INTRODUCTION
Esophageal cancer is one of the least studied and deadliest cancers. Tobacco smoke is the most firmly established risk factor for esophageal cancer and has been associated with the development of esophageal squamous cell carcinoma and adenocarcinoma; gastroesophageal reflux with bile acid is another important cause of adenocarcinoma[1-3]. Molecularly, the expression of retinoic acid receptor-β2 (RAR-β2) is frequently lost in premalignant and malignant esophageal tissues, and benzo[a]pyrene, a carcinogen present in tobacco and environmental pollution, and bile acid, a tumor promoter for gastrointestinal cancer, may be responsible for its loss. Restoration of RAR-β2 expression suppressed esophageal cancer cell growth and induced apoptosis in vitro and tumor formation in vivo; these effects were correlated with decreased expression of activator protein 1 and cyclooxygenase-2 (COX-2) and phosphorylated extracellular-signal-regulated kinases (Erk1/2)[4]. Moreover, the novel retinoid receptor-induced gene-1 (RRIG1), which is a downstream gene of RAR-β2, participates in regulating the effects of RAR-β2 on cell growth and gene expression[5,6]. RRIG1 protein binds to and inhibits a small GTPase RhoA activity. Restoration of RRIG1 expression inhibits RhoA activation and, consequently, reduces tumor cell colony formation, invasion, and proliferation, which are correlated with inhibition of Erk1/2 phosphorylation, COX-2, and cyclin D1 expression[5,6]. These genes together may form a novel molecular pathway that involves RAR-β2-induced RRIG1 expression and suppression of RhoA/Erk1/2/AP-1/COX2[4]. Therefore, targeting this molecular pathway should translate into better control of esophageal cancer.
Cancer chemoprevention was defined as the use of natural, synthetic, or biologic chemicals (such as drugs and food supplements) in the prevention, suppression, or delay of the carcinogenesis process[6]. Several clinical trials of different drugs have been conducted in an attempt to prevent esophageal cancer. The first class of agents tried was the retinoids[7]. The use of retinoids was based on the fact that vitamin A deficiency was found in esophageal cancer patients and the fact that this deficiency induced hyperkeratotic changes in the esophageal mucosa of experimental animals[8,9]. A clinical trial using N-4-(ethoxycarbophenyl) retinamide demonstrated that cancer incidence in the treatment group with severe esophageal dysplasia was reduced by 43.2% compared with that in the placebo group[10]. However, the results from two other trials conducted in Linxian, China, by the National Cancer Institute were inconclusive[11,12]. Our in vitro study demonstrated that esophageal cancer cells which do not express RAR-β2 are resistant to all-trans retinoic acid[13]. In animal experiments, dietary N-(4-hydroxyphenyl) retinamide enhanced tumorigenesis in response to N-nitrosomethylbenzylamine in the rat esophagus by increasing tumor initiation events[14]. Moreover, 13-cis RA did not reduce NBMA-induced esophageal tumor multiplicity in rats[15].
Non-steroidal anti-inflammatory drugs (NSAIDs) were also tested for the prevention of esophageal cancer[16]. Epidemiological and experimental studies indicated that NSAIDs decreased esophageal cancer incidence[17-21]. Our in vitro data showed that aspirin and NS398 induced apoptosis in esophageal cancer cells, which correlated with their ability to inhibit COX-2 enzymatic activity and upregulate the expression of 15-LOX-1 and -2[22-25]. However, during clinical trials of these agents in the chemoprevention of colorectal cancer, it was reported that Vioxx (rofecoxib) and Celebrex (celecoxib) induced cardiovascular events, which raised safety concerns about the high-dose and long-term use of these drugs in cancer prevention[26,27].
However, to date, most preclinical and clinical chemoprevention studies of human cancers have been focused on targeting a single gene, which showed limited activities in vitro and in vivo[3,16]. In this study, we aimed to target multiple genes in a molecular pathway using combinations of drugs to determine whether this approach is more effective than single-drug treatments in the inhibition of esophageal cancer.
MATERIALS AND METHODS
Cell culture and drug treatment
The human esophageal cancer cell lines SKGT-4 and TE-8 used in our previous studies[13,22] were grown in Dulbecco’s modified Eagle’s minimal essential medium (DMEM), supplemented with 10% fetal bovine serum (FCS), at 37 °C in a humidified atmosphere of 95% air and 5% CO2. For drug treatment, these cells were grown in monolayer overnight and then treated with or without curcumin, (-)-epigallocatechin-3-gallate (EGCG), lovastatin, and combinations of these agents for up to 5 days. The drugs were dissolved in dimethyl sulfoxide (DMSO) and then diluted before use. The concentration of lovastatin was 2 or 4 μmol/L, curcumin was 20 or 40 μmol/L, and EGCG was 20 or 40 μmol/L (all from LKT Laboratories, Inc., St. Paul, MN, United States). The concentrations for the drug combinations were the same as those used individually. For the methyl thiazolyl tetrazolium (MTT) assay, 20 μL of MTT (5 mg/mL, Sigma, St Louis, MO, United States) was added to each well of the 96-well plates and incubated for an additional 4 h. After the growth medium was removed, 100 μL of DMSO was added to the wells to dissolve the MTT crystal, and the optical densities were measured with an automated spectrophotometric plate reader at a single wavelength of 540 nm. The percentage of cell growth was calculated using the formula: % control = ODt/ODc × 100, where ODt and ODc are the optical densities for treated and control cells, respectively. The data were then analyzed statistically using the Student’s t test.
Tumor cell invasion assay
Boyden chambers coated with Matrigel were obtained from BD Biosciences (Bedford, MA, United States) for assaying tumor cell invasion ability[6]. Esophageal cancer cells SKGT-4 and TE-8 were first starved in medium without FCS overnight, and the cells (5 × 104) were resuspended in the FCS-free medium and placed in the top chambers in triplicate. The medium in the top chambers contained lovastatin (4 μmol/L), curcumin (40 μmol/L), EGCG (40 μmol/L), or their combinations. The lower chamber was filled with DMEM and 10% FCS as the chemoattractant and incubated for 48 h. The upper surface was then wiped with a cotton swab to remove the remaining cells. The cells which invaded the Matrigel and attached to the lower surface of the filter were fixed and stained with 1% crystal violet solution. The cells in the reverse side were photographed (5 microscopic fields at 100 × magnification per chamber). The cells in the photographs were then counted, and the data were summarized as mean ± SD and presented as a percentage of the controls (mean ± SD). The data were then analyzed statistically using the Student’s t test.
Protein extraction and Western blotting
The cells were grown and treated with or without the drugs for 2 d. After that, total cellular protein was extracted as described previously[5,6,13,22-25]. Samples containing 50 μg of protein from each treatment were then separated by 10%-14% on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred electrophoretically to a Hybond-C nitrocellulose membrane (GE-Healthcare, Arlington Heights, IL, United States) at 500 mA for 2 h at 4 °C. The membrane was subsequently stained with 0.5% Ponceau S containing 1% acetic acid to confirm that the proteins were loaded equally and to verify transfer efficiency. Next, the membranes were subjected to Western blotting by overnight incubation in a blocking solution containing 5% bovine skimmed milk and 0.1% Tween 20 in phosphate-buffered saline (PBS) at 4 °C. The next day, the membranes were first incubated with primary antibodies and then with horse anti-mouse or goat anti-rabbit secondary antibodies (GE Healthcare) for enhanced chemiluminescence detection of antibody signals. The antibodies used were anti-Ki67 (Vector Laboratories, Burlingame, CA, United States), anti-phosphorylated Erk1/2 (Cell Signaling Technology, Danvers, MA, United States), anti-COX-2 (BD Transduction Laboratories, Lexington, KY, United States), and anti-β-actin (Sigma-Aldrich, St. Louis, MO, United States).
Animal experiments
An animal usage procedure was approved by our Institutional Animal Care and Usage protocol. Esophageal cancer SKGT-4 cells were grown and treated with or without these drugs for 3 d before injection (the doses were the same as above). Nu/nu nude mice (6-8 wk of age) were treated with or without curcumin (50 μg/kg per day), EGCG (50 μg/kg per day), lovastatin (50 μg/kg per day), and their combinations (the same doses used individually) orally for two days and then subcutaneously injected in the right flank through a 22-gauge needle with 2 × 106 tumor cells mixed with 50% Matrigel (BD Biosciences) for a total volume of 200 μL per mouse. The animals were then continuously treated with or without these drugs orally 5 d/wk for an additional 30 dand monitored for tumor formation and growth daily. The tumor mass volumes, measured weekly with a vernier caliper, were calculated as follows: length × width2/2. At the end of the experiments, the tumor xenografts were taken excised, weighed and the results summarized.
Esophageal cancer tissue samples
Our institutional review board (IRB) approved our protocol for the use of patient tissue samples in this study, which included 156 consecutive patients with available paraffin blocks who had undergone esophagectomy without preoperative chemotherapy or radiotherapy between the years 1986 and 1997 at The University of Texas M.D. Anderson Cancer Center.
Immunohistochemistry
Human esophageal cancer tissue specimens and tumor xenografts from the nude mice were resected and subjected to tissue processing, these samples were embedded in paraffin and 4-μmol/L-thick sections were prepared for immunohistochemical analyses of Ki67, phosphorylated Erk1/2, and COX-2 expression. Briefly, the sections were de-paraffinized twice in xylene for 10 min each and rehydrated in a series of ethanol (100%-50%) and were then subjected to antigen retrieval by cooking in a pressure cooker with 0.01 mol/L citric buffer for 10 min and H2O2 treatment to eliminate endogenous tissue peroxidase activity. The tissue sections were then incubated with 100 μL of 20% normal horse or goat serum in PBS and anti-Ki67 (1:50), phosphorylated Erk1/2 (1:50), or COX-2 (1:50) diluted in PBS overnight. The next day, the sections were washed with PBS three times and once with PBS containing 0.1% Tween 20 and further incubated with a second antibody (Horse anti-mouse IgG or Goat-anti rabbit IgG from Vector, Laboratories) for 30 min. After washing with PBS, the sections were then incubated with ABC solution (Vector) in the dark for 30 min and 9-ethylcarbazol-3-amine buffer for 15 min for color development. The sections were counterstained with hematoxylin for 30 s, covered with a cover slip and then reviewed and scored under a microscope as positive or negative staining (10% or more tumor cells with positive staining were counted as positive staining).
RESULTS
Reduction of tumor cell viability by curcumin, EGCG, lovastatin and their combinations
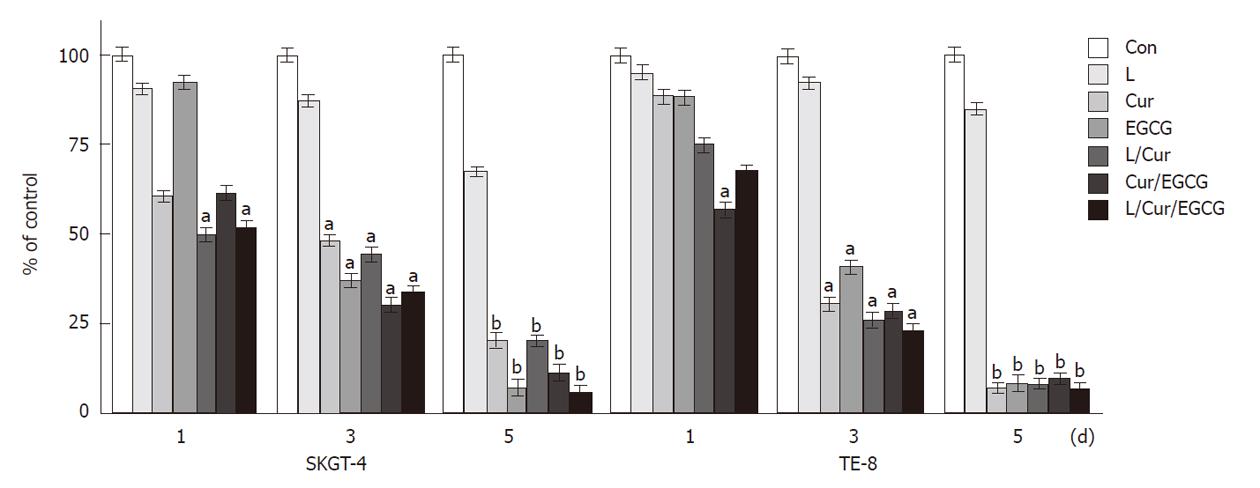
We first determined the effects of curcumin, EGCG and lovastatin individually and their combinations on the suppression of esophageal cancer cell growth by treating esophageal cancer TE-8 and SKGT-4 cell lines with two different doses (curcumin at 20 and 40 μmol/L, EGCG at 20 and 40 μmol/L, lovastatin at 2 and 4 μmol/L and these individual drug doses were used for combination treatments) for up to 5 d. The doses selected were based on previous studies[28-37]. Our data showed that lovastatin (4 μmol/L), curcumin (40 μmol/L), EGCG (40 μmol/L) and their combinations significantly reduced tumor cell viability (Figure 1).
Figure 1 Suppression of esophageal cancer cell growth by curcumin, (-)-epigallocatechin-3-gallate, lovastatin and their combinations.
Esophageal cancer SKGT-4 and TE-8 cells were grown in monolayer overnight and then treated with or without curcumin (Cur) (40 μmol/L), (-)-epigallocatechin-3-gallate (EGCG) (40 μmol/L), lovastatin (L) (4 μmol/L) and their combinations for up to 5 d. Methyl thiazolyl tetrazolium assays were then carried out to detect changes in cell viability (see Methods section). The experiments were repeated three times and the results are summarized as a % of the control (Con) (mean ± SD) and analyzed statistically using the Student’s t test. aP < 0.05, bP < 0.01.
Suppression of tumor cell invasion by curcumin, EGCG, lovastatin and their combinations
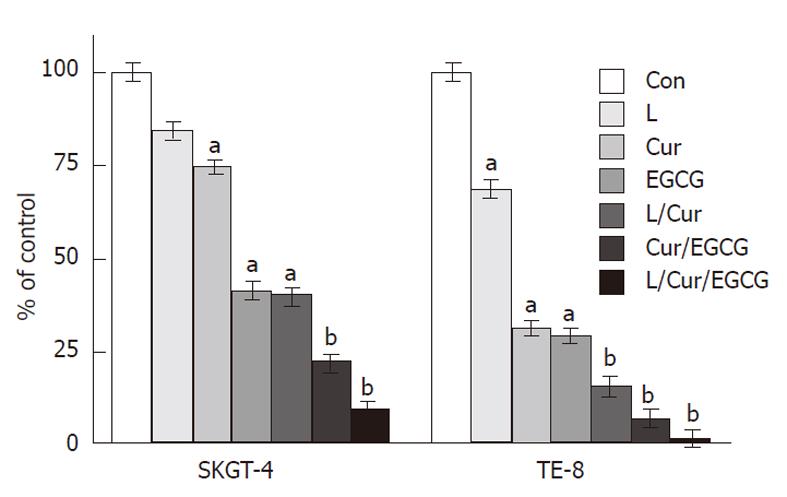
We next determined the effects of these three drugs on the regulation of esophageal cancer cell invasion capacity and found that lovastatin (4 μmol/L), curcumin (40 μmol/L), EGCG (40 μmol/L), and their combinations significantly reduced tumor cell invasion (Figure 2).
Figure 2 Suppression of tumor cell invasion by curcumin, (-)-epigallocatechin-3-gallate, lovastatin and their combinations.
Esophageal cancer SKGT-4 and TE-8 cells were grown and treated with or without curcumin (Cur) (40 μmol/L), (-)-epigallocatechin-3-gallate (EGCG) (40 μmol/L), lovastatin (L) (4 μmol/L) and their combinations in monolayer for 3 d and then subjected to cell invasion assays in Boyden chambers containing Matrigel for 48 h. The invasive cells were stained with 1% crystal violet solution, counted and the results are summarized as a % of the control (Con) (mean ± SD). The data were then analyzed statistically using the Student’s t test. aP < 0.05, bP < 0.01.
Modulation of gene expression by curcumin, EGCG, lovastatin and their combinations
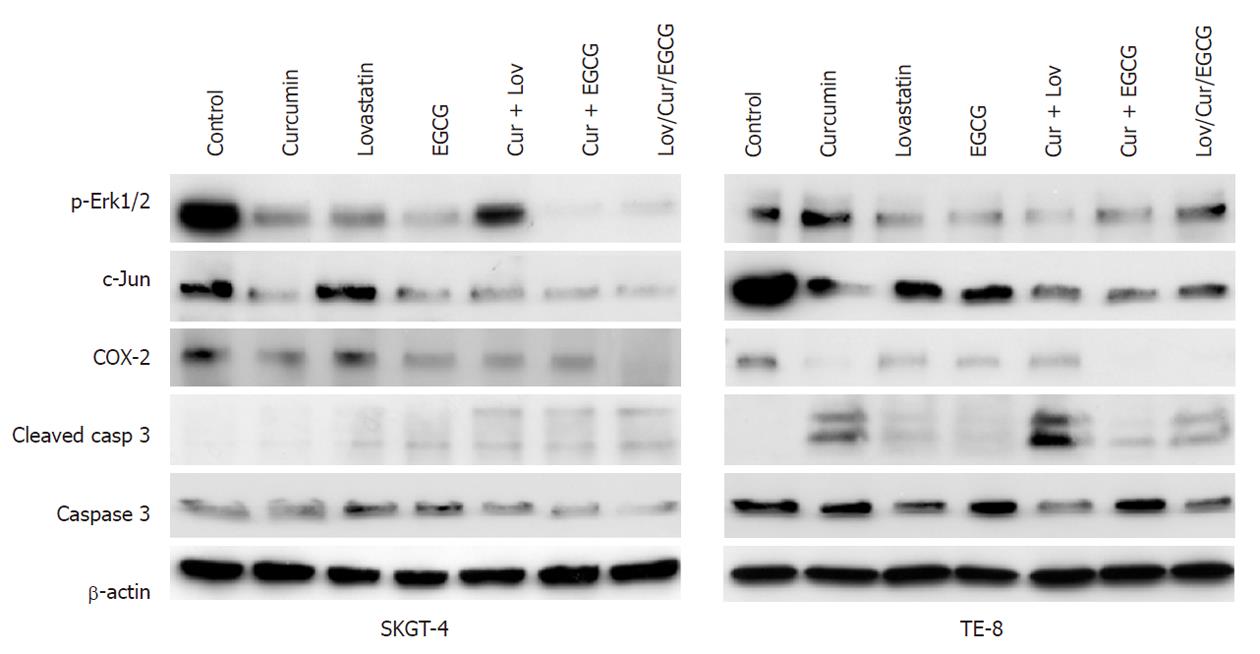
We then assessed the regulation of gene expression by these three drugs and found that lovastatin (4 μmol/L), curcumin (40 μmol/L), EGCG (40 μmol/L) and their combinations downregulated the expression of p-Erk1/2, c-Jun and COX-2, but upregulated activated caspase 3 expression in esophageal cancer SKGT-4 and TE-8 cells (Figure 3).
