Published online May 28, 2012. doi: 10.3748/wjg.v18.i20.2493
Revised: December 2, 2011
Accepted: April 28, 2012
Published online: May 28, 2012
AIM: To estimate the cost-benefit of endoscopic screening strategies of esophageal cancer (EC) in high-risk areas of China.
METHODS: Markov model-based analyses were conducted to compare the net present values (NPVs) and the benefit-cost ratios (BCRs) of 12 EC endoscopic screening strategies. Strategies varied according to the targeted screening age, screening frequencies, and follow-up intervals. Model parameters were collected from population-based studies in China, published literatures, and surveillance data.
RESULTS: Compared with non-screening outcomes, all strategies with hypothetical 100 000 subjects saved life years. Among five dominant strategies determined by the incremental cost-effectiveness analysis, screening once at age 50 years incurred the lowest NPV (international dollar-I$55 million) and BCR (2.52). Screening six times between 40-70 years at a 5-year interval [i.e., six times(40)f-strategy] yielded the highest NPV (I$99 million) and BCR (3.06). Compared with six times(40)f-strategy, screening thrice between 40-70 years at a 10-year interval resulted in relatively lower NPV, but the same BCR.
CONCLUSION: EC endoscopic screening is cost-beneficial in high-risk areas of China. Policy-makers should consider the cost-benefit, population acceptance, and local economic status when choosing suitable screening strategies.
- Citation: Yang J, Wei WQ, Niu J, Liu ZC, Yang CX, Qiao YL. Cost-benefit analysis of esophageal cancer endoscopic screening in high-risk areas of China. World J Gastroenterol 2012; 18(20): 2493-2501
- URL: https://www.wjgnet.com/1007-9327/full/v18/i20/2493.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i20.2493
Esophageal cancer (EC) is the eighth most common cancer and the sixth most common cause of cancer death worldwide[1]. Although the mortality of EC has sharply reduced over the last three decades, EC remains the fourth leading cause of cancer death in China with a mortality of 15.21/100 000[2]. According to the “Third National Retrospective Sampling Survey of Death Causes Report in 2004-2005 of China”, EC continues to be the major public health burden in some high-risk areas, where the mortality of EC was three times higher than the average of the country. EC is a fatal disease, with a 5-year survival rate of less than 20% even in developed countries[3,4].
To explore suitable control measures in high-risk areas of China, a great number of EC screening studies using endoscopic examinations (i.e., endoscopy with mucosal iodine staining and index biopsy as a screening technology, combined with pathological examination for confirming and staging the disease) have been conducted for several decades[5-10]. Through early detection and subsequent treatment, the 5-year survival rate of EC increased to 86%[10]. Furthermore, obvious reductions in incidence and mortality rates of EC were observed under endoscopic screening[11].
A national screening program for EC in high-risk areas has become available in 73 sites of 27 provinces of China based on evidence from previous studies. Nevertheless, due to lacking comprehensive health economic evaluations on such programs, two key public health questions remain to be answered: is the endoscopic screening cost-beneficial in the long run? Should we use the same screening strategy in both developed and developing high-risk areas of China?
The objective of this paper is to explore appropriate screening strategies for EC in high-risk areas of China from the health economic perspective by comparing the long-term cost-benefits of 12 endoscopic screening alternatives. It will provide valuable data for policy makers to make decisions on the current screening program.
A Markov model was constructed to evaluate the cost-benefit of different screening strategies for EC. In each strategy simulation, a hypothetical cohort with 100 000 participants entered the model at age 40 years and were followed up until the age of 70 years. Costs and benefits were all discounted at an annual rate of 3%[12]. TreeAge Pro 2009 Suite by TreeAge Software Inc. was used for all analysis.
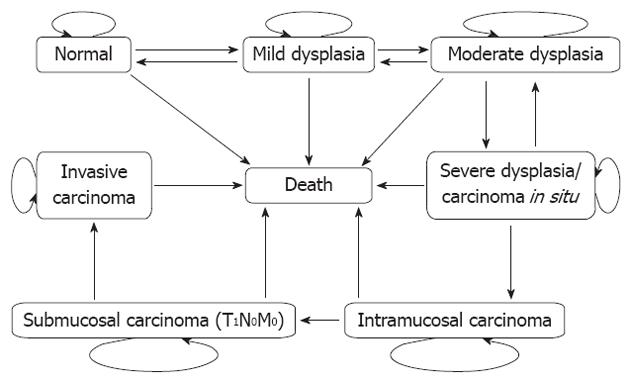
The natural history of EC was categorized as the following health status: normal, mild dysplasia (mD), moderate dysplasia (MD), severe dysplasia/carcinoma in situ (SD/CIS), intramucosal carcinoma (IC), submucosal carcinoma (T1N0M0) (SC), invasive carcinoma (INC), and death. Figure 1 depicts the detailed transition processes of EC in the Markov model. Each rectangle represents a health state. During a Markov cycle (1 year), one could transit from his/her current health state to another (indicated by arrows between different states) or remain in the same state (indicated by half-circle arrows on the rectangles). Prior to the development of IC, the condition could spontaneously regress. Once IC developed, no regression could occur. We used the model to evaluate all screening alternatives and non-screening outcomes.
In the context of lacking guidelines for EC screening worldwide, we explored 12 screening strategies using endoscopic examinations. These strategies were performed at varying starting age for screening (40, 45 or 50 years), screening frequency (once, twice, thrice, or six times in the lifetime), and intervals of follow-up for mD and MD cases (e.g., 5 or 3 years). The strategies were listed as “t(y)nf/t(y)f”, where t denotes the screening frequency, y represents the starting age of screening, nf means we do not follow up the mD and MD cases diagnosed by screening, and f means the mD and MD cases are followed up every 5 years and 3 years, respectively. For twice and thrice screening strategies in the lifetime, the screening intervals were 10 years; for six times screening strategy, the screening intervals were 5 years.
Screening, diagnosis, and treatment procedures for the strategies were all based on the current practice manuals. The participants were screened using endoscopy with iodine staining. If endoscopy revealed a suspected lesion (mD or worse), index biopsy combined with pathological examination were performed consecutively. The detailed procedures of endoscopy were the same with those in the literature[13]. For SD/CIS and IC cases detected by screening, Endoscopic Mucosal Resection and/or Argon Plasma Coagulation served as the standard treatment. For detected patients with SC or worse, therapies included esophagectomy, radiotherapy, and other routine treatments. Subjects who were not screened would be diagnosed and treated if they presented with symptomatic EC. All patients were followed up once by endoscopy in the first year after treatment.
The data used in the model were compiled from a variety of sources: (1) the results of our prospective cohort study based on the EC chemoprevention trial of selenomethionine and celecoxib in “early detection of EC” (EDEC) program; (2) the results of our population-based screening project “Early Detection and Early Treatment of EC in Demonstration Centers in China” (EDETEC); (3) surveillance data; (4) published literatures; and (5) unpublished data.
In the chemoprevention trial of EDEC program, 2213 asymptomatic adults from Linzhou County, Henan Province of China, underwent an endoscopic screening in 1999[14]. Among them, 2189 participants who had histological diagnoses at the baseline evaluations were surveilled until 2007. The primary end-point was the occurrence of EC, confirmed by village doctors through checking the histological diagnoses in medical records. The project EDETEC covering 11 high-risk areas of EC was launched by the Chinese central government in 2005. The purpose was to increase the early detection and treatment rate as well as the 5-year survival rate of EC, and to improve the screening, early detection and treatment program and so forth[15].
At the initial model cycle, a hypothetical cohort of 100 000 participants was distributed among various health states based on the proportion of each pathologic stage of EC in the 40-44 years age group. The proportions were calculated in terms of the screening results of Linzhou County in the project EDETEC between 2005 and 2008. Among the 8267 asymptomatic participants aged 40-69 years, 8.2% cases were identified as mD in the 40-44 years age group. Full details are presented in Tables 1 and 2.
| Parameters | Value | Parameters | Value | Parameters | Value1 |
| Initial probability2 | Transition probability-continued | Compliance of screening2 | 67% (30%-100%) | ||
| Normal | 0.8895 | SD/CIS | see Table 2 | Sensitivity of endoscopy[6] | 96% (92%-99%) |
| mD | 0.0820 | IC | see Table 2 | Specificity of endoscopy[6] | 90% (59%-100%) |
| MD | 0.0180 | INC | Screening cost (I$ per capita)2 | ||
| SD/CIS | 0.0090 | Recovering to post-INC | 0.7696 | Direct cost | 61.50 (37.00-119.00) |
| IC | 0.0008 | Relapsing to INC after treatment | 0.2304 | Indirect cost | 8.31 (8.09-8.53) |
| SC | 0.0005 | After treatment4 | Treatment cost (I$ per capita)[24]2 | ||
| INC | 0.0002 | Post-SD/CIS | Direct cost | ||
| Transition probability[9,16-21]2,3 | Recovering to normal | 0.9950 | SD/CIS | 1292 (1114-1565) | |
| Normal | Relapsing to SD/CIS | 0.0050 | IC | 1292 (1114-1565) | |
| Remaining normal | 0.9760 | Post-IC | SC | 1818 (1519-2799) | |
| Progression to mD | 0.0240 | Remaining post-IC | 0.9450 | INC-screening group | 2767 (2332-4031) |
| mD | Relapsing to IC | 0.0500 | INC-control group | 4888 (4333-6396) | |
| Regression to normal | 0.0500 | Relapsing and progression to SC | 0.0050 | Indirect cost | |
| Remaining mD | 0.9000 | Post-SC | SD/CIS | 1654 (1341-1968) | |
| Progression to MD | 0.0500 | Remaining post-SC | 0.8500 | IC | 1654 (1341-1968) |
| MD | Relapsing to SC | 0.0500 | SC | 3369 (2872-3866) | |
| Regression to mD | 0.0800 | Relapsing and progression to INC | 0.1000 | INC-screening group | 3369 (2872-3866) |
| Remaining MD | 0.8000 | Post-INC (Same with “INC”) | INC-control group | 5526 (4584-6466) | |
| Progression to SD/CIS | 0.1200 | Death probability | see Table 2 | Discount rate[12]5 | 3% (0%-6%) |
| Parameters | Value | |||||
| 40-yr | 45-yr | 50-yr | 55-yr | 60-yr | 65-69-yr | |
| Transition probability | ||||||
| SD/CIS | ||||||
| Regression to MD | 0.17 | 0.15 | 0.14 | 0.12 | 0.11 | 0.09 |
| Remaining SD/CIS | 0.75 | 0.75 | 0.74 | 0.74 | 0.73 | 0.73 |
| Progression to IC | 0.08 | 0.10 | 0.12 | 0.14 | 0.16 | 0.18 |
| IC | ||||||
| Remaining IC | 0.60 | 0.50 | 0.40 | 0.20 | 0.15 | 0.13 |
| Progression to SC | 0.40 | 0.50 | 0.60 | 0.80 | 0.85 | 0.87 |
| SC | ||||||
| Remaining SC | 0.80 | 0.70 | 0.55 | 0.20 | 0.17 | 0.15 |
| Progression to INC | 0.20 | 0.30 | 0.45 | 0.80 | 0.83 | 0.85 |
| Death probability | ||||||
| Non-esophageal-cancer mortality | 0.002270 | 0.003073 | 0.007054 | 0.017061 | 0.019744 | 0.024105 |
| All-cause mortality | 0.002438 | 0.003383 | 0.007967 | 0.019559 | 0.021985 | 0.027370 |
| Case fatality rate of esophageal cancer | 0.581700 | 0.581700 | 0.581700 | 0.581700 | 0.581700 | 0.581700 |
In each cycle of a Markov process, the transitions among health states occurred with annual transition probabilities. They were estimated in terms of: (1) published literatures[9,16-21]; and (2) the results of both EDEC and EDETEC projects in Linzhou County (Tables 1 and 2).
