Published online May 21, 2012. doi: 10.3748/wjg.v18.i19.2434
Revised: June 15, 2011
Accepted: April 12, 2012
Published online: May 21, 2012
Enteropathy-associated T-cell lymphoma (EATL) is a rare peripheral T-cell lymphoma classified into 2 types, with or without celiac disease, based on histology. Type 2 EATL is less commonly associated with celiac disease, in which cells are characterized by being monomorphic and small- to medium-sized. Cells are characterized by CD8 and CD56 expression and c-MYC oncogene locus gain. We present an atypical case of type 2 EATL in the jejunum, with human T-lymphotropic virus-1 that was CD4- CD8+ CD56- CD30- CD25- TIA-1+ and granzyme B+ on immunohistological staining. It also displayed translocation of chromosome 8p24 (c-MYC), as determined by fluorescent in situ hybridization. Mucosal spreading and intraepithelial invasion by lymphoma with villous atrophy were detected adjacent to the mucosal layer. The lymphoma may be derived from intraepithelial CD8+ T cells, similar to celiac disease.
- Citation: Okumura K, Ikebe M, Shimokama T, Takeshita M, Kinjo N, Sugimachi K, Higashi H. An unusual enteropathy-associated T-cell lymphoma with MYC translocation arising in a Japanese patient: A case report. World J Gastroenterol 2012; 18(19): 2434-2437
- URL: https://www.wjgnet.com/1007-9327/full/v18/i19/2434.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i19.2434
Gastrointestinal lymphomas account for 4%-20% of all non-Hodgkin’s lymphomas. Enteropathy-associated T-cell lymphoma (EATL) is a primary extranodal T-cell lymphoma arising in the gastrointestinal tract, a rare subtype of peripheral T-cell lymphoma, and accounts for less than 1% of all non-Hodgkin’s lymphomas[1-3]. There are two different sub-classifications: type 1 EATL (with) and type 2 EATL (without) celiac disease[1,4]. The disease is seen with greater frequency in areas with a high prevalence of celiac disease, but it is rare in Japan[5]. Type 2 EATL accounts for 10%-20% of cases, and is less commonly associated with celiac disease[1,4]. The tumor cells are monomorphic and small- to medium-sized, with infiltration of the intestinal crypt epithelium without inflammation[1,4]. Type 2 EATL often occurs without a history of celiac disease and shows strong expression of CD56 (> 90%) and CD8 (80%) based on immunohistochemical staining[1]. EATL generally has a poor prognosis because it is often diagnosed late, has spread, and is therefore refractory to treatment. We report a case with human T-lymphotropic virus-1 (HTLV-1) in which a type 2 EATL was CD8+, CD56-, T cell restricted intracellular antigen-1 (TIA-1)+ on immunohistological staining, and possessed a unique chromosomal change as determined by fluorescent in situ hybridization (FISH).
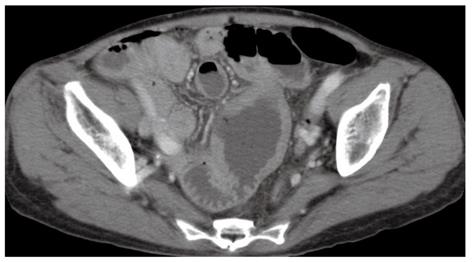
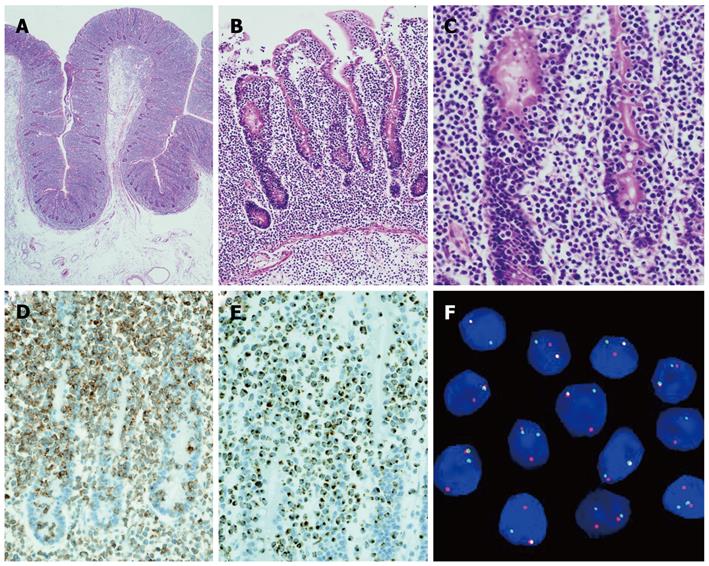
A 66-year-old woman with a past medical history of hypertension presented to the emergency department with a sudden onset of severe abdominal pain. Her weight was 43.5 kg and body mass was 19.7 kg/m2. A physical examination revealed a high fever (39 °C) with chills; diffuse, severe, constant abdominal pain; and muscular guarding. She had experienced abdominal distension for a month, but did not have diarrhea, weight loss, a history of malnutrition, or a food intolerance. We could not detect any palpable peripheral lymph nodes. The laboratory data on admission showed mild leukocytosis (white blood cell count of 10 400/mm3) and hypoproteinemia (total protein 5.4 g/dL). Persistent hypoproteinemia was recognized at 4 mo prior, based on previous medical records. No atypical lymphocytes were detected in the peripheral blood. A computed tomography showed free air and wall thickening in the pseudo-aneurysmally dilated small bowel within the pelvic area, with a defect of the intestinal wall (Figure 