Published online May 21, 2012. doi: 10.3748/wjg.v18.i19.2402
Revised: February 20, 2012
Accepted: March 9, 2012
Published online: May 21, 2012
AIM: To study the risk factors for liver metastasis and the prognosis in patients with human epidermal growth factor receptor 2 (HER2) over-expressing gastric cancer (GC).
METHODS: A total of 84 GC patients recruited from the General Hospital of the People’s Liberation Army (PLA) between 2003 and 2010 were randomly enrolled in this study. HER2 expression was detected by immunohistochemistry in 84 GC patients with liver metastases. The study group consisted of 66 men and 18 women, with an average age of 54 years (range: 19-74 years). Liver metastasis was diagnosed by magnetic resonance imaging or computed tomography. Patients were followed-up and predictive factors of liver metastasis were evaluated.
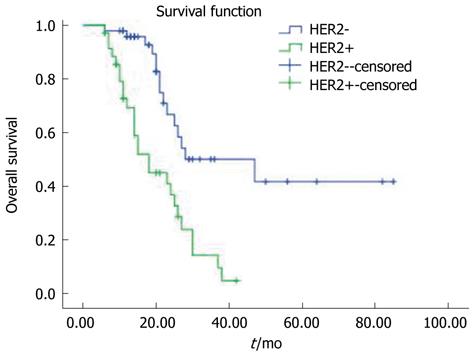
RESULTS: The median follow-up period was 47 mo (range: 6-85 mo). The characteristics of 35 (25.7%) patients with HER2 over-expression of liver metastatic GC are presented. HER2 over-expression was detected in 23 out of 49 (46.9%) patients with intestinal GC, and 9 out of 35 (25.7%) patients with diffuse GC. 29 out of 59 (49.2%) patients aged < 60 years were HER2-positive, while 8 out of 25 (32%) patients aged ≥ 60 were HER2-positive; a significant difference (P < 0.05). Univariate analysis (log-rank test) showed that HER2 over-expression, sex, Lauren classification, differentiation and disease-free interval were correlated with poor survival (P < 0.05). Survival analysis with a survival curve showed that HER2 over-expression was significantly relevant, with a reduced survival time in GC patients with liver metastases (P < 0.01). 2-year survival was not associated with the patient’s age. A disease-free survival longer than 12 mo has a significant association with extended overall survival (OS) in GC patients with liver metastases. The median survival time after the diagnosis of liver metastases was 18 mo [95% confidence interval (CI): 9.07-26.94] among HER2 positive GC patients with liver metastases. In comparison, for 49 (69.4%) out of 84 HER2 negative patients with liver metastatic GC, the median survival time was 47 mo (95% CI: 19.37-74.63). In patients with HER2 positive liver metastatic GC, the median OS was significantly shorter than in HER2 negative patients (median, 20.32 mo; 95% CI: 16.51-24.13 vs median, 50.14 mo; 95% CI: 37.83-62.45; P < 0.01).
CONCLUSION: HER2 over-expressing GC patients with liver metastases have a poor prognosis. Overall survival was significantly lower in HER2 positive patients. HER2-overexpression is correlated with a lower survival rate.
- Citation: Dang HZ, Yu Y, Jiao SC. Prognosis of HER2 over-expressing gastric cancer patients with liver metastasis. World J Gastroenterol 2012; 18(19): 2402-2407
- URL: https://www.wjgnet.com/1007-9327/full/v18/i19/2402.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i19.2402
Although the incidence of gastric cancer (GC) has slightly declined in the past several decades, it remains the second leading cause of cancer death worldwide, especially in Asia[1]. GC with human epidermal growth factor receptor 2 (HER2) over-expression accounts for 9%-38% of GC, and HER2 is mainly expressed on the cell membrane. Yan et al[2] studied HER2/neu protein expression and gene amplification in gastric carcinoma and their relationship. They recommended that all samples with immunohistochemistry (IHC) as HER2 expression should be analyzed with fluorescence in situ hybridization and found that the detection of HER2 gene amplification can assess the malignant biological behaviors and prognosis of gastric cancer. Zhang et al[3] found HER2 over-expression in 18.6% of GC and HER2 located mostly on the membrane, which was consistent with what was previously reported. Yu et al[4] examined the expressions of Grb2 and HER2 in normal gastric mucosa, primary gastric cancer, and lymph node metastatic foci in 1143 GC patients by using tissue microarray assay, which suggested the importance of Grb2 and HER2 in GC development and progression. Matsubara et al[5] investigated 86 patients who received first-line chemotherapy for advanced GC. Their results showed that patients with high HER2 expression had a longer survival time than those with low HER2 expression. However, there is controversy with regard to the hypothesis that HER2 expression serves as a factor in predicting the prognosis of GC patients[6-10]. In a prospective study of 63 patients with resectable CG (in which follow-up was performed for 40.7 mo), membranous epidermal growth factor receptor (EGFR) was detected by random and double blind assays, and cytosolic HER2 by immunoenzymatic assay. Results showed that high expression of EGFR and HER2 in cancers were associated with a poor outcome in patients with resectable GC[11].
Detection of micrometastatic foci may allow for a more accurate assessment of the prognosis, along with aiding in the selection of candidates for intensive chemotherapy among GC patients. Bone marrow micrometastasis has been shown to influence the prognosis of GC patients[12,13]. Matsunami et al[14] used ion mobility spectrometry to improve detection sensitivity in their study, and their results showed that only 30% of patients showed the development of bone marrow micrometastasis. The positive rate was lower than that in Western patients, but similar to those of the Japanese[15]. In the present study, we investigated the prognosis of GC patients with HER2 over-expression in the liver metastatic foci in Chinese patients.
A total of 84 GC patients recruited from the General Hospital of the People’s Liberation Army (PLA) between 2003 and 2010 were randomly enrolled in this study. All patients underwent radical dissection and chemotherapy, and/or radiotherapy. The study group consisted of 66 men and 18 women, with an average age of 54 years (range: 19-74 years). GC tissue and adjacent normal tissue were collected for pathologic examination. The detailed pathologic results were obtained from the Department of Pathology of the General Hospital of the PLA.
The histologic type of GC was defined according to Lauren’s classification. This classification divides GC into intestinal (in which well-formed tubules were found) and diffuse (in which diffuse tumor infiltration and signet ring cells were noted, but well-formed tubules were absent) types. In our study, 53 patients were pathologically diagnosed with intestinal GC and 31 with diffuse GC.
Patients were followed-up for 6-85 mo. Informed consent was obtained before the study, and the protocol was approved by the Clinical Research Ethics Committee of the General Hospital of the PLA.
Formalin-fixed and paraffin-embedded sections (5 μm) were deparaffinized in xylene, rehydrated through an ethanol series, and treated for 10 min in a microwave oven at 98 °C for antigen retrieval. Sections were then blocked in 3% hydrogen peroxidase, followed by incubation with a protein-blocking agent. Sections were treated with fetal bovine serum (10%), with or without the HER2 antibody (1:100), for 30 min at 37 °C then counterstained with hematoxylin and mounted. Omission of the primary antibody was used as a negative control for tissue sections. Anti-HER2 monoclonal antibody (Dako Herceptin Test kit; Dako, Glostrup, Denmark), and Dako EnVisionTM Kit (Dakocytomation, Dako) were used for IHC. The sections were examined microscopically and interpreted in a blinded fashion by two pathologists. HER2 protein expression on the cell membrane was scored according to the following criteria: 0: No staining or < 10% of tumor cells; 1+: Faint/barely perceptible partial staining in > 10% of tumor cells; 2+: Weak to moderate staining of the entire membrane or cytoplasm in > 10% of tumor cells; 3+: Strong staining in > 10% of tumor cells. Tumors with scores of 0 and 1+ were evaluated as negative while those with scores 2+ and 3+ were positive.
A total of 35 GC patients with HER2-positive liver metastases were included in this study. Liver metastatic GC was pathologically diagnosed by pathologists at the National Comprehensive Cancer Network. Patients with clinical symptoms suggesting liver metastasis were diagnosed by abdominal magnetic resonance imaging (MRI) or computed tomography (CT) clinical information was collected from medical records, including the date of initial diagnosis of GC, the development of liver metastases, the date of chemotherapy, and death or the last follow-up, as well as disease sites at the start of chemotherapy and the details of treatment. We also evaluated the clinical response of other metastatic diseases according to the Response Evaluation Criteria in Solid Tumors at the time of diagnosis of liver metastasis. The baseline characteristics and prognostic factors for GC were reviewed, including age, sex, differentiation and GC type, and disease-free interval. Overall survival (OS) served as a main outcome.
SPSS version 17.0 software package (SPSS, Chicago, IL, United States) was used for statistical analysis. Frequency comparisons of HER2 expression status and clinicopathologic variables were performed with the F test (two-sided). Log-rank test was used for survival analysis. Survival curves were computed according to the Kaplan-Meier method. A value of P < 0.05 was considered statistically significant.
The median follow-up period was 47 mo (range: 6-85 mo). The characteristics of 35 (25.7%) patients with HER2 over-expression in liver metastatic GC are presented in Table 1. The results showed no significant differences between the sexes, although significant associations were observed between intestinal and diffuse (P < 0.05) types of GC. Over-expression of HER2 was observed as being higher for patients younger than 60 (P < 0.05).
| Parameter | Patient (n) | HER2 + patients (n) | Ratio (%) | Pvalue |
| Age (yr) | ||||
| < 60 | 59 | 29 | 49.2 | < 0.05 |
| ≥ 60 | 25 | 8 | 32 | |
| Gender | ||||
| Male | 66 | 28 | 42.4 | > 0.05 |
| Female | 18 | 7 | 38.9 | |
| Tumor type | ||||
| Intestinal | 49 | 23 | 46.9 | < 0.05 |
| Diffuse | 35 | 9 | 25.7 |
HER2 over-expression was detected in 23 out of 49 (46.9%) patients with intestinal GC and 9 out of 35 (25.7%) patients with diffuse GC. The proportion of patients with HER2 over-expression was higher in patients with GC of the intestinal-type than in those with GC of the diffuse-type (P < 0.05). In addition, 29 out of 59 (49.2%) patients aged < 60 years were HER2-positive, while 8 out of 25 (32%) patients aged ≥ 60 were HER2-positive, which was a significant difference (P < 0.05). However, there was no significant difference with regards to gender for HER2 over-expression (Table 1).
Univariate analysis (log-rank test) showed that HER2 over-expression, sex, Lauren classification, differentiation and disease-free interval were correlated with poor survival (P < 0.05), while age was not correlated with 2-year survival (P > 0.05) (Table 2). Survival analysis with a survival curve (Figure 1) showed that HER2 over-expression was significantly relevant with decreased survival time in GC patients with liver metastases (P < 0.01).
| Parameter | Patient (n) | 2-yr survival (%) | Pvalue |
| Age (yr) | > 0.05 | ||
| < 60 | 56 | 66.1 | |
| ≥ 60 | 28 | 60.7 | |
| Gender | < 0.05 | ||
| Male | 66 | 78.6 | |
| Female | 18 | 21.4 | |
| HER2 over-expression | < 0.05 | ||
| Absent | 49 | 58.3 | |
| Present | 35 | 41.7 | |
| Lauren classification | < 0.05 | ||
| Intestinal | 49 | 58.3 | |
| Diffuse | 35 | 41.7 | |
| Differentiation | < 0.05 | ||
| High/middle | 4 | 80 | |
| Low/null | 50 | 63.3 | |
| Disease-free interval (n = 84) | < 0.05 | ||
| ≥ 12 mo | 63 | 7 | |
| < 12 mo | 19 | 22.6 |
2-year survival was not associated with a patient’s age. A disease-free survival (DFS) longer than 12 mo had significant association with an extended OS in GC patients with liver metastasis (Table 2).
The median survival time after diagnosis of liver metastases was 18 mo [95% confidence interval (CI): 9.07-26.94] among those HER2 positive with liver metastases in GC. In comparison, for 49 (69.4%) out of 84 HER2 negative patients with liver metastatic GC, the median survival time was 47 mo (95% CI: 19.37-74.63).
In patients with HER2 positive liver metastatic GC, the median OS was significantly shorter than in HER2 negative patients (median, 20.32 mo; 95% CI: 16.51-24.13 mo vs median, 50.14 mo; 95% CI: 37.83-62.45 mo; P < 0.01).
The HER2, an important member of the HER family, is encoded by a gene located on chromosome 17q21. During tumorigenesis, HER2 has been identified to act as an oncogene to modulate the proliferation, invasion and apoptosis of tumor cells. In cancer formation, HER2 acts as an oncogene to regulate the proliferation, invasion, and apoptosis of cancer cells. HER2 over-expression was reported in 9%-38% of GC, and was mainly found on the cell membrane[16]. The concordance between protein and mRNA over-expression of HER2 was recently elucidated in tumorigenesis, especially in cancers scored 3+ by IHC[17].