It is believed that persons with SD/CIS or lesser abnormality may not die from EC and that patients with IC or SC may die from all causes including EC. In patients with INC, we assumed that they may mainly die from EC. Therefore, in our model, the corresponding death probabilities for three different populations above were converted from non-esophageal-cancer mortality, all-cause mortality, and case fatality rate of EC, respectively. All age-specific mortality rates were obtained from the death registry reports of Linzhou County between 2004 and 2006. And they were converted to probabilities by the formula: Probability = 1 - Exp (-rt), where “r” represents the rate and ‘t’ denotes the time (Tables 1 and 2).
The compliance of EC screening in different settings varied from 33.4% to 77.1%[22]. In the EDETEC program, the screening compliance of EC in Linzhou County during 2005-2008 was 67% (8267/12 294), which was used as a baseline in this analysis (Table 1).
In our model, both screening and treatment costs included direct and indirect costs, which were calculated from a societal perspective. Direct costs referred to those associated with drugs, disposable supplies, equipment and facilities, staff, etc. In this study, we used costs rather than charges. And they were collected using Micro-costing methods in the EDETEC program[23]. Indirect cost was also estimated from our EDETEC program, including those related to transportation, accommodation, and the productivity losses of both patients and their caregivers[24,25]. Considering differences in purchasing power, costs were presented in 2008 international dollars (I$).
Screening cost per capita using endoscopic examination was I$69.81. In screening group, the treatment costs for patients with SD/CIS or worse ranged from I$2964 to I$6136. In control group, the treatment cost for INC cases was I$10 414, much higher than that in screening group (Table 1).
According to a previous study in Linzhou County, the sensitivity and specificity of endoscopic examination were 96% and 90%, respectively[6] (Table 1). For individuals diagnosed as having precancerous lesions or EC (i.e., SD/CIS or worse), we assumed that they would complete the entire treatment procedures.
The basic outcomes of the model were total costs (including screening costs and treatment costs) and expected life years. Then the net present values (NPVs) and the benefit-cost ratios (BCRs) were calculated under each of the strategies (for a hypothetical cohort of 100 000 subjects followed up from 40 years to 70 years of age).
For each screening cohort, the benefit consisted of the treatment cost averted and productivity gains from screening programs[25], and counted by the formula: benefit = GDP per capita of Linzhou in 2008 (I$6542) × (life years of screening cohort - life years of “non-screening” group) + treatment cost of “non-screening” group. The NPV was the benefit minus the total cost of the screening group; the BCR equaled to the benefit divided by the total cost. The strategies with a NPV > 0 and a BCR > 1 were considered cost-beneficial.
In addition, the screening alternatives were compared using an incremental cost-effectiveness analysis. The strategies that were more expensive and gained fewer life years (dominated), or less costly and less cost-effective (extended dominated) than an alternative were excluded.
Given the uncertainty about some parameters, univariate sensitivity analyses were used to assess the robustness of the model results by varying the values of screening compliance, discount rate, screening cost, treatment cost, sensitivity and specificity of endoscopy within reasonable ranges (Table 1).
Based on the established natural-history model, the validity of the Markov model was assessed by comparing the model-predicted age-specific incidence and the age-specific proportion of each stage of EC with the observed data in real-world conditions.
Compared with non-screening outcomes, the screening strategies could save life years of 2539-15 384 for a hypothetical population of 100 000, with NPVs of I$24 million-I$99 million and BCRs of 1.61-3.06. Strategies with higher screening frequencies were more cost-beneficial than those with lower screening frequencies (Table 3).
| Screening strategies t(y)nf/ t(y)f1 | Life years | Life years saved (LYS) | Costs (I$) | ICER (I$/LYS) | Benefit (I$) | NPV (I$ ) | BCR |
| Non-screening | 1 811 125 | - | 46 354 958 | D | - | - | - |
| Once(40)nf | 1 813 664 | 2539 | 39 133 890 | D | 62 964 854 | 23 830 964 | 1.61 |
| Once(45)nf | 1 814 180 | 3055 | 38 213 022 | D | 66 340 477 | 28 127 455 | 1.74 |
| Once(50)nf | 1 814 634 | 3509 | 36 989 316 | D | 69 310 502 | 32 321 186 | 1.87 |
| Once(40)f | 1 817 922 | 6797 | 38 007 700 | D | 90 820 285 | 52 812 585 | 2.39 |
| Once(45)f | 1 818 783 | 7658 | 36 792 906 | ED | 96 452 865 | 59 659 959 | 2.62 |
| Once(50)f | 1 817 966 | 6841 | 36 117 125 | / | 91 108 128 | 54 991 003 | 2.52 |
| Twice(40)f | 1 822 516 | 11 391 | 39 532 080 | 940 | 120 873 795 | 81 341 715 | 3.06 |
| Twice(45)f | 1 821 595 | 10 470 | 38 665 956 | 702 | 114 848 701 | 76 182 745 | 2.97 |
| Twice(50)f | 1 819 124 | 7999 | 38 261 433 | ED | 98 683 654 | 60 422 221 | 2.58 |
| Thrice(40)f | 1 823 528 | 12 403 | 41 665 346 | 2108 | 127 494 203 | 85 828 857 | 3.06 |
| Thrice(45)f | 1 821 827 | 10 702 | 40 775 616 | D | 116 366 423 | 75 590 807 | 2.85 |
| Six times(40)f | 1 826 509 | 15 384 | 48 042 566 | 2139 | 146 995 621 | 98 953 055 | 3.06 |
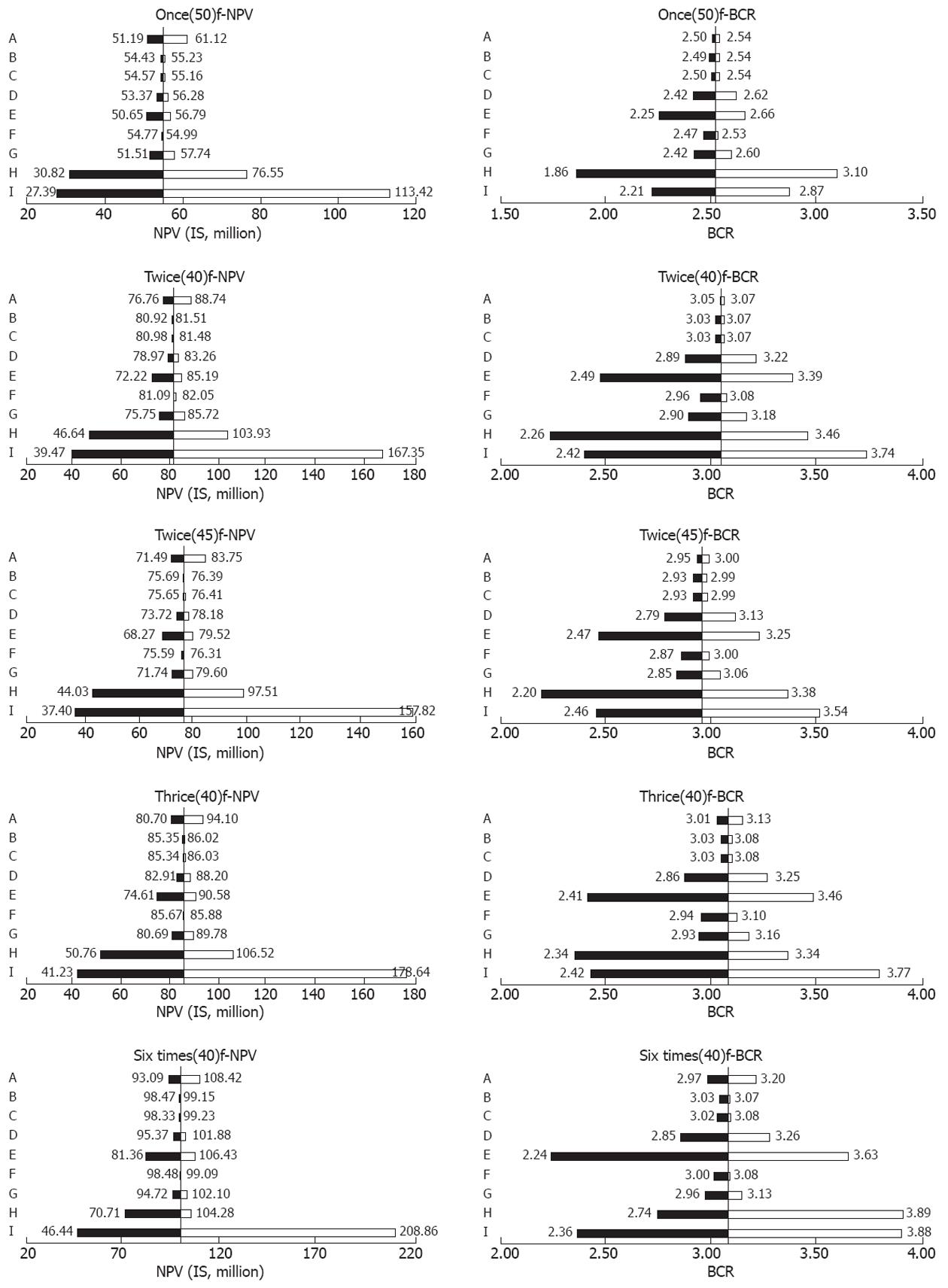
When compared with each other, it indicated that the once(50)f-, twice(40)f-, twice(45)f-, thrice(40)f-, and six times(40)f-strategies were cost-effective, dominating or extended dominating others. In other words, other strategies cost more and saved fewer lives, and were excluded. Among the cost-effective screening alternatives, the once(50)f-strategy saved the lowest life years of 6841, and resulted in the fewest NPV of I$55 million and BCR of 2.52. The highest life years saved were observed in the six times(40)f-strategy, with the maximum NPV of I$99 million and BCR of 3.06. Compared with six times(40)f-strategy, the thrice(40)f-strategy saved fewer life years and yielded lower NPV, but had the same BCR.
When the sensitivity and specificity of endoscopy, screening and treatment costs, discount rate, and screening compliance were changed once at a time (Table 1), once(50)f-, twice(40)f-, twice(45)f-, thrice(40)f-, and six times(40)f-strategies kept dominant. Uncertainty in those parameters had little effect on the choice of cost-effective strategies.
NPVs and BCRs changed obviously with screening cost, compliance, and discount rate under all cost-effective strategies. Both NPVs and BCRs were relatively less affected by the treatment cost, sensitivity and specificity of endoscopic examination. No matter how these parameters varied within the ranges, the results showed that screening was cost-beneficial with positive NPVs and BCRs > 1. In general, our results were robust (Figure 2).
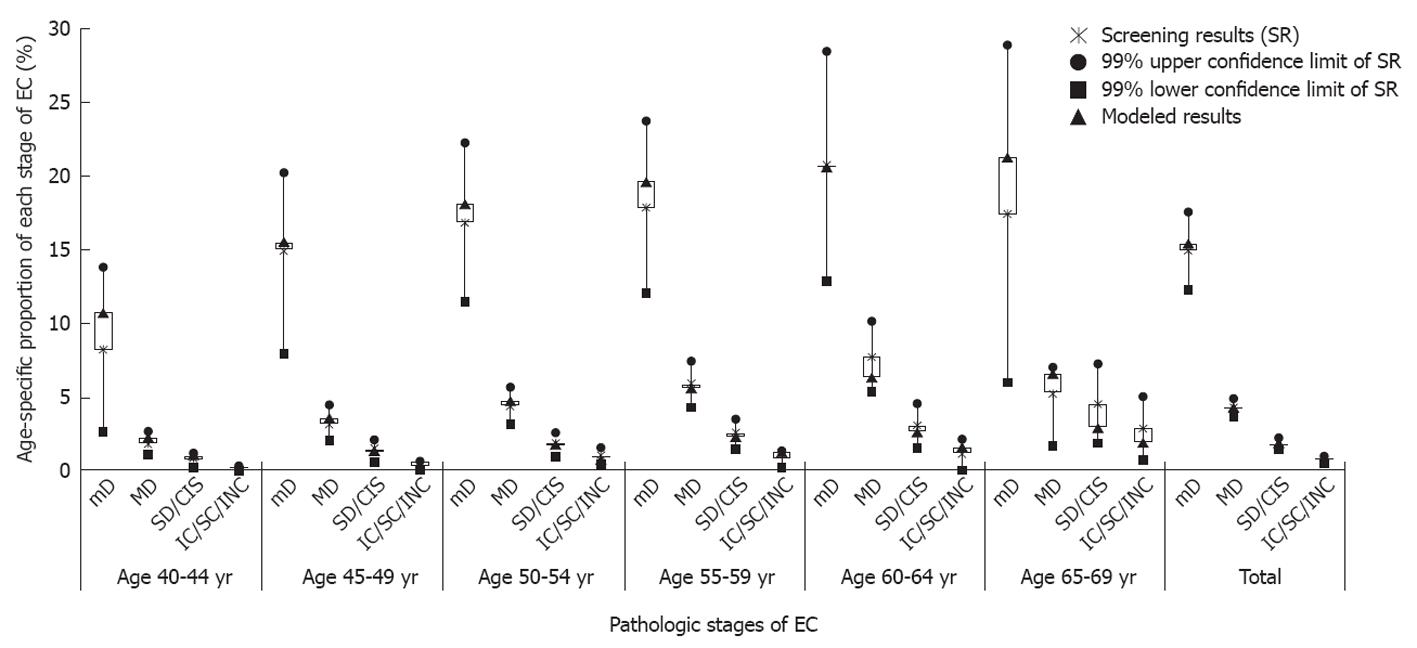
Comparison of incidence: The cancer registry report in Linzhou County during 2004-2006 showed that the age-specific incidence rates of EC were 47.44 per 100 000, 247.77 per 100 000, and 398.00 per 100 000 for the age groups of 40-49, 50-59, and 60-69 years, respectively. The corresponding model-predicted rates were 46.19/100 000, 248.14/100 000, and 424.78/100 000, respectively. The modeled estimates were about 94%-103% of the observed rates.
Comparison of proportions: First and most important, in any age group, the proportion of each histological grade of EC predicted by model was quite close to the screening results of the EDETEC program in Linzhou County during 2005-2008. And the estimated proportions were within the 99% confidence intervals of the observed data. Secondly, in each age group, the proportions decreased with the severity of the disease. And the proportions of mD ranked first. Last but not the least, for each pathologic grade of EC, the proportions increased with age, and reached the top in the 65-69 year-old group. Such tendency fit the characteristics of natural history of EC, and was also in agreement with the previous reports in other high-risk areas of China[26,27]. In summary, the validity of the model was satisfactory (Figure 3).
It was the first comprehensive cost-benefit assessment for the EC screening using endoscopic examination in China. Compared with no screening, all 12 screening strategies covering a hypothetical population of 100 000 resulted in substantial NPVs and high BCRs. However, when compared with each other, only five strategies were cost-effective based on the incremental cost-effectiveness analysis. Among all cost-effective strategies, screening once at age 50 yielded the lowest NPV (I$55 million) and BCR (2.52). Screening six times for those between 40-70 years of age at a 5-year interval yielded the highest NPV (I$99 million) and BCR (3.06). Compared with the six times(40)f-strategy, screening thrice between 40-70 years of age at a 10-year interval saved fewer life years and produced lower NPV, but had the same BCR. Under these strategies, the mD and MD cases diagnosed by screening were followed up every 5 years and 3 years, respectively; all patients with SD/CIS or worse found by screening were treated, and followed up once by endoscopy in the first year after treatment. The validation assessment and the sensitivity analysis showed that our results were reliable.
