickly perform one-step placement of FCSEMS with an 8F-diameter delivery device. In this study, although bile leakage occurred during the procedure, all patients had only mild peritonitis and were treated with conservative management, such as antibiotics and nothing by mouth.
If the gap between the bile duct and the GI tract grows farther after EUS guided biliary drainage with stent, early migration of stent into the abdominal cavity may occur, particularly in the case of a transgastric approach. A fatal complication due to sepsis after development of large fistula related to stent migration into the abdominal cavity has recently been reported[26]. However, bile leakage via the relatively large fistula can make bile peritonitis and the bile duct collapse. Therefore, it may be difficult or impossible to carry out EUS-BD in these patients. In such a case, PTBD or emergency surgery must also be considered. To prevent severe complications, an endoscopist must have considerable knowledge about periduodenal and hepatobiliary anatomy in linear array EUS. Also, the procedure must be performed quickly and efficiently with appropriately sized stents. When selecting a stent, stent structure, axial force and shortening of stent should be considered.
In conclusion, EUS-guided transgastric or transduodenal biliary drainage with one-step placement of an FCSEMS may be a promising alternative method to PTBD. It may be a reasonable, feasible, and promising minimally invasive endoscopic approach in selected patients as indicated as above. However, several complications can occur, and special attention of the potential complications such as peritonitis and migration are needed. Therefore, additional experience and the development of new comfortable accessories for this procedure are needed. Also, a large case series and prospective trials are needed to further assess this technique.
For palliation of patients with malignant biliary obstruction, percutaneous biliary drainage (PTBD) is a classic method in some patients when endoscopic biliary drainage is unsuccessful because of failed biliary cannulation or tumor infiltration, which limits the endoscopic approach to the major papilla. Recently endoscopic ultrasonography-guided biliary drainage (EUS-BD) with plastic stent or uncovered self-expandable metal stent (SEMS) has been introduced as an alternative to PTBD in these cases of biliary obstruction.
The authors thought that a nitinol fully covered SEMS (FCSEMS) with a large bore diameter may have advantages over the plastic stent and other types of metal stent.
Technical and functional success with FCSEMS for palliation of patients with malignant biliary obstruction was respectively 92.3% (12/13) and 91.7% (11/12). Although there was early mild complication such as one case of immediate stent migration and 2 cases of bile peritonitis, all complications improved with conservative treatment.
EUS-guided transgastric or transduodenal biliary drainage with one-step placement of a nitinol FCSEMS may be a promising alternative method to PTBD. Additional experience and the development of new comfortable accessories for this procedure are needed. Also, a large case series and prospective trials are needed to further assess this technique.
There are 2 approaches for EUS-BD, the transgastric route and the transduodenal route. EUS-guided hepaticogastrostomy was performed through the transgastric route, and EUS-guided choledochoduodenostomy through the transduodenal route.
The authors reported high success rate with this challenging procedure. They clearly demonstrated that extrahepatic approach is easier and safer than intrahepatic approach, Although technical expertise is important, the authors demonstrated more than 90% technical success with low major complication rates. This paper motivates talented endoscopists to solve biliary obstruction via EUS guided technics.
Peer reviewer: Yucel Ustundag, Professor, Department of Gastroenterology, Zongudak Karaelmas University Hospital, Kozlu Zonguldak Turkey, 67600 Zonguldak, Turkey
S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Fogel EL, Sherman S, Devereaux BM, Lehman GA. Therapeutic biliary endoscopy. Endoscopy. 2001;33:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Yee AC, Ho CS. Complications of percutaneous biliary drainage: benign vs malignant diseases. AJR Am J Roentgenol. 1987;148:1207-1209. [PubMed] |
| 3. | Bories E, Pesenti C, Caillol F, Lopes C, Giovannini M. Transgastric endoscopic ultrasonography-guided biliary drainage: results of a pilot study. Endoscopy. 2007;39:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | Burmester E, Niehaus J, Leineweber T, Huetteroth T. EUS-cholangio-drainage of the bile duct: report of 4 cases. Gastrointest Endosc. 2003;57:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 196] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Giovannini M, Pesenti C, Rolland AL, Moutardier V, Delpero JR. Endoscopic ultrasound-guided drainage of pancreatic pseudocysts or pancreatic abscesses using a therapeutic echo endoscope. Endoscopy. 2001;33:473-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 179] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Will U, Thieme A, Fueldner F, Gerlach R, Wanzar I, Meyer F. Treatment of biliary obstruction in selected patients by endoscopic ultrasonography (EUS)-guided transluminal biliary drainage. Endoscopy. 2007;39:292-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Yamao K, Bhatia V, Mizuno N, Sawaki A, Ishikawa H, Tajika M, Hoki N, Shimizu Y, Ashida R, Fukami N. EUS-guided choledochoduodenostomy for palliative biliary drainage in patients with malignant biliary obstruction: results of long-term follow-up. Endoscopy. 2008;40:340-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Yamao K, Sawaki A, Takahashi K, Imaoka H, Ashida R, Mizuno N. EUS-guided choledochoduodenostomy for palliative biliary drainage in case of papillary obstruction: report of 2 cases. Gastrointest Endosc. 2006;64:663-667. [PubMed] |
| 9. | Park do H, Koo JE, Oh J, Lee YH, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with one-step placement of a fully covered metal stent for malignant biliary obstruction: a prospective feasibility study. Am J Gastroenterol. 2009;104:2168-2174. [PubMed] |
| 10. | Rogart JN, Boghos A, Rossi F, Al-Hashem H, Siddiqui UD, Jamidar P, Aslanian H. Analysis of endoscopic management of occluded metal biliary stents at a single tertiary care center. Gastrointest Endosc. 2008;68:676-682. [PubMed] |
| 11. | van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, van der Harst E, Kubben FJ, Gerritsen JJ, Greve JW, Gerhards MF, de Hingh IH. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129-137. [PubMed] |
| 12. | Itoi T, Itokawa F, Sofuni A, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Moriyasu F. Endoscopic ultrasound-guided choledochoduodenostomy in patients with failed endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2008;14:6078-6082. [PubMed] |
| 13. | Püspök A, Lomoschitz F, Dejaco C, Hejna M, Sautner T, Gangl A. Endoscopic ultrasound guided therapy of benign and malignant biliary obstruction: a case series. Am J Gastroenterol. 2005;100:1743-1747. [PubMed] |
| 14. | Tarantino I, Barresi L, Repici A, Traina M. EUS-guided biliary drainage: a case series. Endoscopy. 2008;40:336-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. Interventional EUS-guided cholangiography: evaluation of a technique in evolution. Gastrointest Endosc. 2006;64:52-59. [PubMed] |
| 16. | Pessa ME, Hawkins IF, Vogel SB. The treatment of acute cholangitis. Percutaneous transhepatic biliary drainage before definitive therapy. Ann Surg. 1987;205:389-392. [PubMed] |
| 17. | Savides TJ, Varadarajulu S, Palazzo L. EUS 2008 Working Group document: evaluation of EUS-guided hepaticogastrostomy. Gastrointest Endosc. 2009;69:S3-S7. [PubMed] |
| 18. | Itoi T, Yamao K. EUS 2008 Working Group document: evaluation of EUS-guided choledochoduodenostomy (with video). Gastrointest Endosc. 2009;69:S8-12. [PubMed] |
| 19. | May A, Ell C. A new self-expanding nitinol stent (JoStent SelfX) for palliation of malignant biliary obstruction: a pilot study. Endoscopy. 2004;36:329-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340:1488-1492. [PubMed] |
| 21. | Isayama H, Komatsu Y, Tsujino T, Sasahira N, Hirano K, Toda N, Nakai Y, Yamamoto N, Tada M, Yoshida H. A prospective randomised study of "covered" versus "uncovered" diamond stents for the management of distal malignant biliary obstruction. Gut. 2004;53:729-734. [PubMed] |
| 22. | Kullman E, Frozanpor F, Söderlund C, Linder S, Sandström P, Lindhoff-Larsson A, Toth E, Lindell G, Jonas E, Freedman J. Covered versus uncovered self-expandable nitinol stents in the palliative treatment of malignant distal biliary obstruction: results from a randomized, multicenter study. Gastrointest Endosc. 2010;72:915-923. [PubMed] |
| 23. | Kahaleh M, Tokar J, Conaway MR, Brock A, Le T, Adams RB, Yeaton P. Efficacy and complications of covered Wallstents in malignant distal biliary obstruction. Gastrointest Endosc. 2005;61:528-533. [PubMed] |
| 24. | Yang KY, Ryu JK, Seo JK, Woo SM, Park JK, Kim YT, Yoon YB. A comparison of the Niti-D biliary uncovered stent and the uncovered Wallstent in malignant biliary obstruction. Gastrointest Endosc. 2009;70:45-51. [PubMed] |
| 25. | Nakai Y, Isayama H, Komatsu Y, Tsujino T, Toda N, Sasahira N, Yamamoto N, Hirano K, Tada M, Yoshida H. Efficacy and safety of the covered Wallstent in patients with distal malignant biliary obstruction. Gastrointest Endosc. 2005;62:742-748. [PubMed] |
| 26. | Martins FP, Rossini LG, Ferrari AP. Migration of a covered metallic stent following endoscopic ultrasound-guided hepaticogastrostomy: fatal complication. Endoscopy. 2010;42 Suppl 2:E126-E127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Artifon EL, Chaves DM, Ishioka S, Souza TF, Matuguma SE, Sakai P. Echoguided hepatico-gastrostomy: a case report. Clinics (Sao Paulo). 2007;62:799-802. [PubMed] |
| 28. | Park do H, Song TJ, Eum J, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided hepaticogastrostomy with a fully covered metal stent as the biliary diversion technique for an occluded biliary metal stent after a failed ERCP (with videos). Gastrointest Endosc. 2010;71:413-419. [PubMed] |
| 29. | Siddiqui AA, Sreenarasimhaiah J, Lara LF, Harford W, Lee C, Eloubeidi MA. Endoscopic ultrasound-guided transduodenal placement of a fully covered metal stent for palliative biliary drainage in patients with malignant biliary obstruction. Surg Endosc. 2011;25:549-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |