INTRODUCTION
Cervical inlet patch (CIP) is characterized by the presence of heterotopic columnar gastric mucosa in the upper esophagus, most commonly located just below the upper esophageal sphincter (UES). Other sites for heterotopic gastric mucosa have been reported in the duodenum, jejunum, cystic duct, ampulla of vater, gallbladder, rectum and the anus[1-7], but their etiology and pathological significance remain unclear. The incidence of CIP has been reported from as low as 1%, to as much as 10% of endoscopic cases in different adult studies[8,9]. A large autopsy series of 1000 children demonstrated a prevalence of 4.5%[10]. During esophagogastroduodenoscopy (EGD), the region just below the UES is often quickly traversed after overcoming the initial resistance. CIP is usually best seen at the end of an EGD exam while withdrawing back through the esophagus and specifically looking for the condition. One study found almost a 6-8 fold increase in the incidence, from 0.3% to 2.3%, depending upon the endoscopist’s awareness of this entity and thoroughness of examination[11]. Although generally asymptomatic, CIP can present with dysphagia[12], stricture[13], ulcers[14], bleeding[15] or fistula[16]. It is unclear whether CIP is congenital or acquired. One postulate is that CIP originates from incomplete embryonic replacement of the stratified epithelium, which normally starts at the 4th month of gestation. The greater incidence of CIP seen in pediatric populations and in the upper esophageal pouch of children with tracheo-esophageal fistula supports this hypothesis[10,17,18]. Also, immunohistochemical studies suggest an embryologic origin for CIP on account of differences in endocrine markers such as serotonin, glucagon, pancreatic polypeptide, somatostatin and neurotensin in histologic specimens of CIP and Barrett’s esophagus (BE)[19].
A second postulate is that CIP, especially as noted in adults, is an acquired metaplastic change occurring in the squamous mucosa of the esophagus and is associated with predisposing factors for gastroesophageal reflux disease (GERD), such as sliding hiatal hernia[20]. Its incidence is up to four-fold higher in patients having BE[21] and CIP was found in almost a third of patients having dysplastic BE or adenocarcinoma[22]. Thus, long-standing acid reflux is thought to lead to columnar metaplasia in the upper esophagus, similar to BE. Several reports suggest that CIP may progress to adenocarcinoma[23-26].
In this study, we evaluate whether optical coherence tomography (OCT) can assess epithelial differences in CIP compared to normal esophagus, BE, normal stomach and duodenum. OCT is an emerging medical imaging technology that enables micron-scale, cross-sectional, and three-dimensional (3D) imaging of biological tissues in situ and in real-time[27,28]. OCT is similar to ultrasound B-mode imaging, except that echoes of light, instead of sound, are used to achieve micron-scale image resolutions. In vivo endoscopic OCT imaging was first demonstrated in rabbit gastrointestinal (GI) and respiratory tracts in 1997[29], and was quickly adopted by multiple groups for investigations in the human GI tract[30-36]. A prospective study involving 121 patients demonstrated 97% sensitivity and 92% specificity for diagnosing BE[33]. Our group has developed a portable, catheter-based prototype OCT system, where the OCT probe can be passed through the accessory channel of a standard endoscope, and achieves imaging speeds of up to 100 000 axial scans per second with axial resolutions of 5 μm to 7 μm in tissues[37]. Real-time cross-sectional OCT image display and 3D capture capabilities were demonstrated in animals[37], and humans[36,38,39]. 3D-OCT volumetric imaging enables the synthesis of en face views (similar to magnification endoscopy images), the generation of virtual cross-sectional images with arbitrary orientation, the average of multiple frames to reduce speckle and improve contrast, and quantitative measurements of tissue morphology. To our knowledge, this is the first description of in vivo CIP microstructure using OCT.
MATERIALS AND METHODS
Imaging protocol
This study was conducted at the Veterans Affairs Boston Healthcare System (VABHS), in compliance with an approved protocol by the institutional review boards at VABHS, Harvard Medical School and Massachusetts Institute of Technology. Five male Caucasian patients undergoing regular EGD at VABHS from August 2009 to April 2011 were enrolled in this study. This includes one patient with CIP, one representative patient with BE and three representative normal subjects. The representative patient with BE and normal subjects were selected from a large cohort of subjects who were imaged using OCT for another study. White light video endoscopy was performed using the Evis Extra III high definition system (Olympus America, Center Valley, PA), and endoscopic 3D-OCT images were obtained using the system described below.
Optical coherence tomography system
The 3D-OCT endomicroscopy system was developed in collaboration with LightLab Imaging - St Jude Medical, Inc. and is similar to the system previously described by our group[37]. A Fourier Domain Mode Locking swept laser with a center wavelength of 1310 nm and average output power of 42 mW at a sweep repetition rate of 59 kHz was used as the light source. The full-width-half-maximum bandwidth of the laser sweep was about 120 nm, which supports about 5 μm axial resolution in tissue. The system sensitivity was 103 dB with 13 mW of incident power. The imaging probe, with an outer diameter of 2.5 mm, was introduced through a standard working channel of a high-definition endoscope (Olympus GIF-Q180) to enable simultaneous video endoscopy and 3D-OCT imaging examination. The output beam from the probe was focused to a 15 μm spot and was emitted at an angle of about 80 °C from the probe axis by a prism. The internal optics in the probe was rotated rapidly for radial scanning at 60 (or 70) frames per second (fps). Each image frame had about 512 × 1000 pixels at 60 fps (or about 512 × 900 pixels at 70 fps). To acquire a spirally scanned, volumetric OCT data set of the GI tract, the probe was pulled back at 1.0 mm/s along the sheath, which corresponds to a frame-to-frame spacing of 14-17 μm. At this image acquisition speed, a 20 mm × 8 mm × 2 mm 3D-OCT data set was acquired in 20 s.
Individual 2D-OCT frames were displayed on screen for real-time preview. The volumetric data sets were acquired and streamed to a hard drive. During post-processing, each 2D radial frame was unwrapped to create a rectangular frame. A custom program was written to detect the surface of the plastic probe sheath in each frame, which is used to flatten the image. The flattened 3D-OCT data sets were then loaded into Amira (ResolveRT, Mercury Computer Systems) for 3D rendering and visualization in different orthogonal imaging planes.
Histology analysis