1). Laparotomy was performed and showed a pseudo-aneurysmally dilated and perforated jejunum 80 cm from the ligament of Treitz, with massive purulent ascites fluid. We performed a segmental resection of the lesion and a side-to-side anastomosis. The resected jejunum was generally thickened and there was diffuse transmural infiltration by monomorphic and medium-sized atypical lymphoid cells, with villous atrophy of the intestinal glands (Figure 2A). Intramucosal spreading of lymphoma was found in an adjacent mucosal layer, and many atypical intraepithelial lymphocytes were detected (Figure 2B and C). Immunohistochemical staining was positive for CD3, CD7, CD8, TIA-1 granzyme B, c-MYC and Ki-67, and negative for CD4, CD5, CD20, CD25, CD30, CD56, and CCR4 (Figure 2D and E). FISH analysis revealed translocation of chromosome 8q24 (c-MYC) (Figure 2F). Polymerase chain reaction analysis of TCRγ gene rearrangements were performed using the BIOMED-2 procedure[5]. Rearrangement bands were detected in 4 VγIf, Vγ9, Vγ10, Vγ11, and Jγ1.1/2.1, and Jγ1.3/2.3 consensus primers.
Based on these findings, we made a diagnosis of type 2 EATL. On postoperative day 10, the patient was discharged without any complications. After the diagnosis, we performed additional laboratory tests. Serum soluble interleukin-2 receptor was elevated (822 U/mL), and serum HTLV-1 antibodies were positive. 67 Gallium scintigraphy and fluorodeoxyglucose positron-emission tomography did not show any site of involvement. Bone marrow biopsy did not reveal malignant cells. Based on these results, we diagnosed clinical stage 2E. The patient received high-dose chemotherapy with autologous hematopoietic cells 6 mo after surgery.
EATL is a rare subtype of peripheral T-cell lymphoma, with an incidence of 0.25% of all lymphomas in Japan[3,6]. Since gluten allergy is uncommon in Japan, it is extremely rare for celiac disease to be a basal disorder, and type 2 EATL is more common than type 1 EATL[5]. EATL most commonly arises in the proximal jejunum, and typically presents with abdominal pain. It is often associated with intestinal obstruction, perforation, or bleeding, and is diagnosed by histology tests. Type 2 EATL consists of monomorphic and small- to medium-sized tumor cells that frequently express CD8 and CD56[3]. The prognosis of EATL is usually poor based on results of conventional combination chemotherapy, although high-dose chemotherapy with autologous hematopoietic cell transplantation may have longer survival rates[2,7].
The pathological findings of this case showed monomorphic, small- to medium-sized tumor cells with villous atrophy of the mucosa. Combined with the mucosal spreading and increased intraepithelial lymphocytes (IELs), we made a diagnosis of type 2 EATL. Type 2 EATL is usually positive for CD8 and CD56 (> 90%), but this patient was CD56 negative[3]. Several studies have suggested that the Epstein-Barr virus plays an etiological role in EATL pathogenesis, but this is still a subject of debate. In this case, Epstein-Barr encoded RNAs and latent membrane proteins were not expressed, suggesting that Epstein-Barr virus was not involved in tumor proliferation. Test results showed HTLV-1 infection based on serological antibodies, but the tumor cells were different from adult T cell lymphoma/leukemia immunophenotype features with respect to CD4, CD8, CD25, CCR4 and cytotoxic proteins[8]. FISH analysis, however, showed gene translocation at 8q24, which is the c-MYC oncogene locus. It has been suggested that c-MYC protein plays a role in lymphoid proliferation and the apoptotic pathway in malignant lymphomas[9]. In type 2 EATL pathogenesis, about 70% of cases show a gain of 8q24, suggesting c-MYC is an important transcription factor in EATL[4]. We present here the first EATL case with translocation of chromosome 8q24 (c-MYC region).
Some studies suggested chronic inflammation might contribute to the development of neoplastic lymphocyte growth in EATL[4,10]. Celiac disease is characterized by villous atrophy, crypt hyperplasia, and an increased invasion of CD8+ IEL. Celiac disease may progress to type 1 EATL[10], especially in patients with refractory celiac disease, in which IELs have aberrant phenotypes and genetic alterations due to persistent inflammation[4,10]. This is supported by the fact that refractory celiac disease and type 1 EATL rarely show aberrant CD8 expression[4,10]. This case showed protein-losing enteropathy and lymphoma cells with IEL-like features in the mucosa, and villous atrophy. We considered the possibility of persistent enteropathy, which is consistent with hypoproteinemia 4 mo before the surgery. Celiac-like enteropathy, rare in Japan, is consistent with the clinical symptoms, and in this case, persistent enteropathy might cause CD8+ IELs to transform to neoplastic cells via c-MYC. The immunophenotype of this case was different from typical type 2 EATL cases, regarding CD3+ CD4- CD8+ CD30- CD56-.
In summary, we report the unique case of a 66-year-old HTLV-1 woman diagnosed with type 2 EATL, which was CD3+ CD4- CD8+ CD30- CD56- on immunostaining, and displayed translocation of the c-MYC region.
Peer reviewer: Mitsunori Yamakawa, Professor, Department of Pathological Diagnostics, Yamagata University, Faculty of Medicine, 2-2-2 Iida-Nishi, Yamagata 990-9585, Japan
S- Editor Cheng JX L- Editor Rutherford A E- Editor Zhang DN
| 1. | Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC Press 2008; 439. |
| 2. | Gale J, Simmonds PD, Mead GM, Sweetenham JW, Wright DH. Enteropathy-type intestinal T-cell lymphoma: clinical features and treatment of 31 patients in a single center. J Clin Oncol. 2000;18:795-803. [PubMed] |
| 3. | Zettl A, deLeeuw R, Haralambieva E, Mueller-Hermelink HK. Enteropathy-type T-cell lymphoma. Am J Clin Pathol. 2007;127:701-706. [PubMed] |
| 4. | Deleeuw RJ, Zettl A, Klinker E, Haralambieva E, Trottier M, Chari R, Ge Y, Gascoyne RD, Chott A, Müller-Hermelink HK. Whole-genome analysis and HLA genotyping of enteropathy-type T-cell lymphoma reveals 2 distinct lymphoma subtypes. Gastroenterology. 2007;132:1902-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Takeshita M, Nakamura S, Kikuma K, Nakayama Y, Nimura S, Yao T, Urabe S, Ogawara S, Yonemasu H, Matsushita Y. Pathological and immunohistological findings and genetic aberrations of intestinal enteropathy-associated T cell lymphoma in Japan. Histopathology. 2011;58:395-407. [PubMed] |
| 6. | Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer. 2003;97:2462-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 199] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Sieniawski M, Angamuthu N, Boyd K, Chasty R, Davies J, Forsyth P, Jack F, Lyons S, Mounter P, Revell P. Evaluation of enteropathy-associated T-cell lymphoma comparing standard therapies with a novel regimen including autologous stem cell transplantation. Blood. 2010;115:3664-3670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | Tsukasaki K, Hermine O, Bazarbachi A, Ratner L, Ramos JC, Harrington W, O'Mahony D, Janik JE, Bittencourt AL, Taylor GP. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 409] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 9. | Knudsen A. The influence of the reserve albumin concentration and pH on the cephalocaudal progression of jaundice in newborns. Early Hum Dev. 1991;25:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | de Mascarel A, Belleannée G, Stanislas S, Merlio C, Parrens M, Laharie D, Dubus P, Merlio JP. Mucosal intraepithelial T-lymphocytes in refractory celiac disease: a neoplastic population with a variable CD8 phenotype. Am J Surg Pathol. 2008;32:744-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |