Published online Jan 14, 2012. doi: 10.3748/wjg.v18.i2.192
Revised: August 11, 2011
Accepted: August 15, 2011
Published online: January 14, 2012
Chronic hepatobiliary inflammatory diseases are not widely acknowledged as underlying disorders of systemic AA amyloidosis, except epidemic schistosomiasis. Among them, primary sclerosing cholangitis (PSC) might initiate amyloid A protein deposition in diverse tissues, giving rise to systemic amyloidosis, due to a progressive and unresolved inflammatory process, and its possible association with inflammatory bowel diseases. Nevertheless, only one such case has been reported in the literature to date. We report a 69-year-old Japanese woman with cirrhosis who was diagnosed with PSC complicated with systemic AA amyloidosis, without any evidence of other inflammatory disorders. As a result of cholestasis in conjunction with biliary strictures and increased serum IgG4, the presence of IgG4+ plasma cells was examined systemically, resulting in unexpected documentation of Congo-red-positive amyloid deposits, but not IgG4+ plasma cells, in the liver, stomach and salivary glands. Elevated serum IgG4 is the hallmark of IgG4-related disease, including IgG4-associated cholangitis, but it has also been demonstrated in certain patients with PSC. Amyloid A deposits in multiple organs associated with an indolent clinical course that progresses over many years might have a diagnostic value in discriminating PSC from IgG4-associated cholangitis.
- Citation: Kato T, Komori A, Bae SK, Migita K, Ito M, Motoyoshi Y, Abiru S, Ishibashi H. Concurrent systemic AA amyloidosis can discriminate primary sclerosing cholangitis from IgG4-associated cholangitis. World J Gastroenterol 2012; 18(2): 192-196
- URL: https://www.wjgnet.com/1007-9327/full/v18/i2/192.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i2.192
Primary sclerosing cholangitis (PSC) is an intractable fibro-inflammatory disease of the bile ducts that is characterized by biliary strictures without any underlying insults, e.g., immunodeficiency, ischemia, and biliary toxins[1]. PSC usually follows an indolent but progressive course, which results in eventual death or liver transplantation in the majority of patients. A single center study in Germany demonstrated that the estimated median survival from the time of diagnosis to death or time of liver transplantation was 9.6 years; 39.6% of patients underwent liver transplantation, while 14.3% of them developed hepatobiliary malignancies[2]. Definite diagnosis is thus required in cases with suspected lesions, especially to discriminate PSC from IgG4-associated cholangitis (IAC); a recently defined disorder with better prognosis[3-5].
IAC consists of a biliary stricture that responds to or improves with corticosteroid therapy[6], and is recognized as one of a variety of IgG4-related disease that exhibits a wide range of clinical manifestations. The clinical diagnostic criteria for IgG4-related disease consists of three parts: namely, enlarged/thickened lesions in one or more organs; elevated serum IgG4 levels (≥ 135 mg/dL); and histopathological findings[5]. Although IgG4 levels are usually higher in patients with IAC than in those with PSC, raised serum IgG4 levels have been recently reported in 9%-36% of patients with PSC[7,8]. Therefore, the identification of IgG4+ plasma cell infiltrates in the bile duct as well as in other organs[5,9] is important in making a diagnosis.
In this report, we describe a patient with cirrhotic PSC who had elevated levels of serum IgG4. Multiple organ biopsies were performed to obtain a definitive diagnosis and rule out IAC. Unexpectedly, Congo-red-positive amyloid deposits, but not IgG4+ plasma cells, were demonstrated in the liver, stomach and salivary glands. Subsequently, raised levels of serum amyloid A protein (SAA) were confirmed, resulting in a diagnosis of PSC complicated with systemic AA amyloidosis, despite the absence of known genetic susceptibility[10]. This is the second report describing the concurrence of systemic AA amyloidosis in PSC. A sustained acute phase response involving the overproduction of SAA over a period of many years is likely to characterize indolent hepatobiliary inflammation in PSC, but not in IAC.