Previously, two similar investigations presented BCRs of 4 and 4-12 for EC screening in China, which were higher than those in our analysis[25,28]. Explanations of the discrepancy from our estimates were: (1) Liu et al[28] and Wei et al[25] investigated 40-69 year old asymptomatic persons using cross sectional analyses, while we conducted a hypothetic birth cohort analysis, and followed up the cohort from 40 years to 70 years of age. Previous studies did not consider that some “normal/mild/moderate” cases defined by screening would progress and suffer from EC in the following life years[9,19-20,29]. A prospective study found that 23.7% mD and 50% MD cases developed EC during the 13-year follow-up[19]. The treatment costs may be very high for these EC patients. Neglecting them would overestimate BCRs; (2) Compared with non-screening, most of the EC patients in the screening group were diagnosed at earlier stages (87% vs 8%)[24]. As a result, the treatment cost per capita for EC patients in the screening group was lower than that in “non-screening” group. According to the formula of BCR, we found that BCR was positively associated with the difference of treatment cost per person between screening and control groups. Unlike Wei et al[25], we estimated the costs from the perspective of resource expenditure other than hospital charges. The difference of treatment costs between the groups in our study was much smaller than that in prior studies. That could account for the difference of BCRs to some extent; and (3) The costs and benefits were not discounted in previous studies[25,28]. Our sensitivity analysis showed that the discount rates were inversely associated with BCRs. And the BCRs of almost all strategies increased to nearly 4 when the discount rate declined to zero.
As the most widely used summary measures in health economic evaluations, the NPV and BCR are used to determine the return on any investment. Our study demonstrated that an investment of I$ 36 117 125 would result in a return of I$ 54 991 003 under once(50)f-strategy. These economic benefits resulted from a reduction in the incidence and mortality of EC, and the productivity gains of the prolonged life years through early detection and subsequent treatment. Our results revealed that the return increased with the screening frequency, and the six times 40)f-strategy resulted in the highest NPV. Although thrice(40)f-strategy yielded lower benefits, it was much less costly than the six times(40)f-strategy. It means that the thrice(40)f-strategy was a suitable alternative for the six times(40)f-strategy if there was an emphasis on capital constraints.
In addition to cost-benefit outcomes, some other factors should be considered when choosing reasonable screening strategy in different settings. First of all, endoscopy is an invasive examination. Concerns related to the high frequency of screening (e.g., six times in the lifetime) can lead to the great deduction of the compliance if it is not appropriately addressed, especially in the areas with low compliance at the time of initial screening, such as some villages of Ci County (33.7%)[22]. Moreover, the total costs of the screening strategy, life years saved, local economic level, and health resource status should also be weighed and balanced by policy makers. In summary, we recommended that once(50)f-strategy which was the cheapest would be suitable in underdeveloped settings with inadequate health resources, and that thrice(40)f-strategy which could save more life years would be preferable in developed settings with adequate health resources.
One issue needed to be emphasized in our analysis was that most data used in our model were calculated from specific epidemiological data of Linzhou, the highest incidence area of EC worldwide. A great number of endoscopic screenings in this area have been performed since the 1980s, and systematic cancer incidence and death registration have been established. Therefore, the related data from Linzhou County were available and reliable. Our sensitivity analyses displayed that variation in some important parameters within wide ranges did not have a significant effect on our results. This further confirmed that our evaluation results mainly based on data from Linzhou were objective and applicable for other similar high-risk areas in China.
It is known that the cost-benefit of screening for EC (or any other cancer) is highly dependent on the incidence (and subsequent mortality) of that particular cancer. Based on our model prediction and area-specific incidence of EC in Cancer Registry Annual Report of China in 2004, we preliminarily and roughly estimated the cost-benefit of screening program in moderate-(around the national average level of EC incidence, 15.22/100 000), and low-risk areas (less than half of the national average level for EC incidence, 7.61/100 000). In moderate-risk areas, the BCRs ranged from 1.09-1.59, and screening once at age 50 incurred the highest BCR. In low-risk areas, only the strategy of screening once at age 50 remained cost-beneficial (with a highest BCR of 1.09). The results revealed that in moderate- or low-risk areas, screening program was not so cost-beneficial as that in high-risk areas. The screening once at age 50 was relatively preferable. Therefore, our results should be prudential to be used in moderate- or low-risk areas. However, more researches are needed in the future.
Our analysis had several limitations. First, the screening and treatment costs did not include program costs, which might account for a large part of the total costs[12]. The underestimation of costs may result in overrating the benefits of screening strategies, whereas the one-way sensitivity analysis of costs found that even when the costs were increased by over 20%, the screening was still considered as cost-beneficial. Second, in this study, the transition probabilities of all health states should change with age. However, those of normal, mD and MD states were fixed due to the unavailability of the data, which could affect the models’ results to some extent. Hence, further studies on the natural history of EC appear warranted. Finally, although we performed one-way sensitivity analyses to evaluate the impact of each uncertainty on the results, we could not quantify the total impact of combinations of the parameter values. We did not conduct a multivariate probabilistic sensitivity analysis, since data on the probability distributions of variables were unavailable. This may more or less influence the outcomes of the sensitivity analysis.
In conclusion, EC endoscopic screening is cost-bene-ficial in high-risk areas of China. The strategy with once screening at age 50 years in the lifetime is the cheapest but saves fewer life years. If decision makers wish to save more life years and get more benefit, the strategy of thrice screening from 40 years of age at an interval of 10 years would be preferable. In different high-risk areas of EC, policy makers should consider the cost-benefit of screening, acceptability in the population, local health resources and economic level when choosing appropriate screening strategies.
The authors thank Professor Guo-Qing Wang for providing suggestions for the transition probabilities and the natural history of EC.
Esophageal cancer (EC) remains the fourth-leading cause of cancer death in China, and continues to be the major public health burden in some high-risk areas. Previous studies found that EC screening program using endoscopic examination (i.e., endoscopy with mucosal iodine staining and index biopsy as a screening technology, combined with pathological examination for confirming and staging the disease) could increase the 5-year survival rate, decrease the incidence and mortality of EC. A national screening program for EC in high-risk areas has become available in 73 sites of 27 provinces of China. Nevertheless, the health economic effects in the long run on such programs remain unknown. And whether screening strategy is suitable in regions with different health resources and economic level is not clear.
To assess the cost-benefit of screening program in the long run, large-population-based perspective studies are difficult and expensive to conduct, and results would be obtained in decades. Instead, in the area of health economic evaluation for secondary prevention of cancer, the research hotspot is to use Markov model to explore suitable strategies which are cost-effective and cost-beneficial.
Previous researches with regard to cost-benefit analyses of EC screening program in China were cross-sectional studies without follow-up, and only evaluated the health economic effects of one screening strategy which is used currently. The authors conducted a hypothetic birth cohort analysis and followed up the cohort from 40 years to 70 years of age on the basis of Markov model, and compared 12 hypothetic screening strategies (different at starting age of screening, screening intervals, etc.) so as to explore preferable screening strategies in different areas.
The study results suggest that EC endoscopic screening is cost-beneficial in high-risk areas of China. The strategy, screening once at age 50 years in the lifetime, is the cheapest but saves fewer life years. If decision makers wish to save more life years and get more benefit, the strategy, screening thrice from 40 years of age at an interval of 10 years, would be preferable. The results will provide policy makers important information on updating such screening program in high-risk areas.
Markov model: Markov model is considered as a powerful tool for simulating the development process of chronic diseases. In Markov models, health states passed through by patients are defined separately; and then through modeling on the basis of a system of transitional probability among states within a cycle (usually 1 year), the development of diseases and the medical resources used in population could be estimated; Cost-benefit analysis: Cost-benefit analysis is a systematic process for calculating and comparing benefits and costs of a project to see whether the benefits outweigh the costs for two purposes: (1) to determine if it is a sound investment; and (2) to see how it compares with alternate projects.
The authors present the results of a decision analysis of endoscopic screening for esophageal squamous cell cancer for a high-risk region in China. They conclude that endoscopic screening, compared to no screening, is cost-effective, with several different screening schedules that could be used. Overall, this is a nicely done study and is well-written.
Peer reviewer: Julian Abrams, MD, MS, Assistant Professor of Clinical Medicine, Division of Digestive and Liver Diseases, Columbia University Medical Center, 622 W 168th Street, PH 20-303, New York, NY 10032, United States
S- Editor Shi ZF L- Editor Ma JY E- Editor Zheng XM
| 1. | World Health Organization. GLOBOCAN 2008-Oesophageal Cancer Incidence and Mortality Worldwide in 2008 summary. Available from: http://globocan.iarc.fr/factsheets/cancers/oesophagus.asp. |
| 2. | Wei WQ, Yang J, Zhang SW, Chen WQ, Qiao YL. [Analysis of the esophageal cancer mortality in 2004 - 2005 and its trends during last 30 years in China]. Zhonghua Yufang Yixue Zazhi. 2010;44:398-402. |
| 3. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. |
| 4. | Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, Grosclaude P, Hédelin G, Matsuda T, Møller H. EUROCARE-3: survival of cancer patients diagnosed 1990-94--results and commentary. Ann Oncol. 2003;14 Suppl 5:v61-118. |
| 5. | Guanrei Y, Songliang Q. Endoscopic surveys in high-risk and low-risk populations for esophageal cancer in China with special reference to precursors of esophageal cancer. Endoscopy. 1987;19:91-95. |
| 6. | Dawsey SM, Fleischer DE, Wang GQ, Zhou B, Kidwell JA, Lu N, Lewin KJ, Roth MJ, Tio TL, Taylor PR. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer. 1998;83:220-231. |
| 7. | Wang GQ. [30-year experiences on early detection and treatment of esophageal cancer in high risk areas]. Zhongguo Yixue Kexueyuan Xuebao. 2001;23:69-72. |
| 8. | Lu XJ, Chen ZF, Guo CL, Li SS, Bai WL, Jin GL, Wang YX, Meng FS, Gao F, Hou J. Endoscopic survey of esophageal cancer in a high-risk area of China. World J Gastroenterol. 2004;10:2931-2935. |
| 9. | Wang SJ, Zhang LW, Wen DG, Li YS, Yu WF, Wang XL, Wang JH, Li SP, Ma CF, Li YW. [Analysis of endoscopic screening for the natural history of precancerous lesions in esophagus and cardia]. Zhongguo Zhongliu Linchuang. 2007;34:370-373. |
| 10. | Wang GQ, Jiao GG, Chang FB, Fang WH, Song JX, Lu N, Lin DM, Xie YQ, Yang L. Long-term results of operation for 420 patients with early squamous cell esophageal carcinoma discovered by screening. Ann Thorac Surg. 2004;77:1740-1744. |
| 11. | Qiao YL, Hou J, Yang L, He YT, Liu YY, Li LD, Li SS, Lian SY, Dong ZW. [The trends and preventive strategies of esophageal cancer in high-risk areas of Taihang Mountains, China]. Zhongguo Yixue Kexueyuan Xuebao. 2001;23:10-14. |
| 12. | World Health Organization. WHO-CHOICE. Available from: http://www.who.int/choice/publications/p_2003_generalised_cea.pdf. |
| 13. | Roth MJ, Liu SF, Dawsey SM, Zhou B, Copeland C, Wang GQ, Solomon D, Baker SG, Giffen CA, Taylor PR. Cytologic detection of esophageal squamous cell carcinoma and precursor lesions using balloon and sponge samplers in asymptomatic adults in Linxian, China. Cancer. 1997;80:2047-2059. |
| 14. | Limburg PJ, Wei W, Ahnen DJ, Qiao Y, Hawk ET, Wang G, Giffen CA, Wang G, Roth MJ, Lu N. Randomized, placebo-controlled, esophageal squamous cell cancer chemoprevention trial of selenomethionine and celecoxib. Gastroenterology. 2005;129:863-873. |
| 15. | Wei WQ, Wang JB, Yang J, Qiao YL. Esophageal cancer. In: Tuncer AM, Moore M, Qiao YL, Yoo KY, Tajima K, Ozgul N, Gultekin M, editors. Asian Pacific Organization for Cancer Prevention. Cancer report 2010; The 5th International APOCP Conference; 2010 Apr 3-7; Istanbul, Turkey. Ankara: MN Medical and Nobel Publishing Company, 2010: 209-214. |
| 16. | Wen DG, Zhang LW, Wang SJ, Li YS, Yu WF, Wang XL, Wang JH, Li SP, Li YW, Wang SP. [Sojourn time observation of esophageal and cardia precancerous lesions by periodic endoscopic screening of 301 subjects in Shexian]. Zhengzhou Daxue Xuebao. 2007;42:62-66. |
| 17. | Jacob P, Kahrilas PJ, Desai T, Hidvegi D, Walloch J, Yokoo H, Gurley AM, Ostrow JD. Natural history and significance of esophageal squamous cell dysplasia. Cancer. 1990;65:2731-2739. |
| 18. | Benedetti G, Sablich R, Vitalba A, Lacchin T, Guido E, Del Bianco T. Intraepithelial carcinoma of the oesophagus: report of 25 cases from north-east Italy. Eur J Surg. 2000;166:622-627. |
| 19. | Wang GQ, Abnet CC, Shen Q, Lewin KJ, Sun XD, Roth MJ, Qiao YL, Mark SD, Dong ZW, Taylor PR. Histological precursors of oesophageal squamous cell carcinoma: results from a 13 year prospective follow up study in a high risk population. Gut. 2005;54:187-192. |
| 20. | Dawsey SM, Lewin KJ, Wang GQ, Liu FS, Nieberg RK, Yu Y, Li JY, Blot WJ, Li B, Taylor PR. Squamous esophageal histology and subsequent risk of squamous cell carcinoma of the esophagus. A prospective follow-up study from Linxian, China. Cancer. 1994;74:1686-1692. |
| 21. | Wang LD, Yang HH, Fan ZM, Lü XD, Wang JK, Liu XL, Sun Z, Jiang YN, He X, Zhou Q. Cytological screening and 15 years’ follow-up (1986-2001) for early esophageal squamous cell carcinoma and precancerous lesions in a high-risk population in Anyang County, Henan Province, Northern China. Cancer Detect Prev. 2005;29:317-322. |
| 22. | Song GH, Meng FS, Chen C, Chen ZF. [Analysis on the factors influencing the compliance to endoscopic screening for early diagnosis and treatment in high-risk area of esophageal cancer]. Zhonghua Liuxingbingxue Zazhi. 2009;30:977-978. |
| 23. | Yang J, Wei WQ, Niu J, He YT, Liu ZC, Song GH, Zhao de L, Qiao YL, Yang CX. Estimating the costs of esophageal cancer screening, early diagnosis and treatment in three high risk areas in China. Asian Pac J Cancer Prev. 2011;12:1245-1250. |
| 24. | Lv SH, Li BY, Wei WQ, Shao Y, Niu J, Yang J, Lian SY, Qiao YL, Yang CX. [Cost analysis on esophageal cancer treatment among screening residents in Linzhou of Henan province]. Xiandai Yufang Yixue. 2010;19:3667-3669. |
| 25. | Wei WQ, Yang CX, Lu SH, Yang J, Li BY, Lian SY, Qiao YL. Cost-benefit analysis of screening for esophageal and gastric cardiac cancer. Chin J Cancer. 2011;30:213-218. |
| 26. | Wang GQ, Wei WQ, Lu N, Hao CQ, Lin DM, Zhang HT, Sun YT, Qiao YL, Wang GQ, Dong ZW. [Significance of screening by iodine staining of endoscopic examination in the area of high incidence of esophageal carcinoma]. Ai Zheng. 2003;22:175-177. |
| 27. | He Z, Zhao Y, Guo C, Liu Y, Sun M, Liu F, Wang X, Guo F, Chen K, Gao L. Prevalence and risk factors for esophageal squamous cell cancer and precursor lesions in Anyang, China: a population-based endoscopic survey. Br J Cancer. 2010;103:1085-1088. |
| 28. | Liu ZR, Wei WQ, Huang YQ, Qiao YL, Wu M, Dong ZW. [Economic evaluation of “early detection and treatment of esophageal cancer”]. Ai Zheng. 2006;25:200-203. |
| 29. | Chen ZF, Wang GQ, Hou J, Zhang JH, Song GH, Qiao CY, Li SS, Chen C. [Follow-up results of esophageal squamous severe dysplasia in 158 cases]. Zhongguo Zhongliu Linchuang. 2004;31:306-308. |