Figure 3 Modulation of gene expression by curcumin, (-)-epigallocatechin-3-gallate, lovastatin and their combinations.
Esophageal cancer SKGT-4 and TE-8 cells were grown in monolayer overnight and treated with or without curcumin (Cur) (40 μmol/L), (-)-epigallocatechin-3-gallate (EGCG) (40 μmol/L), lovastatin (Lov) (4 μmol/L) and their combinations for 2 d and total cellular protein was extracted from the cells and subjected to Western blotting analysis of gene expression. Erk1/2: Extracellular-signal-regulated kinases; COX-2: Cyclooxygenase-2.
Suppression of tumor growth in nude mouse xenografts by curcumin, EGCG, lovastatin and their combinations
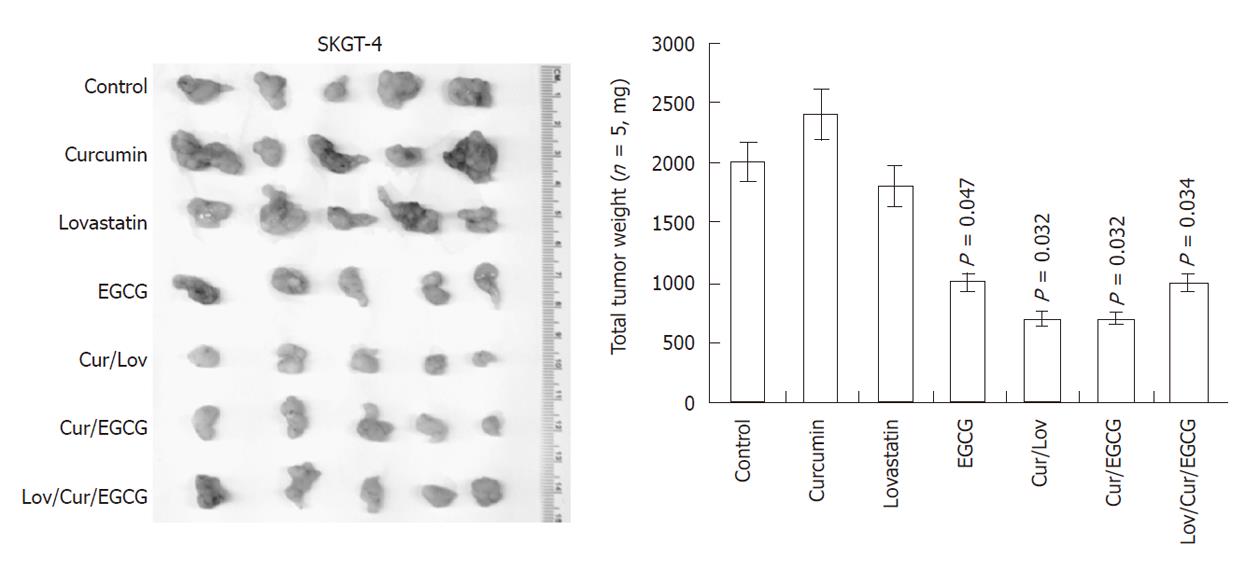
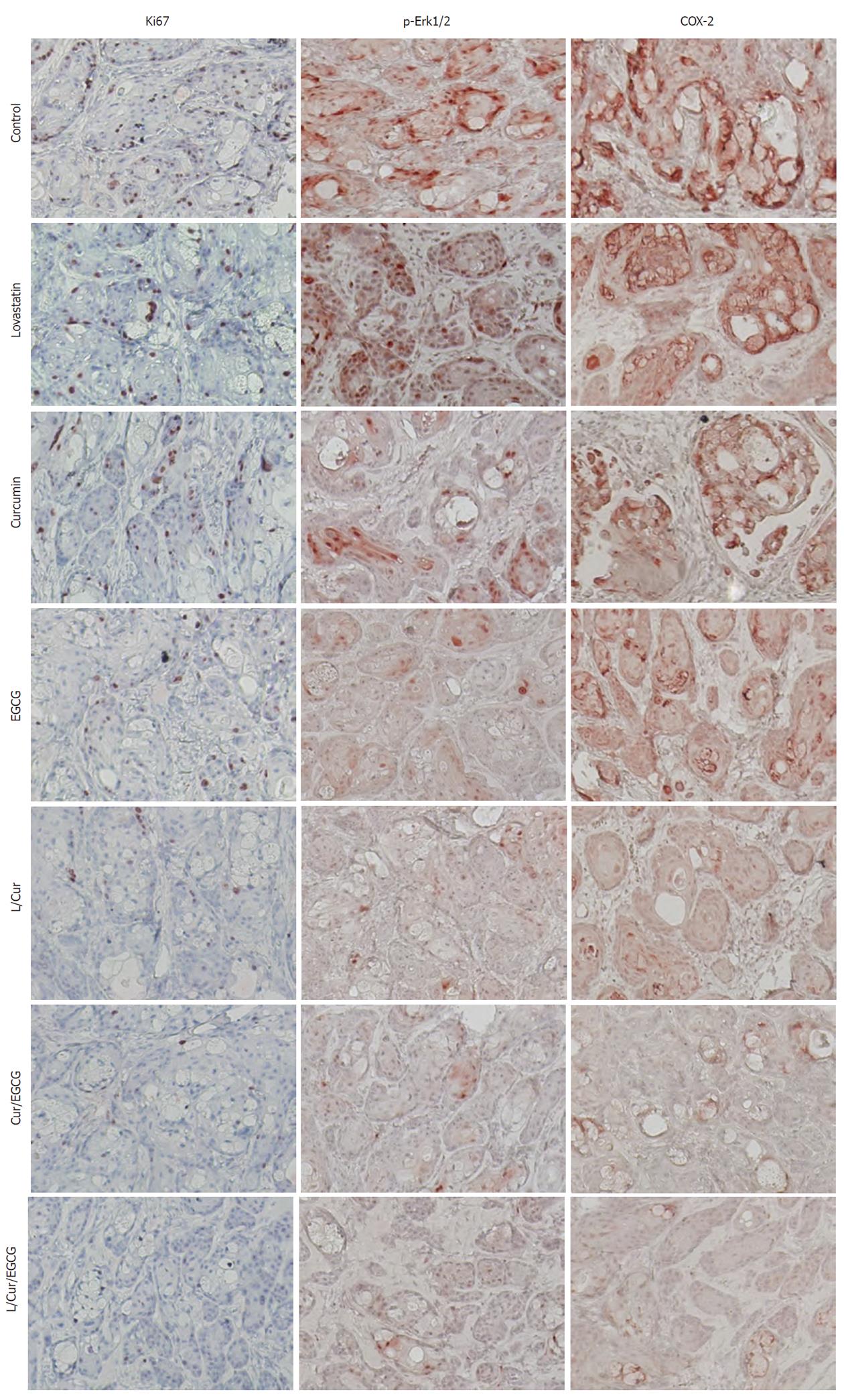
We then performed nude mouse xenograft assays of SKGT-8 cells to determine the effects of the drugs individually and their combinations. We found that lovastatin (50 μg/kg per day), curcumin (50 μg/kg per day), EGCG (50 μg/kg per day) and their combinations at the same doses inhibited tumor growth differently (Figure 4). Treatment with a single drug such as curcumin or lovastatin did not have any effects on tumor formation and growth (Figure 4), although these drugs individually and in combination inhibited expression of Ki67, p-Erk1/2 and COX-2 expression in xenograft tissues (Figure 5).
Figure 4 Inhibition of esophageal cancer cell growth by curcumin, (-)-epigallocatechin-3-gallate, lovastatin and their combinations in nude mouse xenografts.
Esophageal adenocarcinoma SKGT-4 cells were inoculated subcutaneously into nude mice (5 per group). Two days before tumor cell injection, the mice started treatment with or without curcumin (Cur) (50 μg/kg per day), (-)-epigallocatechin-3-gallate (EGCG) (50 μg/kg per day), lovastatin (Lov) (50 μg/kg per day) and their combinations (the same doses as given individually) for 30 d (5 d/wk by oral gavage). At the end of the experiments, the xenograft tumor mass was isolated and weighed and summarized.
Figure 5 Reduced expression of Ki67, phosphorylated extracellular-signal-regulated kinases and cyclooxygenase-2 in xenografts following treatment of mice with or without curcumin, (-)-epigallocatechin-3-gallate, lovastatin and their combinations.
Tumor cell xenografts obtained from the nude mouse experiments were processed and subjected to immunohistochemical analyses of Ki67, phosphorylated extracellular-signal-regulated kinases (Erk1/2) and cyclooxygenase-2 (COX-2) expression. Representative images were obtained in each treatment group. EGCG: (-)-epigallocatechin-3-gallate; L: Lovastatin; Cur: Curcumin.
Expression of phosphorylated Erk1/2 and COX-2 in esophageal cancer tissue specimens
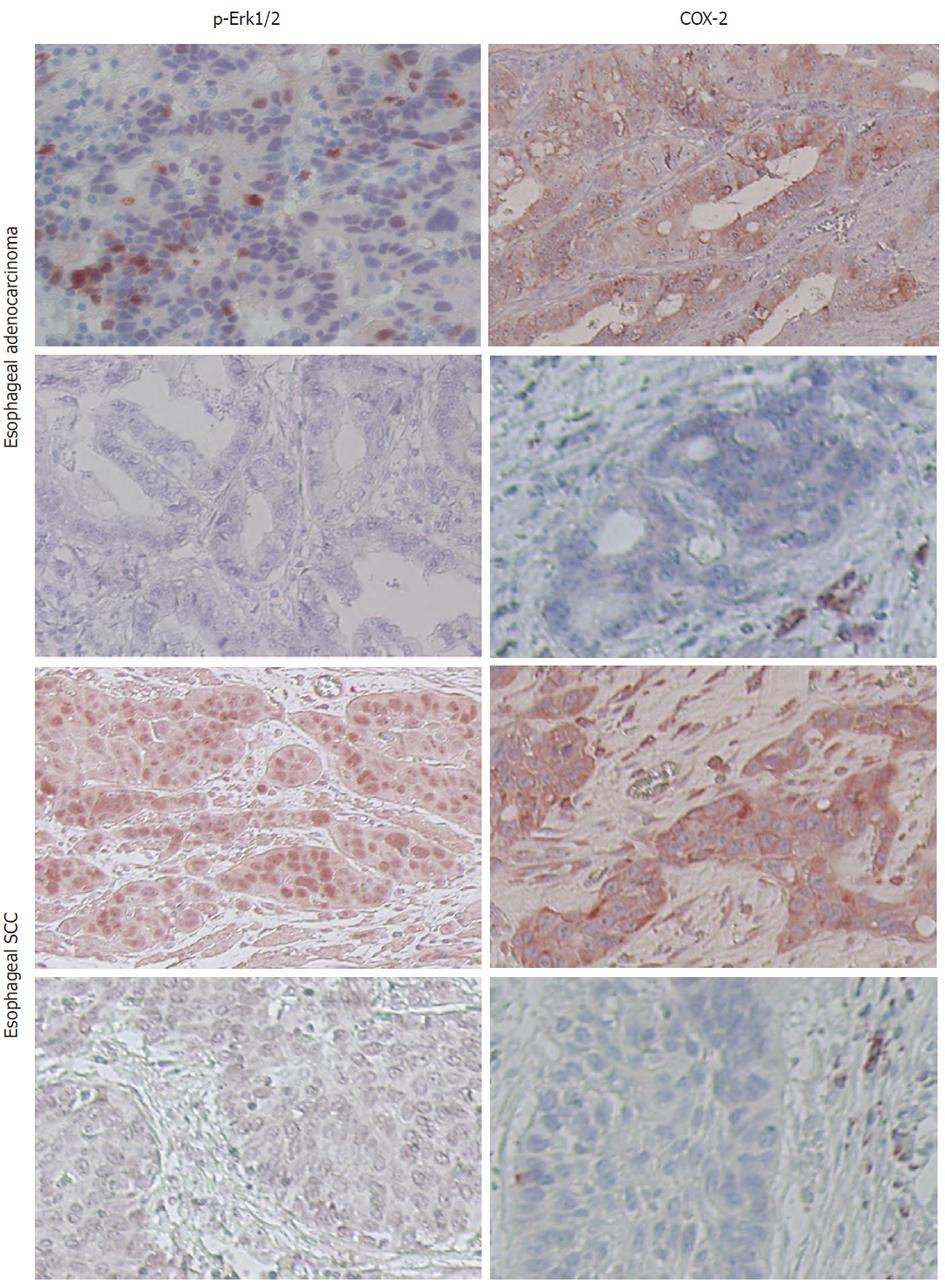
To determine the relevance of p-Erk1/2 and COX-2 expression in human esophageal cancer, we analyzed their expression in esophageal cancer tissue specimens using immunohistochemistry. We found that 77 of 156 (49.4%) tumors expressed phosphorylated Erk1/2 and that 121 of 156 (77.6%) esophageal cancers expressed COX-2 protein. In particular, phosphorylated Erk1/2 was expressed in 23 of 50 (46%) esophageal squamous cell carcinoma (SCC) and in 54 of 106 (50.9%) adenocarcinoma, while COX-2 was expressed in 39 of 50 (78%) esophageal SCC and in 82 of 106 (77.4%) esophageal adenocarcinoma (Figure 6).
Figure 6 Expression of phosphorylated extracellular-signal-regulated kinases and cyclooxygenase-2 in esophageal cancer specimens.
Paraffin sections of esophageal cancer tissues were immunostained with anti-phosphorylated extracellular-signal-regulated kinases (Erk1/2) or cyclooxygenase-2 (COX-2) antibody. Representative images were obtained from these tissue sections. SCC: Squamous cell carcinoma.
DISCUSSION
In the current study, we demonstrated that curcumin, EGCG, lovastatin, and their combinations can significantly reduce the viability and invasion capacity of esophageal cancer cells in vitro. Nevertheless, they were much less effective in vivo in nude mouse xenografts, especially curcumin and lovastatin individually. At the molecular level, these three agents individually or in combination inhibited the expression of phosphorylated Erk1/2, c-Jun, and COX-2 and induced caspase 3 expression in esophageal cancer cells in vitro. In nude mouse xenografts, the expression of p-Erk1/2 and COX-2 was downregulated by these three drugs, especially their combinations. We also analyzed the expression of phosphorylated Erk1/2 and COX-2 in tissue specimens from esophageal cancer patients. The data showed that 49.4% of esophageal cancers expressed phosphorylated Erk1/2 and that 77.6% of cancers expressed COX-2 protein. These data suggest that curcumin, EGCG, and lovastatin inhibit esophageal cancer cell growth in vitro and in nude mouse xenografts possibly through the suppression of phosphorylated Erk1/2, c-Jun and COX-2 expression.
Previous studies have shown the chemopreventive activity of EGCG in suppressing carcinogenesis in several organs, including the esophagus[29,34]. Molecularly, EGCG can suppress the mitotic signal transduction pathway, e.g., inhibit Erk1/2 phosphorylation and anti-AP-1 activity[38]. A recent study demonstrated that EGCG induced a concentration- and time-dependent reversal of hypermethylation of RAR-β2 in esophageal cancer cell lines, resulting in re-expression of RAR-β2[33]. Furthermore, curcumin has been shown to inhibit different cancers at the initiation, promotion, and progression stages in animal models[31,32,38]. Curcumin also suppressed growth and induced apoptosis in numerous types of cancer cells in vitro[38,39]. Although the defined mechanisms of its action require further study, its efficacy appears to be related to the induction of glutathione and glutathione-S-transferase activity, inhibition of lipid peroxidation and arachidonic acid metabolism, and suppression of oxidative DNA adduct formation[32,38,39]. Curcumin can inhibit the activation of NF-κB and the expression of c-Jun, c-Fos, c-Myc, Erk1/2, COX-2, PI3K, Akt, CDKs, and iNOS[31,35,38,39]. Curcumin was also able to suppress cigarette smoke-induced NF-κB activation and COX-2 expression in head and neck SCC and non-small-cell lung cancer cells[31,32]. In esophageal cancer, dietary curcumin can inhibit chemically-induced esophageal carcinogenesis in mice and rats[28,40]. In addition, the statin family of drugs has shown cancer chemopreventive effects[41]. Statins can trigger some tumor cells to undergo apoptosis in vitro and suppress tumor growth in vivo[30,37,41]. Statins also have an antimetastatic property, which is evident in their suppression of tumor cells invasiveness in Matrigel, as well as in animal experiments[42]. In addition, statins, especially at high concentrations, can inhibit capillary tube formation by endothelial cells in vitro and in vivo[33,44]. The effects of statins are thought to be mediated through inhibition of Ras and RhoA activity[41]. Based on these previous studies and reports, we determined the effects of their combinations on suppression of esophageal cancer cell growth in vitro and in nude mouse xenografts by targeting the RAR-β2/Erk1/2/AP1/COX-2 pathway[3]. Indeed, our current study has demonstrated the effects of their combinations in vitro. Molecularly, these three agents were able to regulate the expression of this gene pathway in vitro and in vivo in nude mice. Nevertheless, individually curcumin and lovastatin had no effect on tumor formation and growth in nude mice, even when the highest dose possible was used. This may be due to the bioavailability of curcumin and the induction of COX-2 expression by high dose lovastatin, reported previously[30,39]. However, the current study did not show the induction of COX-2 expression by high dose lovastatin in esophageal cancer in vitro and in nude mice, similar to that seen in prostate cancer[30].
However, there are some limitations in the current study. Firstly, we showed that these three drugs regulated gene expression of the RAR-β2/Erk1/2/AP1/COX-2 pathway, however, previous studies also showed that as chemoprevention agents, these drugs target multiple genes and their pathways in different cancers. Thus, further study is needed to determine the mechanisms of action of these drugs in human cancers. Furthermore, we used established esophageal cancer cell lines to determine the chemopreventive effects of these agents in this study, the results of which may be quite different in comparison to those in premalignant cells in vivo. The xenograft assay tested the effects of these agents in suppressing tumor initiation and growth, but not tumor development per se, although the xenograft assay did test the bioavailability of these agents in vivo. In addition, we did not test whether the doses of these three agents are clinically achievable, and to reduce costs, we utilized a single dose of each agent and their combinations. Thus, future studies are needed to test these agents in a clinical Phase I trial and in animal experiments where more doses and a time course study will be included.