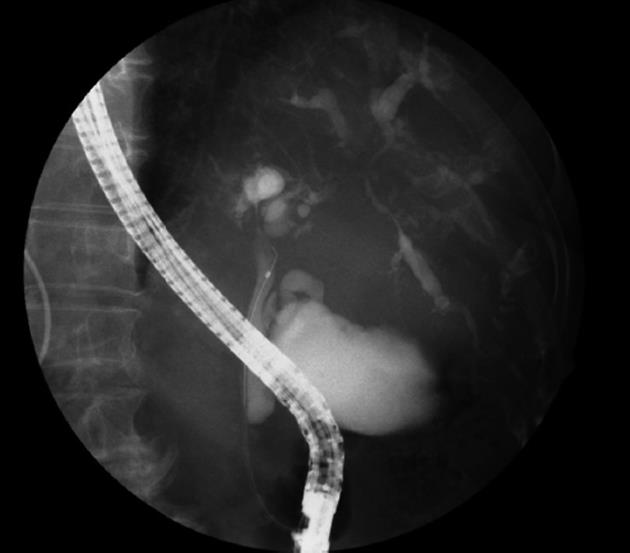
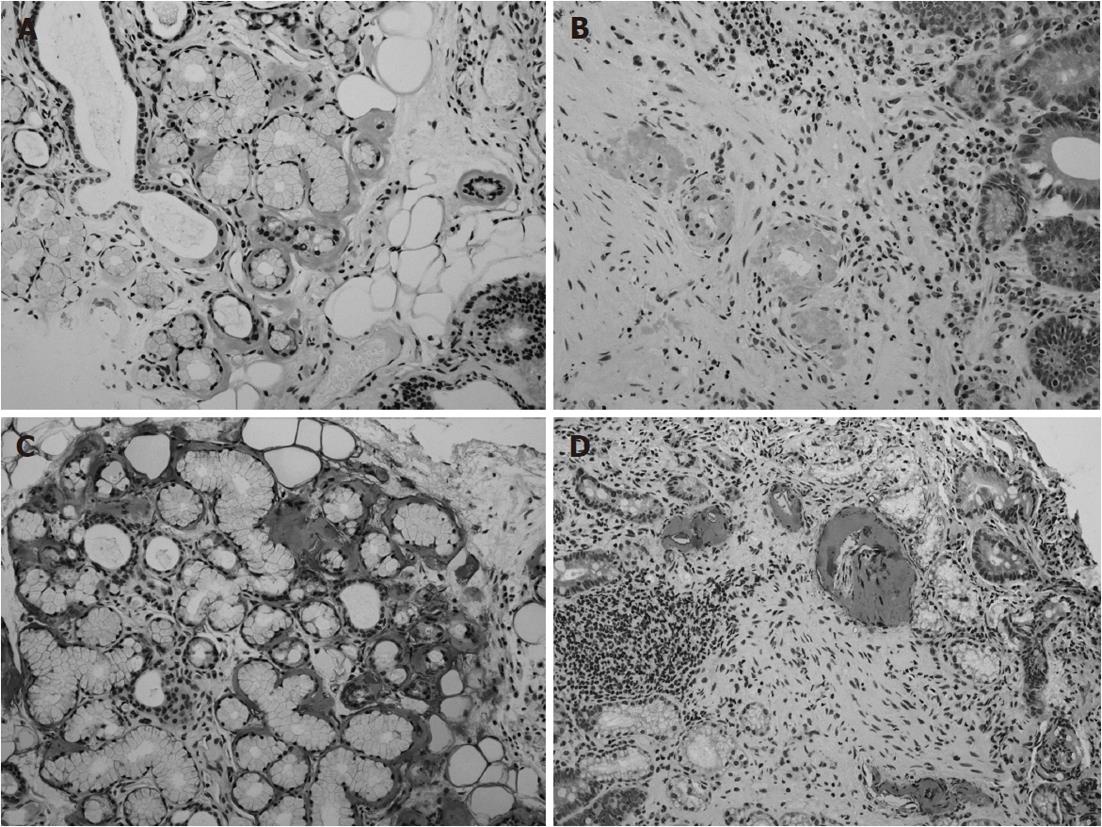
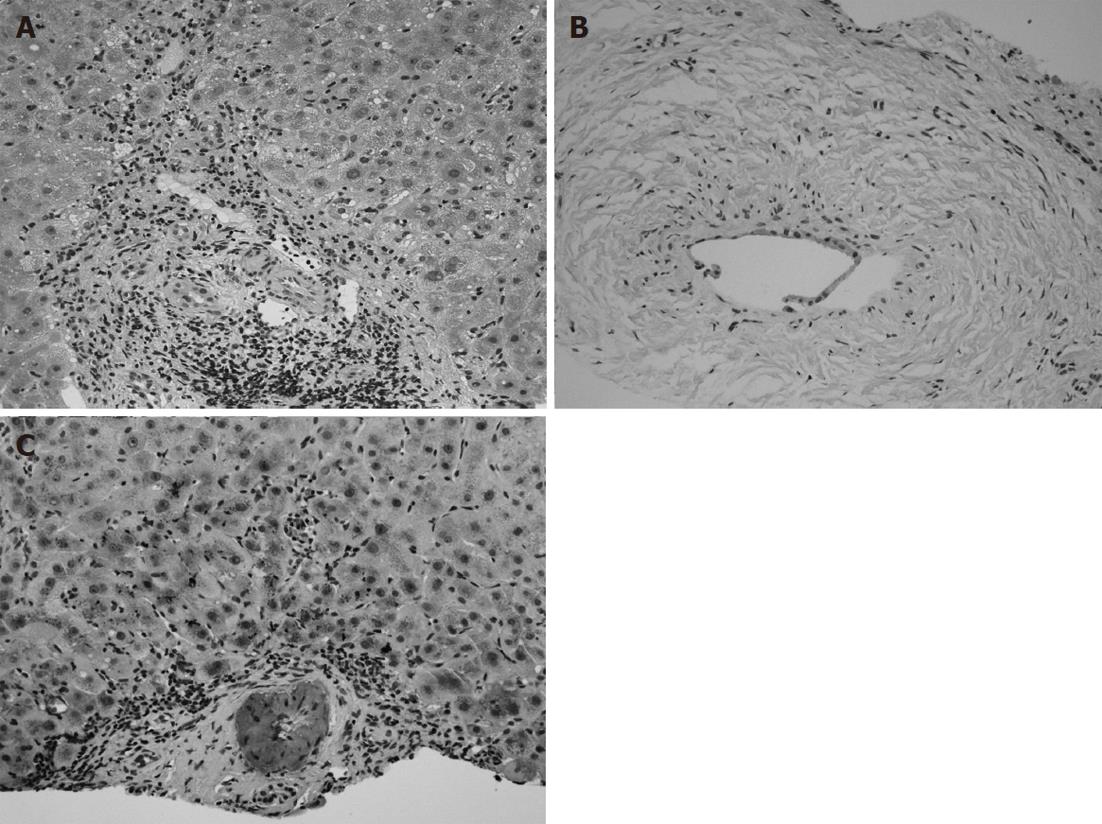
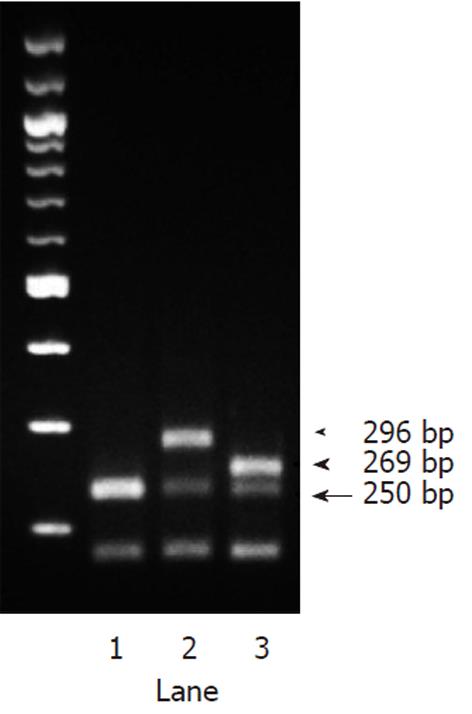
A 69-year-old Japanese woman was referred to our hospital with progressive elevation of cholestatic liver enzymes in October 2009. She had a history of endoscopic sphincteropapillotomy for choledocholithiasis at age 47 years, at which time, PSC was also suspected due to the irregularity of the extra- and intrahepatic bile duct walls, as revealed by endoscopic retrograde cholangiopancreatography (ERCP). Her cholestatic liver tests subsequently remained abnormal despite removal of all gallstones, indicating the presence of PSC. She had never been immunocompromised. Fatigue, pruritis, and abdominal fullness had worsened even after administration of ursodeoxycholic acid (600 mg/d; 13.2 mg/kg body weight) and she was therefore admitted to our hospital in October 2010. On admission, mild jaundice was apparent, and blood tests revealed elevated liver enzymes along with increased acute phase proteins, i.e., aspartate aminotransferase 109 IU/L (normal < 37 IU/L), alanine aminotransferase 80 IU/L (normal < 39 IU/L), alkaline phosphatase 871 IU/L (normal < 359 IU/L), γ-glutamyltranspeptidase 72 IU/L (normal < 75 IU/L), total bilirubin 3.2 mg/dL (normal < 1.2 mg/dL), C-reactive protein 2.66 mg/dL (normal < 0.3 mg/dL), and SAA protein 303.9 mg/L (normal < 8.0 mg/L). With a Child-Pugh score of 10, her functional hepatic reserve was reduced and she had moderate ascites. Anti-nuclear, anti-smooth muscle, anti-mitochondrial, and perinuclear anti-neutrophil cytoplasmic antibodies were negative. Serum IgG was 2890 mg/dL (normal < 1700 mg/dL), including elevated IgG4 level of 251 mg/dL (normal < 105 mg/dL). Although serum IgE was 15 400 IU/mL (normal < 361 IU/mL), antibodies against parasites, including liver flukes, were negative. Viral markers for hepatitis B and C were both negative. Abdominal contrast-enhanced computed tomography showed biliary strictures from common hepatic duct to the second to third branches, accompanied by dilatation of the distal bile ducts. Atrophy of the right hepatic lobe in conjunction with collateral vessel formation around the lower esophagus confirmed cirrhosis. The size and contours of the pancreas were normal. ERCP revealed irregularities of the walls of the lower common, hilar and intrahepatic bile ducts, which were accompanied by multiple strictures and a “pruned-tree” appearance in the intrahepatic bile ducts (Figure 1). Deterioration of liver function likely resulted from progression of PSC, but given the equivocal ERCP results and elevated IgG4 levels, we elected to rule out IgG4-related disease, particularly IAC. The biopsied specimen from the distal common bile duct contained only erosive duct mucosa, preventing the visualization of IgG4+ lymphoplasmacytic infiltration. We therefore performed biopsies of the major duodenal papilla[9], the salivary glands, and the gastric mucosa in order to investigate the possibility of multiple organ infiltration of IgG4+ plasma cells. Unexpectedly, IgG4+ plasma cells were scarcely found by immunohistochemistry, while Congo-red-positive, amorphous eosinophilic materials were demonstrated in the subglandular and stroma of the salivary glands and in the submucosal stroma of the stomach (Figure 2A and B). Potassium permanganate sensitivity and positive AA immunohistochemical staining confirmed that these materials were AA amyloid deposits (Figure 2C and D). Subsequent liver histology revealed an increase in lymphoneutrophilic infiltrates with some eosinophils in the portal tracts, interface hepatitis and bridging fibrosis, damaged interlobular bile ducts with collagenous periductal thickening, marked ductular proliferation, and cholate stasis (Figure 3A and B). Amyloid deposition in the vessel walls of the portal tracts was also apparent (Figure 3C). Oral administration of 30 mg/d prednisolone for 1 wk failed to show any beneficial effect on cholestasis, therefore, a diagnosis of IAC turned out to be implausible. Abnormal uptake was not found in positron emission tomography; again, excluding underlying IgG4+-related lymphoproliferative disease, as well as IgE myeloma. The normal colonoscopic appearance of the colonic mucosa, as well as the histology that only indicated nonspecific inflammation of the intestine, excluded a concurrent diagnosis of inflammatory bowel disease. Currently known patterns of genetic susceptibility to systemic AA amyloidosis were found to be absent[10,11]. SAA1 genotyping by polymerase chain reaction-restriction fragment length polymorphism analysis[12] revealed homozygosity of SAA1.5/1.5 (Figure 4), and sequencing of whole Mediterranean fever (MEFV) exons demonstrated no amino acid substitution mutations (data not shown). Based on all of the above data, we diagnosed the patient with PSC complicated by systemic AA amyloidosis.
In this study, we presented a patient with PSC complicated by systemic AA amyloidosis. To the best of our knowledge, this is the second reported case of concurrent PSC and systemic AA amyloidosis, and it included detailed information on pathology as well as on genetic susceptibility to AA amyloidosis.
Sustained overproduction of SAA in association with chronic unresolved inflammation, as demonstrated in our case, is essential for the development of amyloidosis[13]. Nevertheless, susceptibility to AA amyloidosis differs among various diseases. According to Lachmann et al, the most prevailing underlying inflammatory disorder is chronic inflammatory arthritis, followed in descending order by chronic sepsis, periodic fever syndromes, and Crohn’s disease[13]. Although PSC is indolent, progressive inflammatory hepatobiliary disease results in cholestatic cirrhosis. Nonetheless, only one other case of PSC has been reported as a cause of AA amyloidosis[14]. Even assuming that about 6% of AA amyloidosis patients do not have underlying inflammatory disorders[13], AA amyloidosis in our case was likely to be secondary to PSC, as coexisting inflammatory bowel disease was excluded. The patient had neither the SAA locus conferring susceptibility to AA amyloidosis in Japanese rheumatoid arthritis, namely SAA1.3, nor MEFV amino acid substitution mutations that are responsible for familial Mediterranean fever, an autoinflammatory disease. Multiple factors affecting amyloid deposition in tissues, such as amyloid P and apolipoprotein E, might have cooperatively contributed to the pathogenesis of AA amyloidosis in this case.
Regarding the diagnosis of PSC, discrimination of IAC is of primary importance owing to better prognosis of the latter with corticosteroid therapy. Serum IgG4 levels in conjunction with cholangiographic features have clinical relevance in this process. In our case, while the elevated serum IgG4 level favored diagnosis of IAC, the “pruned-tree” appearance of the intrahepatic bile ducts coupled with stenosis of the lower common bile duct were equivocal. Moreover, the fact that 9%-36% of patients with PSC also show mildly elevated IgG4[7,8] indicates the need for additional parameters to facilitate differential diagnosis. The presence of IgG4+ plasma cells in the bile ducts and in other organs is suggestive of IgG4-related disease and thus has more diagnostic specificity[5]. In our case, PSC was confirmed by the absence of IgG4+ plasma cells in the examined organs. On the other hand, unexpected demonstration of Congo-red-positive amyloid deposition in the salivary glands, stomach and liver prompted us to consider the distinct implications of these findings on the differential diagnosis, because AA amyloid found in various organs might have diagnostic specificity for PSC. To the best of our knowledge, there have been no case reports describing coexistence of AA amyloid deposits with IgG4+ plasma cells in IgG4-related diseases. A sustained acute phase response in PSC might be a sufficient cause for AA amyloid deposition. Alternatively, mechanistically distinct and/or mutually exclusive inflammatory processes occurring in PSC and IAC, in the latter case reportedly Th-2-dependent[5], might be responsible for the phenomenon. At any rate, estimation of the incidence of AA amyloidosis in PSC[15], as well as in IAC, in a large cohort is necessary to verify our hypothesis.
The proper treatment of PSC complicated by systemic AA amyloidosis remains to be determined. The aim of treatment of AA amyloidosis is generally considered to be the suppression of underlying inflammatory conditions, thereby reducing SAA concentrations[13]. Immunosuppressive agents including anti-tumor necrosis factor therapies are often administered for this purpose, with the exclusion of conditions involving chronic sepsis, such as bronchiectasis. Renal dysfunction has been reported as a predominant disease manifestation, and progression to end-stage renal failure has been linked to increased mortality of systemic AA amyloidosis[13]. Regression of AA amyloid deposits (as evaluated by serum amyloid P scintigraphy), that is associated with median SAA concentration during anti-inflammatory therapy, is inversely correlated to the outcomes of renal dysfunction[13]. Although the first case of coexisting PSC and systemic AA amyloidosis reported in the literature experienced relief of symptoms and biochemical improvement as a result of the combination of corticosteroid and azathioprine, the patient experienced progression of amyloidosis-induced nephrotic syndrome followed by its reversal after liver transplantation[14]. Maintaining SAA in a low-normal range through use of anti-inflammatory agents was also difficult in our case; short-term use of prednisolone showed no benefit and colchicine did not decrease serum SAA (data not shown). Administration of tocilizumab, a humanized anti-interleukin 6 receptor antibody that strongly suppresses SAA levels, might be promising for patients with secondary AA amyloidosis who are not responding adequately to treatment for underlying inflammation[16].
In summary, we presented a female PSC patient who had concurrent systemic AA amyloidosis. Is AA amyloidogenesis more specific to PSC than IAC? Could AA amyloid deposition discriminate PSC from IAC? Reexamination of AA amyloid deposition in PSC cases in a large cohort will help answer these and other relevant questions.
We would like to thank following colleagues for their help: Yuka Jiuchi and Yumi Maeda for technical assistance; Yuki Naruke and Yumi Mihara for pathological diagnosis; Satoru Hashimoto, Shinya Nagaoka and Hiroshi Yatsuhashi for the diagnostic decision.
Peer reviewers: Atsushi Tanaka, MD, PhD, Associate Professor, Department of Medicine, Teikyo University School of Medicine, 2-11-1, Kaga, Itabashi-ku, Tokyo 173-8605, Japan; Rui Tato Marinho, MD, PhD, Department of Gastroenterology and Hepatology, Hospital Santa Maria, Medical School of Lisbon, Av. Prof. Egas Moniz, 1649-035 Lisboa, Portugal
S- Editor Tian L L- Editor Kerr C E- Editor Li JY
| 1. | Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, Gores GJ. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660-678. [PubMed] |
| 2. | Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: A single center study. Am J Gastroenterol. 2007;102:107-114. [PubMed] |
| 3. | Björnsson E, Chari ST, Smyrk TC, Lindor K. Immunoglobulin G4 associated cholangitis: description of an emerging clinical entity based on review of the literature. Hepatology. 2007;45:1547-1554. [PubMed] |
| 4. | Webster GJ, Pereira SP, Chapman RW. Autoimmune pancreatitis/IgG4-associated cholangitis and primary sclerosing cholangitis--overlapping or separate diseases? J Hepatol. 2009;51:398-402. [PubMed] |
| 5. | Okazaki K, Uchida K, Koyabu M, Miyoshi H, Takaoka M. Recent advances in the concept and diagnosis of autoimmune pancreatitis and IgG4-related disease. J Gastroenterol. 2011;46:277-288. [PubMed] |
| 6. | Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, Topazian MD, Clain JE, Pearson RK, Petersen BT. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706-715. [PubMed] |
| 7. | Hirano K, Kawabe T, Yamamoto N, Nakai Y, Sasahira N, Tsujino T, Toda N, Isayama H, Tada M, Omata M. Serum IgG4 concentrations in pancreatic and biliary diseases. Clin Chim Acta. 2006;367:181-184. [PubMed] |
| 8. | Mendes FD, Jorgensen R, Keach J, Katzmann JA, Smyrk T, Donlinger J, Chari S, Lindor KD. Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2006;101:2070-2075. [PubMed] |
| 9. | Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A. A new diagnostic endoscopic tool for autoimmune pancreatitis. Gastrointest Endosc. 2008;68:358-361. [PubMed] |
| 10. | Nakamura T, Higashi S, Tomoda K, Tsukano M, Baba S, Shono M. Significance of SAA1.3 allele genotype in Japanese patients with amyloidosis secondary to rheumatoid arthritis. Rheumatology (Oxford). 2006;45:43-49. [PubMed] |
| 11. | Lachmann HJ, Sengül B, Yavuzşen TU, Booth DR, Booth SE, Bybee A, Gallimore JR, Soytürk M, Akar S, Tunca M. Clinical and subclinical inflammation in patients with familial Mediterranean fever and in heterozygous carriers of MEFV mutations. Rheumatology (Oxford). 2006;45:746-750. [PubMed] |
| 12. | Yamada T, Wada A, Itoh Y, Itoh K. Serum amyloid A1 alleles and plasma concentrations of serum amyloid A. Amyloid. 1999;6:199-204. [PubMed] |
| 13. | Lachmann HJ, Goodman HJ, Gilbertson JA, Gallimore JR, Sabin CA, Gillmore JD, Hawkins PN. Natural history and outcome in systemic AA amyloidosis. N Engl J Med. 2007;356:2361-2371. [PubMed] |
| 14. | Van Steenbergen W, Braeye L, Harlet R, Nevens F, Fevery J, Desmet V, Roskams T, Pirenne J. Primary sclerosing cholangitis complicated by amyloid A amyloidosis: complete regression of the nephrotic syndrome by liver transplantation. Eur J Gastroenterol Hepatol. 2010;22:1265-1270. [PubMed] |
| 15. | Zen Y, Quaglia A, Portmann B. Immunoglobulin G4-positive plasma cell infiltration in explanted livers for primary sclerosing cholangitis. Histopathology. 2011;58:414-422. [PubMed] |
| 16. | Okuda Y, Takasugi K. Successful use of a humanized anti-interleukin-6 receptor antibody, tocilizumab, to treat amyloid A amyloidosis complicating juvenile idiopathic arthritis. Arthritis Rheum. 2006;54:2997-3000. [PubMed] |