In approximately 25.7% of invasive GC, the HER2 tyrosine kinase receptor is over-expressed. HER2 consists of four different receptors and is associated with cell proliferation, differentiation, and survival. The HER2 over-expressing GC is more aggressive and has a poor prognosis. With respect to the importance of HER2 heterodimer in tumorigenesis, the signaling pathways and downstream effectors of the HER family have become key molecules in exploring strategies for anti-cancer therapy. For example, herceptin, a humanized monoclonal antibody targeting HER2/neu, has been used as a first-line anti-cancer drug in the treatment of breast cancer over-expressing HER2/neu[18,19]. Evidence from preclinical trials also indicated that herceptin could benefit GC patients[20,21].
To date, a high incidence of liver metastases has been observed in patients with HER2 over-expressing GC, which may be attributed to the biological characteristics of GC and the treatments for GC. A retrospective analysis has identified HER2 as a risk factor for the development of liver metastases and relapse. The 5-year incidence of liver metastases is significantly higher in patients with HER2 over-expressing GC. In particular, improvements in the treatment of systemic diseases have enabled patients with HER2 over-expressing metastatic GC to survive for a relatively long period of time. MRI and CT have been routinely used for the diagnosis of liver metastases in these patients and found that the incidence of liver metastases is increasing. However, the association between liver metastases and HER2 expression remains largely unclear.
The results of the present study demonstrated that 25.7% of GC patients with liver metastases had HER2 over-expression, and that HER2 was mostly expressed in the membrane, which is consistent with what has been previously reported. The present study aimed to investigate the predictive factors in GC patients with HER2 over-expressing liver metastases.
GC of the two histologic types (intestinal and diffuse) differs in their epidemiology, pathogenesis, clinical outcome, and even genetic profiling[22]. The study showed tumors of two Puerto Rican (PR) patients with overexpressed Her2/neu and the resulting partial clinical responses motivated us to compare Her2/neu expression in PR (n = 101) and Caucasian non-Hispanic (n = 95) patients. The immunohistochemistry of tumors showed overexpression of P-Stat3, Cyclin D1, and Her2/neu, compared to non-neoplastic mucosa. Her2/neu and EGF-R protein levels were statistically significantly different, with higher levels of both proteins in the PR group. Importantly, Her2/neu expression was strong and diffuse in tumors with signet-ring morphology, while other histo-pathological subtypes showed higher intra-tumoral Her2/neu heterogeneity than typically observed in breast cancer. Targeted therapies in gastric cancer directed at EGF-R and Hers-2/neu pathways warrant further investigation. These therapies may be especially effective in PR patients and in patients with signet-ring cell morphologies with a dismal prognosis[23]. The study showed that overexpression of HIF-1a had no association with clinicopathological status, patient prognosis, or chemosensitivity; the expression of HIF-1a mRNA is up-regulated by a signal transduction pathway from a tyrosine kinase receptor, such as HER2[24]. Our results showed HER2 positivity differed significantly by histologic subtype (intestinal: 46.9%; diffuse: 25.7%). The mechanisms of HER2 over-expression in intestinal-type GC are complex and still largely unclear. Depending on the depth of invasion (T), the involvement of lymph nodes (N), and the presence of distant metastasis (M), the TNM stage is the most important prognostic factor for GC in clinic practice[25]. The role of HER2 over-expression as a prognostic factor in GC is still controversial[26,27]. Recently, increasing evidence has showed a direct correlation between HER2 over-expression and poor survival[28]. In a series of 260 GC patients, Okegawa et al[29] found that HER2 over-expression was an independent factor and correlated with serosal invasion and lymph node metastases. Our results demonstrated that HER2 over-expression was closely associated with a lower survival rate in GC patients with liver metastases (P < 0.01). HER2 may become a novel molecule in tumorigenesis of GC and a potential candidate for molecule-targeted therapy, especially in diffuse-type GC.