Standard hematoxylin and eosin (H and E) histology was performed by the pathology service at VABHS on biopsy or endoscopic mucosal resection (EMR) specimens in order to compare and validate the 3D-OCT data. Photomicrographs of the H and E slides were taken under a standard Olympus BX40 microscope using a 4× objective.
RESULTS
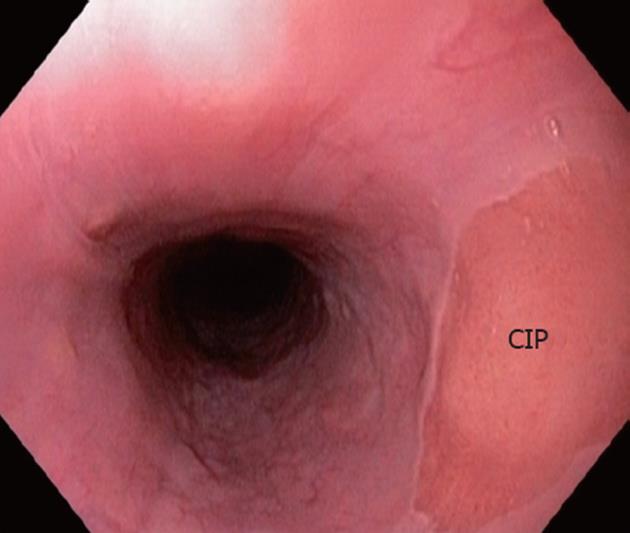
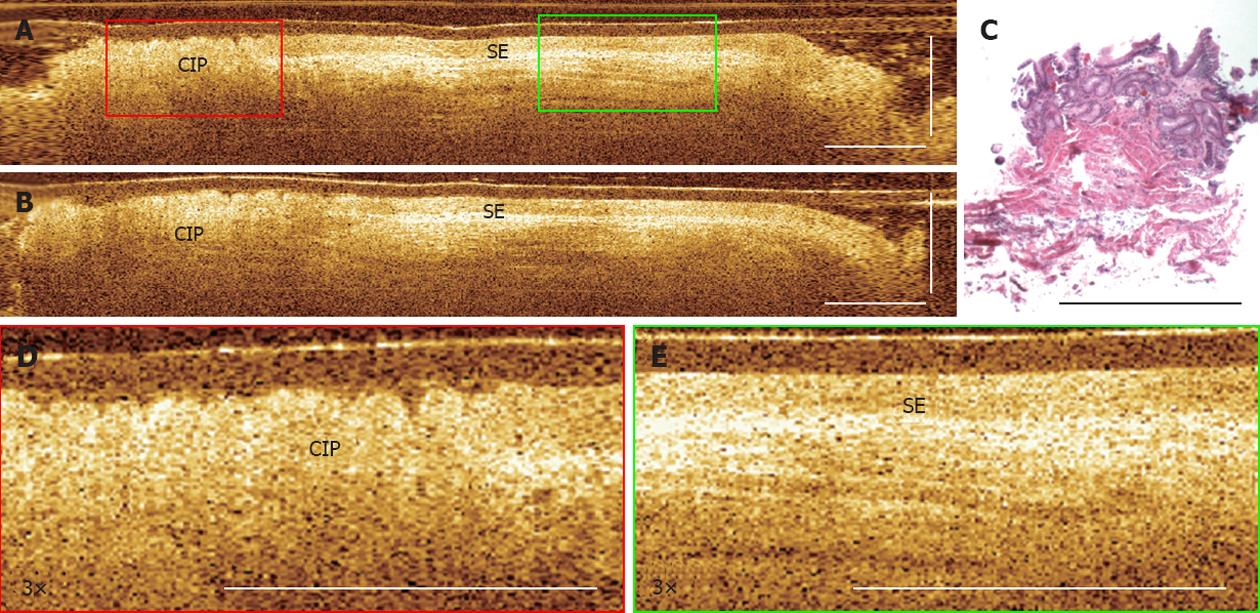
The endoscopic view of a CIP in a 68-year old patient referred for endoscopic treatment for long-segment BE is shown in Figure 1. During retraction of the endoscope, a pink circular lesion was observed under white light endoscopy in the upper esophagus (about 20 cm from the tooth). The histology of the biopsies taken from the lesion later confirmed the finding of CIP (Figure 2C). Endoscopic OCT imaging was performed over the CIP under direct simultaneous visualization with a white light endoscope. From cross-sectional OCT images shown in Figure 2A and B, regions with CIP and the adjacent squamous epithelium can be identified. In addition, the CIP region clearly shows shallower light penetration compared with the adjacent normal esophagus. This is similar to typical images from normal gastric mucosa of representative other subjects. Zoomed views shown in Figure 2D and E clearly demonstrate columnar and squamous epithelium in the CIP and the adjacent normal esophagus, respectively. The columnar features observed in the CIP are consistent with the corresponding H and E histology shown in Figure 2C.
Figure 1 Endoscopic view of cervical inlet patch.
Figure 2 Endoscopic optical coherence tomography imaging of cervical inlet patch.
A: Cross-sectional optical coherence tomography images of cervical inlet patch (CIP); B: Adjacent squamous epithelium, respectively; C: Corresponding hematoxylin and eosin histology obtained from a biopsy at the CIP site; D: 3× magnification of the CIP; E: Squamous epithelium (SE) region marked in (A). Scale bars: 1 mm.
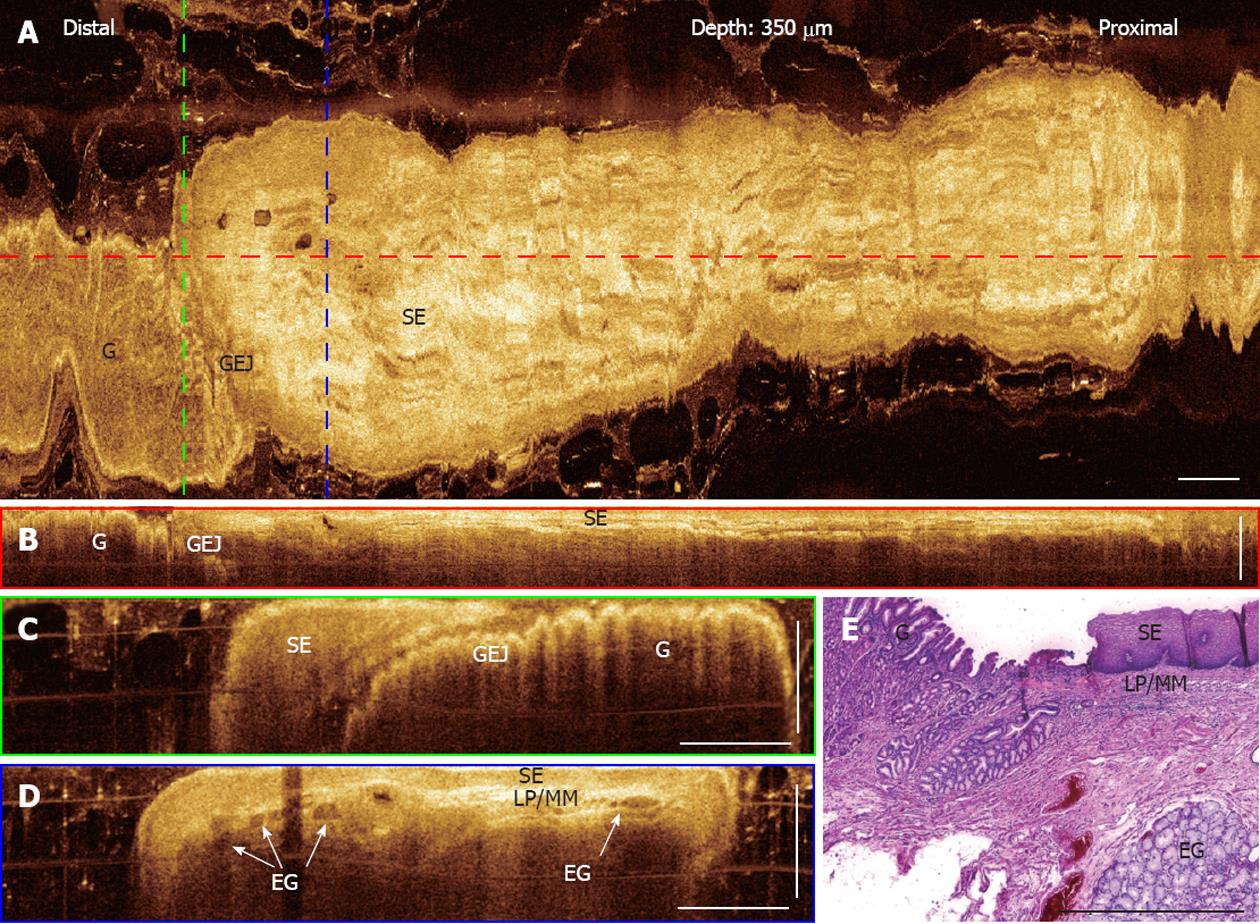
For comparison, OCT images of a normal gastro-esophageal junction (GEJ) obtained from a representative patient with chronic heartburn symptom (Figure 3). The en face OCT projection image at 350 μm underneath the tissue surface clearly shows the GEJ. The OCT imaging probe scans a large field (20 mm × 8 mm) on the tissue, which is about 100× larger compared to the region sampled by a standard biopsy (1-2 mm2). Regions with gastric glandular mucosa (left) and esophageal squamous mucosa (right) exhibit clearly different patterns. Cross-sectional OCT images in Figure 3B-D show the GEJ and esophageal squamous epithelium along the probe pullback and rotation directions, respectively. The GEJ, squamous epithelium, lamina propria/muscularis mucosa, and esophageal glands underneath the squamous epithelium are clearly observed. Features observed in OCT images also match the representative histology of a normal GEJ shown in Figure 3E.
Figure 3 Three-dimensional-optical coherence tomography images of a normal gastro-esophageal junction.
A: En face projection optical coherence tomography (OCT) image at a depth of 350 μm; B: Regions with gastric mucosa and squamous mucosa show distinct features; Cross-sectional OCT image along the probe pullback direction showing the gastro-esophageal junction (GEJ) and normal squamous epithelium (SE) clearly; C, D: Cross-sectional images of the GEJ and SE, corresponding to the green and blue dashed lines marked in (A), respectively. Structures, such as SE, lamina propria (LP)/muscularis mucosa (MM), esophageal glands (arrows) (EG), and gastric mucosa, can be clearly identified; E: Representative histology at the GEJ. Scale bars: 1 mm.
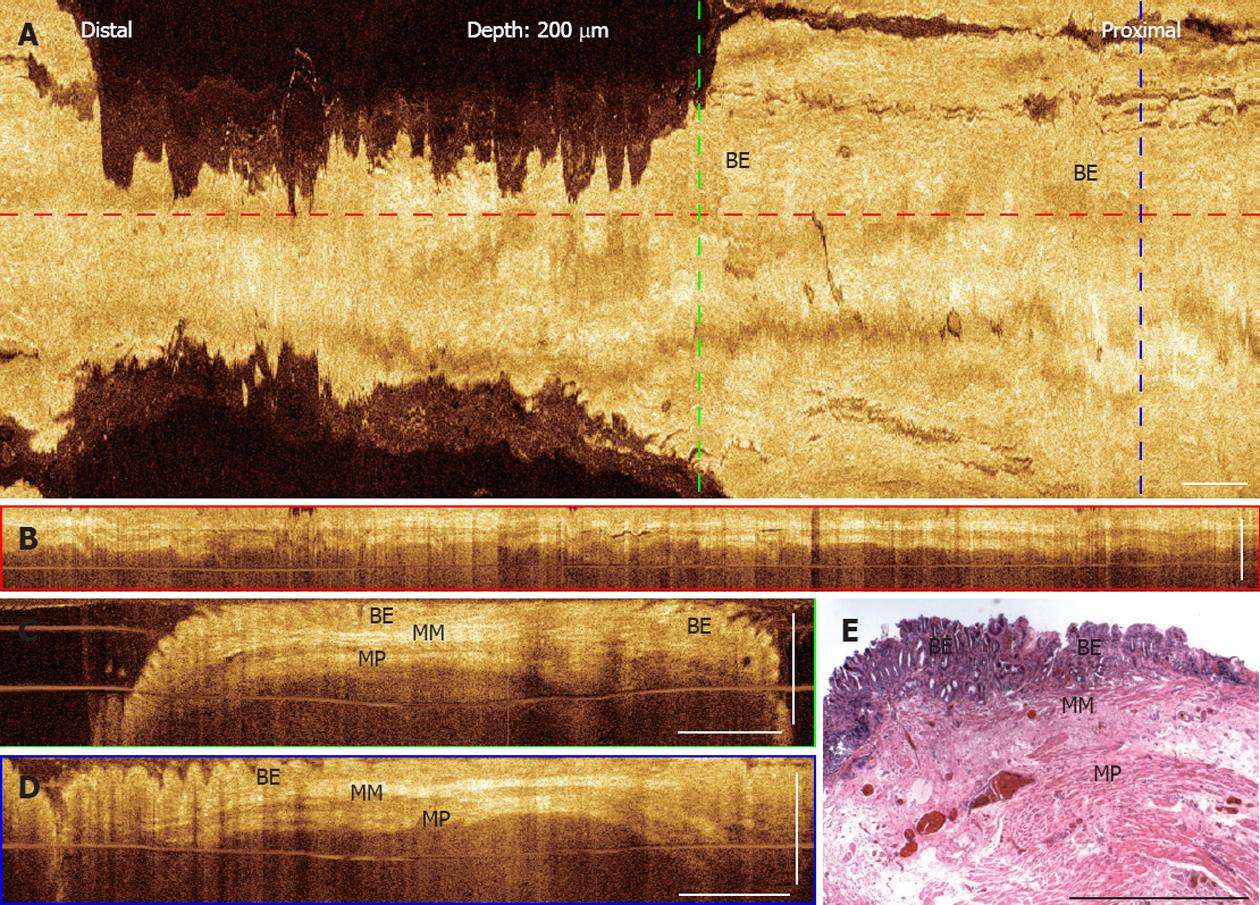
3D-OCT images from a representative patient with a long segment BE confirmed with histology (Figure 4). The en face projection OCT image at 200 μm underneath the tissue surface shows a similar angulated pattern compared with the en face image shown in the gastric mucosa (Figure 4A). Cross-sectional OCT images (Figure 4B and D) clearly show layered structures, where the original squamous mucosa in the esophagus is replaced by the columnar BE mucosa. Two hyper-scattering layers are observed underneath the BE mucosa, where the top layer corresponds to the newly formed muscularis mucosa layer which replaces the lamina propria, and the bottom layer corresponds to the muscularis propria. These OCT features are confirmed with corresponding histology of an EMR specimen obtained at the imaging area in the same patient.
Figure 4 Three-dimensional-optical coherence tomography images of a long segment Barrett's esophagus.
A: En face projection optical coherence tomography (OCT) image at a depth of 200 μm; B: Cross-sectional OCT images of the long segment Barrett's esophagus (BE) along the probe pullback direction; C, D: Cross-sectional OCT images, corresponding to the green and blue dashed lines marked in (A). BE glands, the muscularis mucosa (MM), and the muscularis propria (MP) layers are clearly seen; D: Histology of an endoscopic mucosal resection specimen obtained from the same subject shows corresponding features observed in the OCT images. Scale bars: 1 mm.
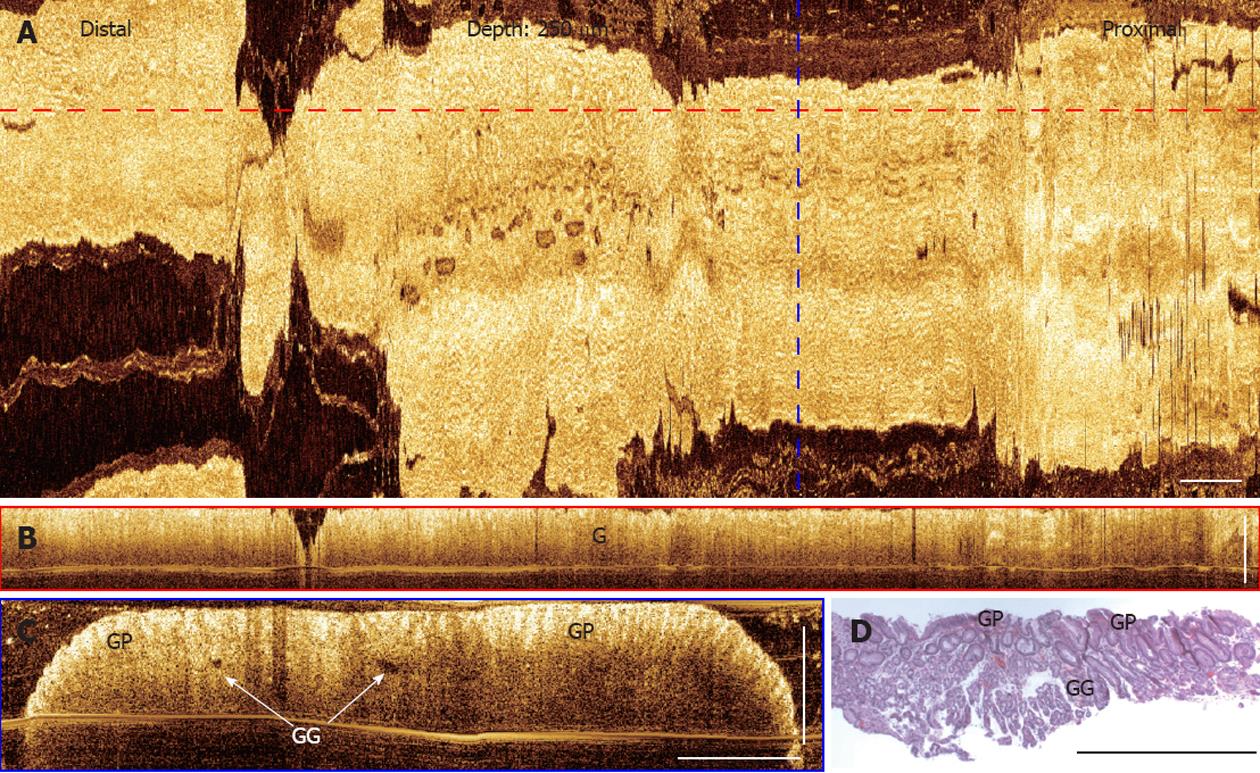
Representative OCT images of normal stomach from a patient with chronic heart burn symptom (Figure 5). The en face projection image (Figure 5A) at a depth of 250 μm under the tissue surface represents the typical angulated gastric glandular mucosa pattern. Cross-sectional OCT images (Figure 5B and C) clearly show the gastric glandular mucosa. Gastric pits and gastric glands can be observed from cross-sectional OCT images and the image features match the representative histology of gastric mucosa shown in Figure 5D. Light penetration in normal gastric tissues is also shallower compared with normal esophagus and BE.
Figure 5 Three-dimensional-optical coherence tomography images of a normal stomach.
A: En face projection optical coherence tomography (OCT) image at a depth of 250 μm; B: Cross-sectional OCT image along the probe pullback direction, corresponding to the red dashed line marked in (A); C: Cross-sectional images of the gastric mucosa, corresponding to the blue dashed line marked in (A). Gastric pits (GP) and gastric glands (arrows) (GG) can be identified; D: Representative histology of a gastric mucosa. Scale bars: 1 mm.
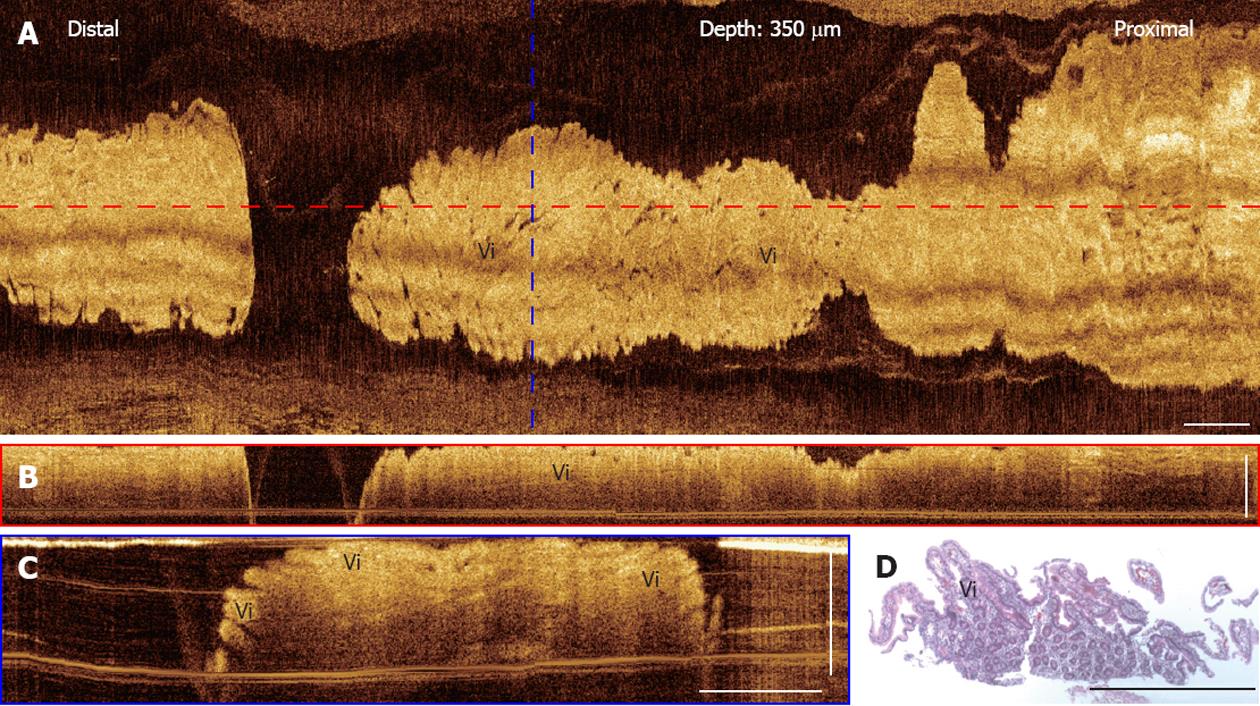
Furthermore, 3D-OCT images of normal duodenum from a patient with chronic heart burn symptom are shown in Figure 6. Distinctive features of the intestinal mucosal villi are observed in the en face OCT projection image (Figure 6A), as well as in the cross-sectional OCT images (Figure 6B and C). The length of individual villi, measured to be around 300-600 μm, matches the corresponding histology shown in Figure 6E. These results demonstrate the feasibility of using OCT to evaluate GI tissue morphology in situ and in real-time.
Figure 6 Three-dimensional-optical coherence tomography images of a normal duodenum.
A: En face projection optical coherence tomography (OCT) image at a depth of 350 μm; B: Cross-sectional OCT images of the duodenum along the probe pullback direction; C: Cross-sectional OCT image, corresponding to the blue dashed line marked in (A); Mucosal villous structures (Vi) in the duodenum are clearly seen; D: Corresponding histology of the duodenum showing the villi. Scale bars: 1 mm.
DISCUSSION
CIP is an under-appreciated entity in general gastroenterologist’s practice. In this study, we present imaging results from OCT, a relatively new imaging technology, to describe the gastric type of epithelial patterns in CIP, as clearly distinct from normal esophageal squamous epithelium, BE, or from normal duodenum. Under OCT, CIP exhibits similar columnar structures compared with normal gastric mucosa, and the imaging depth in both CIP and gastric tissues are low. In practice, obtaining biopsies from CIP in patients with troublesome supra-esophageal or laryngeal symptoms may be difficult owing to poor view just below the UES. OCT may allow “optical biopsy” of the CIP epithelium without the need for obtaining tissue specimens, and may be used to assess changes suspicious for malignancy in the future. Given its small diameter (2.5 mm) and flexibility, the OCT probes may be introduced orally or nasally without an endoscope, and with better tolerance and potentially less motion artifacts. This may further negate the need for sedation, nursing, or use of the endoscopy unit which has implications beyond endoscopy costs.
There are a number of case reports of adenocarcinoma arising from heterotopic gastric mucosa in the upper esophagus[23-26,40]. To our knowledge, 31 cases have been reported in the literature where esophageal adenocarcinoma was found arising from an inlet patch[41-46] and two cases where laryngeal squamous cell carcinoma was found associated with or bordering inlet patches[47]. The pathogenetic link between BE and CIP raises concerns of dysplastic transformation in CIP. Using immunohistochemical markers to address potential cellular origins, Lauwers et al[48] demonstrated similar mechanisms of pathogenesis for CIP and BE on the basis of similarity between immunohistochemical staining patterns between the two entities. Furthermore, the similarity in the expression pattern for cytokeratins 7 and 20 and MUC6 mucin protein were not influenced by the presence or absence of GERD in these CIP patients[48-50]. Based on these findings, Lauwers et al[48] suggested that CIP may arise as a metaplastic change occurring in the esophageal epithelium. In light of these findings and the pathogenetic similarity between CIP and BE based on the cytokeratin expression study by Lauwers, the suspicion that the CIP, at least in adults, arises from local stem cells within the esophagus at or near this “heterotopic” patch is strong. However, unlike BE, a consensus guideline for surveillance of CIP has not been established on account of its relatively low incidence and lack of information on its natural history for dysplastic changes. In this study, we present an alternative approach to evaluate CIP based on OCT imaging. Recently, balloon based OCT probes have been developed in order to allow imaging over the entire esophageal lumen for screening purposes[35,51-53]. The balloon design also helps stabilize the imaging probe to minimize motion artifacts. The advantages of OCT, such as real-time imaging, large area of coverage, and depth resolved imaging, etc., suggest that it may be a useful tool for detection of various GI diseases, including CIP and Barrett’s esophagus.
In addition, OCT enables visualization of the deeper esophageal glands underneath squamous mucosa, which may not be accessible with standard or jumbo biopsy forceps. As suggested by Lauwers et al[48] CIP may arise from submucosal esophageal mucous glands. If these glands are the origin of dysplasia or malignant transformation, 3D-OCT may be uniquely suited for identifying these early dysplastic changes and following them up. Recently, novel methods have been developed to perform OCT imaging with contrast agents, such as gold nanoparticles[54-57], and therefore enable molecular targeted imaging for early cancer detection. In the future, OCT may also be combined with biomarkers, e.g., the superficial expression of Lgr5 in BE. Localization with depth resolution of such markers may help with directed biopsies or targeted ablation.
Presently, there are no commercially available OCT systems for endoscopic applications. The OCT probe used in this study passes through the working channel of a standard white light endoscope, and the entire OCT system is portable and could fit in a standard endoscopy suite to provide complementary real-time information on tissue microstructures during endoscopy.
One limitation of the current study is the small sample size. Multiple normal subjects and patients with BE were imaged and representative results were shown from a large cohort, but only one subject with CIP was available. The objective of this pilot study is to demonstrate the feasibility of in situ imaging of CIP using 3D-OCT and identify characteristic imaging features of CIP compared to other organs in human upper GI tract. One set of representative OCT images from each organ was demonstrated. However, it is not possible to reach any statistical conclusion from this feasibility study.
In conclusion, we demonstrate in situ evaluation of CIP microstructure using endoscopic 3D-OCT. OCT imaging visualized columnar epithelial structures within the CIP region, which clearly contrasted with the squamous epithelium from adjacent normal esophagus, gastric mucosa in the stomach and villous structure in the duodenum. The microstructural features observed with OCT also matched those from H and E histological sections. These results demonstrated the feasibility of using OCT to evaluate GI tissue morphology in situ and in real-time. Since OCT imaging can be performed with small diameter probes introduced orally or nasally, this emerging technology might be used to screen patients with troublesome upper esophageal symptoms for CIP, BE and other changes in the epithelium, even without endoscopy or the need for conscious sedation.
ACKNOWLEDGMENTS
The authors acknowledge the facility support from VA Boston Healthcare System and administrative assistance of Marisa Figueiredo.
COMMENTS
Background
Cervical inlet patch (CIP) is an under-appreciated entity encountered by gastroenterologists in general practice. Although rare, several reports suggest that CIP may progress to adenocarcinoma. Optical coherence tomography (OCT) is an emerging medical imaging technology that enables micron-scale, cross-sectional, and 3D imaging of biological tissues in situ and in real-time.
Research frontiers
This study evaluates whether OCT can optically assess epithelial differences in upper human gastrointestinal (GI) tract in situ.
Innovations and breakthroughs
OCT imaging over the CIP region showed columnar epithelium structure, which clearly contrasted the squamous epithelium structure from adjacent normal esophagus. OCT images obtained from other patients demonstrated distinctive patterns of the normal esophagus, Barrett’s esophagus, normal stomach, and normal duodenum. Microstructures, such as squamous epithelium, lamina propria, muscularis mucosa, muscularis propria, esophageal glands, Barrett’s glands, gastric mucosa, gastric glands, and intestinal mucosal villi were clearly observed with OCT and matched with hematoxylin and eosin histology. OCT may allow real-time “optical biopsy” of the CIP epithelium without the need for obtaining tissue specimens, and may be used to assess suspicious changes of malignancy in the future.
Applications
Given its small diameter and flexibility, the OCT probe may be introduced orally or nasally without an endoscope or need for moderate sedation, and with better tolerance and potentially less motion artifacts compared to endoscopy. In addition, the OCT imaging probe scans an approximately 100× larger field compared to biopsy, and therefore, might be useful in the future to screen for CIP.
Peer review
The paper is a very interesting piece of work exploring the utility of OCT primarily in characterizing cervical inlet patch but also in other esophageal disorders. It provides a reader with the future directions of and technological advances in upper GI endoscopy in an era when a number of non-white light endoscopic contrast techniques are competing for prime time use to more accurately delineate and diagnose pathology.
Peer reviewers: Kevin M Reavis, The Oregon Clinic, 1040 NW 22nd Ave, Suite 560, Portland, OR 97210, United States; Jeff Butterworth, Shrewsbury and Telford Hospitals NHS Trust, 102 The Mount, Shrewsbury SY3 8PH, United Kingdom; John B Marshall, MD, Professor, Division of Gastroenterology, University of Missouri School of Medicine, One Hospital Drive, Columbia, MO 65212, United States
S- Editor Gou SX L- Editor O’Neill M E- Editor Li JY