In our study, liver metastases in GC patients with HER2-overexpression had a shorter DFS (75% vs 22.6%, P < 0.05), which was consistent with what had previously been reported[30]. Tumor type was significantly associated with liver metastases (intestinal 46.9% vs diffuse 25.7%, P < 0.05), but age was not associated with GC. The association between liver and other location metastases suggests that high tumor load contributes to the development of hematogenous metastases, and supports the results in the present study that GC in half of patients was refractory to systemic chemotherapy.
Patients with liver metastases had a shorter survival time than those without, and the median survival time of 18 mo was comparable with previously reported results. In our study, HER2 negative GC patients with liver metastases remained stable, in contrast to other location metastases at the time of diagnosis of liver metastases.
Furthermore, HER2 positive patients with liver metastases exhibited progressive bone and other metastases and 75% of them had a general condition that allowed further systemic therapy, from which most of them benefited. Therefore, although liver metastases seem to appear at the late stage in patients with GC, other metastatic foci are still more or less chemosensitive, and thus treating liver metastases has an important influence on prognosis.
In our study, HER2 over-expressing GC patients seemed to develop symptomatic liver metastases at about 2.5 years after the diagnosis of recurrent or metastatic GC, which poses the question as to whether liver metastases should be monitored. If surveillance is carried out, who would be the candidates and what timing for surveillance should be further investigated? To date, there has been no evidence to suggest that the surveillance of liver metastases in GC patients is beneficial for patient survival or cost-effectiveness.
In conclusion, our findings reveal that the liver metastases in GC patients with HER2-overexpression had a poor prognosis. The low survival was correlated with sex, HER2 over-expression, Lauren classification, differentiation, and disease-free interval. In our future studies, the optimal regimens for chemotherapy will be studied in GC patients with liver metastases who acquire prolonged OS.
The authors thank the patients who took part in this study and department of oncology medicine of the Chinese PLA General Hospital for sending tumor specimens. The authors also appreciated the help from Professor Dai GH and Bai L for selecting the study case and controlling the quality of HER2 IHC.
Gastric cancer (GC) is the second leading cause of cancer death worldwide, especially in Asia. There are many risk factors used for predicting the prognosis of GC patients. A high incidence of liver metastases has been reported among patients with human epidermal growth factor receptor 2 (HER2) over-expressed GC. However, there is some controversy regarding the hypothesis that HER2 expression serves as a factor for predicting the prognosis of GC patients.
HER2, an important member of the HER family, is encoded by a gene located on chromosome 17q21. In cancer formation, HER2 acts as an oncogene to regulate the proliferation, invasion, and apoptosis of cancer cells. The current research hotspot is to study the risk factors for liver metastasis and the prognosis in patients with HER2 over-expressing GC.
Although HER2 is over-expressed about 38% of gastric cancer patients, few studies are available on HER2 status in the liver metastases of gastric carcinoma patients and there is also little data on the evaluated prognosis for Chinese patients. A total of 84 GC patients recruited from the General Hospital of the People’s Liberation Army in China between 2003 and 2010 were randomly enrolled in this study. All patients underwent radical dissection and chemotherapy, and/or radiotherapy. HER2 over-expression was detected with immunohistochemistry in gastric cancer with liver metastasis patients. Liver metastasis was diagnosed by magnetic resonance imaging or computed tomography. The authors also evaluated the clinical response of other metastatic diseases according to the Response Evaluation Criteria in Solid Tumors at the time of diagnosis of liver metastasis. The baseline characteristics and prognostic factors for GC were reviewed, including age, sex, differentiation and GC type, and disease-free interval. Overall survival (OS) served as a main outcome. From the results, the authors drew the conclusion that HER2 over-expressing GC patients with liver metastases have a poor prognosis, and the overall survival was significantly lower in HER2 positive patients.
Immunohistochemistry may be used to screen the HER2 status in gastric carcinoma patients with liver metastasis patients, and to study the risk factors for liver metastasis in gastric cancer. Since the sample size of our study was not large enough, that limited this study’s ability to provide robust evidence. Therefore, larger-scale, multicentric studies are needed to test the results. Even so, the authors believe the current study provides preliminary and powerful data of evaluating the prognosis in patients with HER2 over-expressing GC, and contributes to determining whether target therapy is necessary for gastric carcinoma with liver metastasis.
HER2 is the human epidermal growth factor receptor 2, an important member of the HER family, that is encoded by a gene located on chromosome 17q21. During tumorigenesis, HER2 has been identified to act as an oncogene to modulate the proliferation, invasion and apoptosis of tumor cells.
The authors provide a retrospective study which reviewed 84 patients with HER2 expressing liver metastases in gastric cancer between 2003 and 2010. The study shows that HER2-overexpression has no correlation with liver metastases in gastric cancer. However, OS was obviously lower for HER2 negative patients. HER2-overexpression had correlation to a lower survival rate. Overall, this is an interesting and good study.
Peer reviewer: Keiji Ogura, Tokyo Metropolitan Police Hospital, 4-22-1 Nakano, Tokyo 164-8541, Japan
S- Editor Gou SX L- Editor Rutherford A E- Editor Zhang DN
| 1. | Bulanov D. [Gastric cancer - current state of the problem. Part I. Epidemiology. Pathology. Classification. Staging]. Khirurgiia (. Sofiia). 2007;48-59. [PubMed] |
| 2. | Yan SY, Hu Y, Fan JG, Tao GQ, Lu YM, Cai X, Yu BH, Du YQ. Clinicopathologic significance of HER-2/neu protein expression and gene amplification in gastric carcinoma. World J Gastroenterol. 2011;17:1501-1506. [PubMed] |
| 3. | Zhang XL, Yang YS, Xu DP, Qu JH, Guo MZ, Gong Y, Huang J. Comparative study on overexpression of HER2/neu and HER3 in gastric cancer. World J Surg. 2009;33:2112-2118. [PubMed] |
| 4. | Yu GZ, Chen Y, Wang JJ. Overexpression of Grb2/HER2 signaling in Chinese gastric cancer: their relationship with clinicopathological parameters and prognostic significance. J Cancer Res Clin Oncol. 2009;135:1331-1339. [PubMed] |
| 5. | Matsubara J, Yamada Y, Nakajima TE, Kato K, Hamaguchi T, Shirao K, Shimada Y, Shimoda T. Clinical significance of insulin-like growth factor type 1 receptor and epidermal growth factor receptor in patients with advanced gastric cancer. Oncology. 2008;74:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Tsugawa K, Yonemura Y, Hirono Y, Fushida S, Kaji M, Miwa K, Miyazaki I, Yamamoto H. Amplification of the c-met, c-erbB-2 and epidermal growth factor receptor gene in human gastric cancers: correlation to clinical features. Oncology. 1998;55:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Brien TP, Depowski PL, Sheehan CE, Ross JS, McKenna BJ. Prognostic factors in gastric cancer. Mod Pathol. 1998;11:870-877. [PubMed] |
| 8. | Gürel S, Dolar E, Yerci O, Samli B, Oztürk H, Nak SG, Gülten M, Memik F. The relationship between c-erbB-2 oncogene expression and clinicopathological factors in gastric cancer. J Int Med Res. 1999;27:74-78. [PubMed] |
| 9. | Nakajima M, Sawada H, Yamada Y, Watanabe A, Tatsumi M, Yamashita J, Matsuda M, Sakaguchi T, Hirao T, Nakano H. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85:1894-1902. [PubMed] |
| 10. | Allgayer H, Babic R, Gruetzner KU, Tarabichi A, Schildberg FW, Heiss MM. c-erbB-2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumor-associated protease systems. J Clin Oncol. 2000;18:2201-2209. [PubMed] |
| 11. | García I, Vizoso F, Martín A, Sanz L, Abdel-Lah O, Raigoso P, García-Muñiz JL. Clinical significance of the epidermal growth factor receptor and HER2 receptor in resectable gastric cancer. Ann Surg Oncol. 2003;10:234-241. [PubMed] |
| 12. | Jauch KW, Heiss MM, Gruetzner U, Funke I, Pantel K, Babic R, Eissner HJ, Riethmueller G, Schildberg FW. Prognostic significance of bone marrow micrometastases in patients with gastric cancer. J Clin Oncol. 1996;14:1810-1817. [PubMed] |
| 13. | Maehara Y, Yamamoto M, Oda S, Baba H, Kusumoto T, Ohno S, Ichiyoshi Y, Sugimachi K. Cytokeratin-positive cells in bone marrow for identifying distant micrometastasis of gastric cancer. Br J Cancer. 1996;73:83-87. [PubMed] |
| 14. | Matsunami K, Nakamura T, Oguma H, Kitamura Y, Takasaki K. Detection of bone marrow micrometastasis in gastric cancer patients by immunomagnetic separation. Ann Surg Oncol. 2003;10:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Schlimok G, Funke I, Pantel K, Strobel F, Lindemann F, Witte J, Riethmüller G. Micrometastatic tumour cells in bone marrow of patients with gastric cancer: methodological aspects of detection and prognostic significance. Eur J Cancer. 1991;27:1461-1465. [PubMed] |
| 16. | Tokunaga A, Onda M, Okuda T, Teramoto T, Fujita I, Mizutani T, Kiyama T, Yoshiyuki T, Nishi K, Matsukura N. Clinical significance of epidermal growth factor (EGF), EGF receptor, and c-erbB-2 in human gastric cancer. Cancer. 1995;75:1418-1425. [PubMed] |
| 17. | Yano T, Doi T, Ohtsu A, Boku N, Hashizume K, Nakanishi M, Ochiai A. Comparison of HER2 gene amplification assessed by fluorescence in situ hybridization and HER2 protein expression assessed by immunohistochemistry in gastric cancer. Oncol Rep. 2006;15:65-71. [PubMed] |
| 18. | de Graeff P, Crijns AP, Ten Hoor KA, Klip HG, Hollema H, Oien K, Bartlett JM, Wisman GB, de Bock GH, de Vries EG. The ErbB signalling pathway: protein expression and prognostic value in epithelial ovarian cancer. Br J Cancer. 2008;99:341-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Rusnak DW, Lackey K, Affleck K, Wood ER, Alligood KJ, Rhodes N, Keith BR, Murray DM, Knight WB, Mullin RJ. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1:85-94. [PubMed] |
| 20. | Kim SY, Kim HP, Kim YJ, Oh do Y, Im SA, Lee D, Jong HS, Kim TY, Bang YJ. Trastuzumab inhibits the growth of human gastric cancer cell lines with HER2 amplification synergistically with cisplatin. Int J Oncol. 2008;32:89-95. [PubMed] |
| 21. | Inui T, Asakawa A, Morita Y, Mizuno S, Natori T, Kawaguchi A, Murakami M, Hishikawa Y, Inui A. HER-2 overexpression and targeted treatment by trastuzumab in a very old patient with gastric cancer. J Intern Med. 2006;260:484-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Roder DM. The epidemiology of gastric cancer. Gastric Cancer. 2002;5 Suppl 1:5-11. [PubMed] |
| 23. | Cangiano J, Centeno BA, Garrett CR, Cáceres W, de Jesús A, Lee JH, Pavía O, Jove R, Báez L, Sullivan DM. Signal transduction proteins in tumors from Puerto Rican and Caucasian gastric adenocarcinoma patients: expression differences with potential for specific targeted therapies. Dig Dis Sci. 2008;53:2090-2100. [PubMed] |
| 24. | Urano N, Fujiwara Y, Doki Y, Tsujie M, Yamamoto H, Miyata H, Takiguchi S, Yasuda T, Yano M, Monden M. Overexpression of hypoxia-inducible factor-1 alpha in gastric adenocarcinoma. Gastric Cancer. 2006;9:44-49. [PubMed] |
| 25. | Yamashita K, Sakuramoto S, Kikuchi S, Katada N, Kobayashi N, Watanabe M. Validation of staging systems for gastric cancer. Gastric Cancer. 2008;11:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Tateishi M, Toda T, Minamisono Y, Nagasaki S. Clinicopathological significance of c-erbB-2 protein expression in human gastric carcinoma. J Surg Oncol. 1992;49:209-212. [PubMed] |
| 27. | Sasano H, Date F, Imatani A, Asaki S, Nagura H. Double immunostaining for c-erbB-2 and p53 in human stomach cancer cells. Hum Pathol. 1993;24:584-589. [PubMed] |
| 28. | Fuse N. [Relation of HER2 status and prognosis in gastric cancer patients]. Gan To Kagaku Ryoho. 2011;38:1073-1078. [PubMed] |
| 29. | Okegawa T, Kinjo M, Nutahara K, Higashihara E. Pretreatment serum level of HER2/nue as a prognostic factor in metastatic prostate cancer patients about to undergo endocrine therapy. Int J Urol. 2006;13:1197-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |