Published online May 21, 2012. doi: 10.3748/wjg.v18.i19.2383
Revised: February 10, 2012
Accepted: April 9, 2012
Published online: May 21, 2012
AIM: To investigate the ability of hexahydrocurcumin (HHC) to enhance 5-fluorouracil (5-FU) in inhibiting the growth of HT-29 cells by focusing on cyclooxygenase (COX)-2 expression.
METHODS: Antiproliferative effects of HHC and 5-FU, alone and in combination, on growth of HT-29 human colon cancer cells were assessed using 5-diphenyltetrazolium bromide (MTT) reduction assay. In combination treatment, low doses of 5-FU were used combined with various concentrations of HHC to minimize the toxicity and side effects of 5-FU. The therapeutic effects of these drugs on down-regulation of COX-2 mRNA and protein expression were examined using semi-quantitative reverse transcription–polymerase chain reaction (RT-PCR) and Western blotting analysis.
RESULTS: MTT reduction assay indicated that HHC alone markedly decreased the viability of HT-29 human colon cancer cells compared to control. Semi-quantitative RT-PCR analysis indicated that HHC is a selective COX-2 inhibitor. This finding was supported by the observation that HHC significantly down-regulates COX-2 mRNA expression compared to the control (control: 100.05% ± 0.03% vs HHC: 61.01% ± 0.35%, P < 0.05) but does not alter COX-1 mRNA. In combined treatment, addition of HHC to a low dose of 5-FU exerts a synergistic effect against the growth of HT-29 cells by markedly reducing cell viability to a greater degree than monotherapy. Semi-quantitative RT-PCR indicated that 5-FU at the concentration of 5 μmol/L in combination with HHC at the concentration of 25 μmol/L significantly down-regulates COX-2 mRNA expression when compared with values in cells treated with 5-FU or HHC alone (HHC + 5-FU: 31.93% ± 5.69%, 5-FU: 100.66% ± 4.52% vs HHC: 61.01% ± 0.35%, P < 0.05).
CONCLUSION: HHC together with 5-FU exerts a synergistic effect and may prove chemotherapeutically useful in treating human colon cancer.
- Citation: Srimuangwong K, Tocharus C, Yoysungnoen Chintana P, Suksamrarn A, Tocharus J. Hexahydrocurcumin enhances inhibitory effect of 5-fluorouracil on HT-29 human colon cancer cells. World J Gastroenterol 2012; 18(19): 2383-2389
- URL: https://www.wjgnet.com/1007-9327/full/v18/i19/2383.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i19.2383
Colorectal cancer is a major cause of cancer death in many countries. 5-Fluorouracil (5-FU) is a chemotherapy drug widely used in treating colorectal cancer. However, the toxicity of 5-FU towards normal cells and resistance to this drug are major barriers to successful cancer chemotherapy. Therefore, the combination of 5-FU and other regimens is often practiced to enhance the efficacy of 5-FU and also to reduce its toxicity. Previous in vitro and in vivo studies of colon cancer have reported that 5-FU combined with other regimens, such as genistein[1] and geraniol[2], are more effective than 5-FU treatment alone.
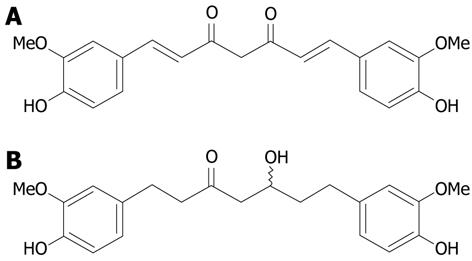
Curcumin (diferuloylmethane, Figure 1), the major yellow pigment in turmeric which is obtained from the rhizome of Curcuma longa L.[3], has been traditionally used in cooking in India and Southeast Asia. It has also been used in both in vitro and in vivo studies as a naturally occurring substance to treat a wide variety of cancers, including ovarian[4], lung[5], skin[6] and colon cancer[7-9]. Curcumin specifically inhibits mRNA and protein expression of cyclooxygenase (COX)-2, which is highly expressed in a variety of human cancers[10-12] including colon cancer, but it does not alter the expression of COX-1, the enzyme that maintains normal gastric mucosa and influences kidney function[13,14]. All these results seem to suggest that curcumin might have minimal toxicity and is safe for the treatment of human colon cancer compared with other traditional chemopreventive agents such as nonsteroidal anti-inflammatory drugs. Although curcumin is a very important agent in preventing and treating colon cancer, its disadvantages include poor solubility and poor absorption in the gastrointestinal tract. Previous reports have indicated that after oral administration of curcumin, about 60% of the dose was absorbed and 38% remained in the large intestine of rats[15] and it is rapidly decomposed in human blood[16]. Curcumin metabolites were synthesized to solve these problems[17].
Hexahydrocurcumin (HHC, Figure 1) is one of the major metabolites of curcumin. Previous studies revealed that this compound exhibits stronger antioxidant activity than curcumin[18]. Moreover, this compound inhibits the biosynthesis of prostaglandin (PGE2) in LPS-stimulated macrophages[19]. PGE2 is a major product of COX-2 enzymes implicated in colorectal carcinogenesis and has been shown to stimulate the growth of human colorectal carcinoma cells. In addition, HHC decreases the level of phorbol ester-induced PGE2 production in human colonic epithelial cells (HCECs) but weakly inhibits COX-2 protein[20].
All these results suggest that HHC down-regulates COX-2 expression, leading us to hypothesize that the HHC-induced suppression of COX-2 expression may improve the effectiveness of the 5-FU conventional chemotherapy drug. The synergistic effect of using 5-FU in combination with curcumin to inhibit the growth of HT-29 human colon cancer cell line has already been demonstrated[21]. The present study aims to evaluate the ability of HHC to enhance 5-FU in inhibiting the growth of HT-29 human colon cancer cells by focusing on the expression of COX-2.
The HT-29 human colon adenocarcinoma cell lines were obtained from the American Type Culture Collection. McCoy’s 5A Media Modified medium, fetal bovine serum (FBS), trypsin, penicillin and streptomycin were purchased from GIBCO-BRL (Gaitherburg, MD). 5-FU and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, United States). TRIzol® Reagent was purchased from Invitrogen (Carlsbad, CA). InProm-II™ reverse transcriptase and GoTag® Flexi DNA polymerase were obtained from Promega (Madison, United States). COX-1 monoclonal antibody (1:1000 dilutions) and COX-2 polyclonal antibody (1:500 dilutions) were obtained from Cayman Chemical (Ann Arbor, MI, United States).
Curcumin was obtained from Curcuma longa as described previously[22]. HHC was synthesized from curcumin by catalytic hydrogenation reaction in ethanol for 5 h, with palladium on charcoal as a catalyst. The product was isolated from tetrahydrocurcumin and octahydrocurcumin by silica gel column chromatography, followed by recrystallization with dichloromethane-n-hexane to give 45% yield of HHC as a white amorphous solid, m.p. 81-82 °C. Tetrahydrocurcumin and octahydrocurcumin were obtained in 28% and 11% yields, respectively, after repeated column chromatography. The spectroscopic (IR, 1H-NMR and mass spectra) data of all the synthesized compounds were identical with those obtained from the previous report[22].
HT-29 cells were cultured in McCoy’s 5A Media Modified containing 10% FBS at 37 °C in 5% CO2 and 90% relative humidity. In all experiments, cells were seeded at 1 × 104 cells per well in 96-well plates for cell viability assay, or 1.5 × 105 cells per well in 6-well plates for RNA extraction and Western blotting analysis. After plating, combinations of 5-FU with HHC were added concurrently to the culture medium. Cells were harvested after various times.
HT-29 cells were plated in 96-well plates with 100 μL medium and were exposed to various concentrations of HHC, 5FU or combination of 5FU and HHC for 24 and 48 h. After incubation, cells were treated with 100 μL MTT solution (final concentration, 0.5 mg/mL) at 37 °C for an additional 4 h. The formazan crystals were solubilized with 100 μL DMSO and then the absorbance was measured at wavelength 540 nm using a microplate reader (Bio-Tek, Instruments, Winooski, VT, United States).
The effectiveness of 5-FU and HHC, alone or in combination, to inhibit growth of HT-29 cells was evaluated by measurement of the combination index (CI)[23], adapted from the method described by Chou et al[24]. The fractional inhibitory concentrations were calculated by dividing the concentration of the drug in the combination at IC50 by the IC50 of the individual drugs. In the following equation, the sum of the dose of 5-FU and the dose of HHC give 50% inhibition of cell growth. CI < 1 indicates a synergistic effect; CI = 1, additive effect; and CI > 1, antagonistic effect. CI = (Dose of 5-FU)/[IC50 (5-FU)] + (Dose of HHC)/[IC50 (HHC)].
After the HT-29 cells were treated for 24 h, total RNA was extracted using TRIzol reagent (Life Technologies, Carlbad, CA, United States). A volume of 1 μg total RNA from each sample was subjected to reverse transcription using ImProm-IITM Reverse Transcription system (Promega Madison, WI, United States). PCR amplification was performed in a reaction volume of 20 μL containing 2 μL of cDNA product and GoTag® DNA polymerase (Promega, United States) using a PerkinElmer 9600 thermal cycler. Amplification of the constitutively-expressed enzyme d-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. The oligonucleotide primers used were: human COX-1, 5′-TGC CCAGCTCCTGGCCCGCCGCTT-3′ (sense), 5′-GTGCATCAACACAGGCGCCTCTTC-3′ (antisense); human COX-2, 5′-TTCAAATGAGATTGTGGAAAAT-3′ (sense), 5′-AGATCATCTCTGCCTGAGTATCTT-3′ (antisense); GAPDH, 5′-TCCCTCAAGATTGTGAGCAA-3′ (sense), and 5′-AAATGAGCCCCAGCCTTCTCC-3′ (antisense). The temperature cycling conditions of amplification were as follows: 15 min at 95 °C, then 25 cycles at 95 °C for 1 min, 55 °C for 1 min, 72 °C for 1 min, and a final extension at 72 °C for 10 min. The amplification products were electrophoresed on a 1.0% agarose gel, visualized by ethidium bromide staining, and photographed gel images were scanned using an image analysis system (Gel Doc 1000; Bio-Rad, Hercules, CA, United States). The intensities of specific COX-1 or COX-2 bands were quantitated in relation to GAPDH bands amplified from the same cDNA using Gene Tools analysis software (Syngene, Cambridge, United Kingdom).
Western blotting analysis was performed to measure protein expression of COX-1, COX-2 or β-actin. Cell lysates were prepared by treating the HT-29 cells in RIPA lysis buffer (1 × PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate and 0.1% SDS) for 30 min. The lysates were sonicated twice for 20 s on ice and then were centrifuged at 10 000 ×g for 10 min to sediment the particulate material. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed under reducing conditions on 8% polyacrylamide gels. The resolved proteins were transferred to PVDF (Millipore, Bedford, MA, United States) and the membranes were incubated with antibodies against human COX-1 or COX-2 (Cayman Chemical Co.) protein in blocking solution. After washing with PBS plus 0.5% Tween-20, the membranes were then incubated with the secondary antibody goat anti-mouse IgG HRP in blocking solution for 2 h at room temperature. After washing, membrane blots were developed using the Immobilon Western (Millipore) chemiluminescence kit and finally exposed to X-ray film.
All values are represented as mean ± SE. Statistical comparisons between different treatment results were analyzed by one-way analysis of variance followed by the Dunnett test. The significance was taken when P-values were less than 0.05.
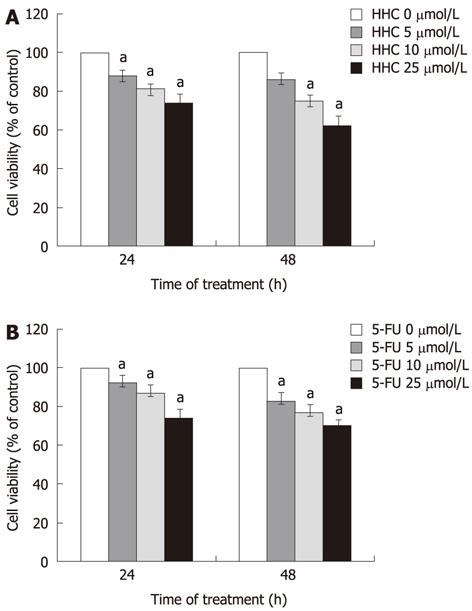
After the HT-29 colon cancer cells were exposed to various concentrations of HHC or 5-FU alone for 24 and 48 h, HHC significantly decreased the viability of HT-29 colon cancer cells as compared to control (Figure 2A, upper panel). The growth inhibition ability of HHC on HT-29 cells was time- and concentration-dependent. A similar result was also observed in the 5-FU-treated group (Figure 2B, lower panel). The half-maximal inhibitory concentration of HHC and 5-FU for inhibiting the growth of HT-29 colon cancer cells at 24 and 48 h exposure was represented by IC50. The respective IC50 values for 24 and 48 h of HHC exposure were 77.05 ± 1.53 and 56.95 ± 2.75, and for 5-FU were 39.13 ± 2.32 and 38 ± 2.21.
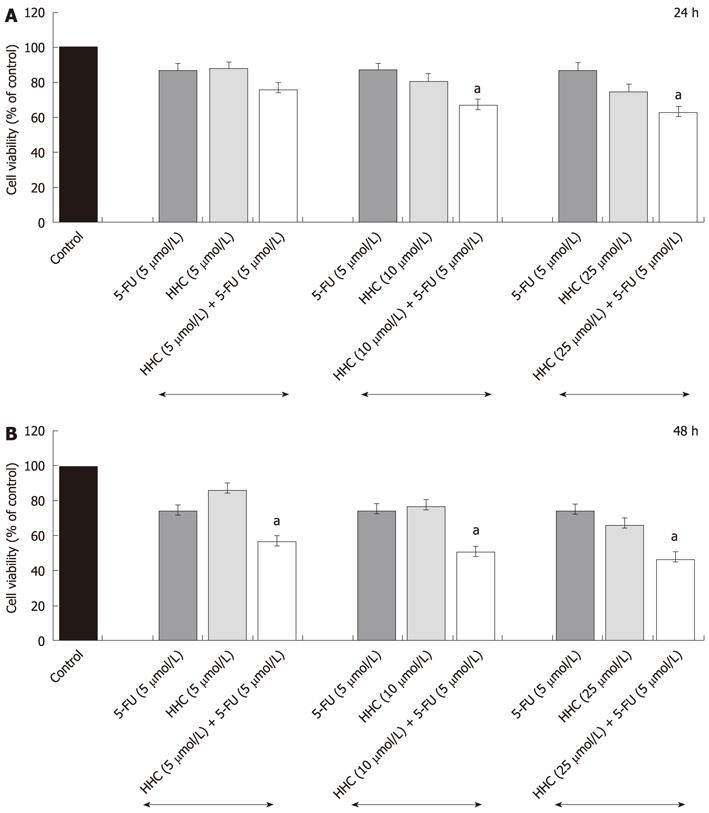
To determine the antiproliferative effect of HHC combined with 5-FU, the HT-29 colon cancer cells were exposed to 5-FU at 5 or 25 μmol/L in combination with HHC at 5 or 25 μmol/L concurrently for 24 h and 48 h and then evaluated by MTT assay. After 24 and 48 h of exposure to two doses of HHC (5 and 25 μmol/L) and high dose of 5-FU (25 μmol/L), marked inhibition of the growth of HT-29 colon cancer cells was observed when compared with those treated with 5-FU or HHC alone. Furthermore, exposure to low dose of 5-FU (5 μmol/L) together with high dose of HHC (25 μmol/L) for 24 and 48 h markedly inhibited the viability of HT-29 colon cancer cells when compared with those treated with HHC or 5-FU alone (Figure 3). It was noteworthy that the inhibitory effect of HHC at 25 μmol/L with a low dose of 5-FU was not different from its effect in combination with a high dose of 5-FU. These results indicated that a low dose of 5-FU could effectively be used in combination with high dose of HHC to inhibit the growth of HT-29 colon cancer cells. In addition, the interactions of these two drugs at 25 μmol/L HHC and 5 μmol/L 5-FU for 24 and 48 h were evaluated by the CI at the IC50 level as described in materials and methods. The combination of 25 μmol/L HHC with 5 μmol/L 5-FU for 24 and 48 h resulted in a synergistic effect (CI < 1) on the inhibition of the growth of HT-29 colon cancer cells when CI = 0.46 ± 0.01 and 0.57 ± 0.02, respectively.
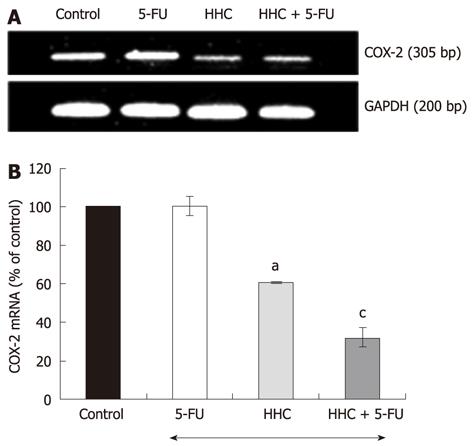
The MTT results showed that a low dose of 5-FU (5 μmol/L) together with HHC at 25 μmol/L decreased the viability of HT-29 colon cancer cells to a markedly greater extent than 5-FU or HHC exposure alone. Moreover, this treatment showed the quantitative synergistic inhibitory effect; therefore, this combination was chosen to further investigate the effects on COX-2 mRNA expression in HT-29 cells. In this study, we observed that 5-FU alone did not decrease the COX-2 mRNA. However, 5-FU combined with HHC markedly reduced the COX-2 expression compared to HHC or 5-FU alone (P < 0.05) (Figure 4). Furthermore, we also observed the combination’s effects on the expression of COX-1 mRNA. The result indicated that the level of COX-1 was not altered by treatment with 5-FU or HHC alone, or their combination.
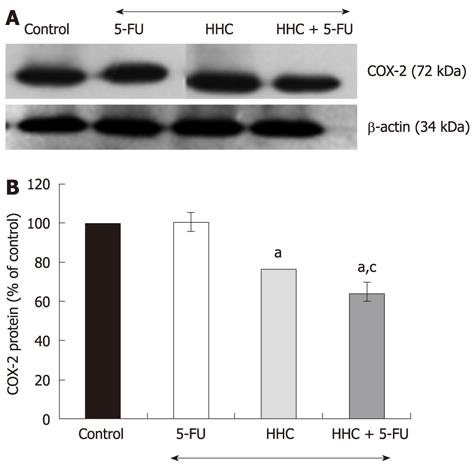
To investigate the combination’s effect on the level of COX-2 protein, HT-29 colon cancer cells were treated with 5-FU (5 μmol/L) together with HHC (25 μmol/L). The results are shown in Figure 5. It is evident that the combination treatment of 5-FU and HHC reduced the COX-2 protein level to a markedly greater degree than did 5-FU or HHC alone (P < 0.05). We further examined the combination’s effect on the level of COX-1 protein. The results show that 5-FU alone, HHC alone or 5-FU together with HHC did not alter the level of COX-1 protein.
The toxicity and the resistance to 5-FU have been major obstacles in successful colorectal cancer chemotherapy. In addition, COX-2 up-regulation is directly involved in colorectal carcinogenesis[25,26]. Therefore, a combined treatment of 5-FU with non-toxic agents that can inhibit COX-2 activity might be useful for treating colon carcinogenesis.
Curcumin, a specific COX-2 inhibitor, is usually used in combination with traditional chemotherapy or other regimens both in vitro, in vivo and also in clinical studies of colon cancer. Many reports have indicated that curcumin enhanced the cytotoxic effect of celecoxib in several human colon cancer cell lines[27]. This effect has also been observed in colorectal cancer models[28]. Moreover, curcumin enhances the cytotoxic effect of 5-FU and decreases the COX-2 protein expression better than single agent treatment[21]. However, several studies over the past three decades have reported that curcumin exhibits poor bioavailability due to poor absorption, low serum levels and rapid metabolism. Therefore, the pharmacological activity of curcumin may be mediated in part by its metabolites[29,30].
In the present study, we investigated the anti-carcinogenic effects of HHC on growth of HT-29 human colon cancer cells. We found that HHC was sufficient for inhibiting the growth of human colon cancer cells by decreasing the viability of HT-29 cells through down-regulation of COX-2 mRNA and protein expression. Moreover, HHC did not alter COX-1 mRNA and protein expression. These results agreed with previous study, which suggested that HHC could sensitize the cancer cells to chemotherapeutic drugs by decreasing phorbol ester-induced PGE2 production in HCECs[20]. PGE2 is a major product of COX-2 enzymes. Moreover, it has been demonstrated that HHC exhibited higher antioxidant activity than its curcumin progenitor[18] and it has been suggested that the hydrogenation at the conjugated double bonds of the central seven-carbon chain and a keto group of the β-diketone of curcumin to HHC markedly enhances antioxidant activity.
Based on these results, it is reasonable to assume that HHC is a safe and effective agent for the treatment of colon cancer, and that this compound is more stable than curcumin. The present investigation deals with the study of the combination effect of HHC with 5-FU chemotherapy drug. Our results indicated that HHC in combination with even a low concentration of 5-FU (5 μmol/L) was sufficient to decrease the viability of HT-29 colon cancer cells. Moreover, this combination showed a synergistic effect on antiproliferation against colon cancer cells (CI < 1) and down-regulated the expression of COX-2 mRNA and protein, compared to either 5-FU or HHC monotherapy. Based on these results, we suggest that the addition of HHC could enhance the inhibiting effects of 5-FU on the growth of HT-29 human colon cancer cells. Moreover, the combination of these drugs that are different in their modes of action could prove much safer and more effective than cancer monotherapy[27]. In addition, this combination did not alter the COX-1 mRNA and protein levels. It is thus reasonable to assume that this therapeutic approach may have no toxic effect and enables the use of 5-FU chemotherapy drug at a lower and safer concentration, which would be highly desirable for long-term treatment of colon cancer.
In conclusion, this study illustrates that HHC is a specific COX-2 inhibitor that plays an important role in carcinogenesis. It has been demonstrated for the first time in this study that the addition of HHC to 5-FU standard chemotherapy in HT-29 human colon cancer cells can enhance the growth inhibition and down-regulation of COX-2. Thus, the combination of these two drugs should have a great potential to be used to treat human colon cancer.
Colorectal cancer is a major cause of cancer death in many countries. The toxicity of 5-fluorouracil (5-FU) chemotherapeutic drug for normal cells and resistance to this drug are major barriers to successful cancer chemotherapy.
Combining 5-FU with other regimens and agents, such as curcumin, is a strategy to enhance the efficacy of 5-FU and also to reduce its toxicity. Curcumin down-regulates cyclooxygenase (COX)-2, which is highly expressed in human colon cancer, but it does not alter the level of COX-1, a housekeeping enzyme. However, curcumin is only slightly absorbed in the gastrointestinal tract due to its poor solubility in water. Therefore, hexahydrocurcumin (HHC), one of the major curcumin metabolites, was synthesized to solve these problems.
This report has highlighted the importance of HHC which could improve the effectiveness of the 5-FU chemotherapeutic drug. In this study, the authors demonstrate that HHC is a specific COX-2 inhibitor. HHC down-regulates COX-2 expression, but it does not alter the level of COX-1. This is the first study to report the anti-carcinogenic effects of HHC on growth of human colon cancer cells. Furthermore, HHC has ability to enhance 5-FU in inhibiting the growth of HT-29 human colon cancer cells and down-regulates the expression of COX-2 mRNA and protein.
This report illustrates the action of HHC combined with 5-FU, and this study may represent a future strategy to provide much safer and more effective treatment for patients with colon cancer.
HHC is one of the major curcumin metabolites. Curcumin is the major yellow pigment in turmeric which is obtained from the rhizome of Curcuma longa L, traditionally used in cooking in India and Southeast Asia.
This is a good descriptive study in which authors investigate ability of HHC to enhance 5-FU in inhibiting the growth of HT-29 cells by focusing on COX-2 expression. The results are interesting and suggest that HHC together with 5-FU exerted a synergistic effect and may prove chemotherapeutically useful in treating human colon cancer.
Peer reviewer: Hisato Nakajima, MD, Department of Gastroenterology and Hepatology, The Jikei University School of Medicine, 3-25-8, Nishi-Shinbashi, Minato-ku, Tokyo 105-8461, Japan
S- Editor Gou SX L- Editor Logan S E- Editor Zheng XM
| 1. | Hwang JT, Ha J, Park OJ. Combination of 5-fluorouracil and genistein induces apoptosis synergistically in chemo-resistant cancer cells through the modulation of AMPK and COX-2 signaling pathways. Biochem Biophys Res Commun. 2005;332:433-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Carnesecchi S, Bras-Gonçalves R, Bradaia A, Zeisel M, Gossé F, Poupon MF, Raul F. Geraniol, a component of plant essential oils, modulates DNA synthesis and potentiates 5-fluorouracil efficacy on human colon tumor xenografts. Cancer Lett. 2004;215:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Chuang SE, Kuo ML, Hsu CH, Chen CR, Lin JK, Lai GM, Hsieh CY, Cheng AL. Curcumin-containing diet inhibits diethylnitrosamine-induced murine hepatocarcinogenesis. Carcinogenesis. 2000;21:331-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 180] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Wahl H, Tan L, Griffith K, Choi M, Liu JR. Curcumin enhances Apo2L/TRAIL-induced apoptosis in chemoresistant ovarian cancer cells. Gynecol Oncol. 2007;105:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Chen YS, Ho CC, Cheng KC, Tyan YS, Hung CF, Tan TW, Chung JG. Curcumin inhibited the arylamines N-acetyltransferase activity, gene expression and DNA adduct formation in human lung cancer cells (A549). Toxicol In Vitro. 2003;17:323-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Kakar SS, Roy D. Curcumin inhibits TPA induced expression of c-fos, c-jun and c-myc proto-oncogenes messenger RNAs in mouse skin. Cancer Lett. 1994;87:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Chauhan DP. Chemotherapeutic potential of curcumin for colorectal cancer. Curr Pharm Des. 2002;8:1695-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Hanif R, Qiao L, Shiff SJ, Rigas B. Curcumin, a natural plant phenolic food additive, inhibits cell proliferation and induces cell cycle changes in colon adenocarcinoma cell lines by a prostaglandin-independent pathway. J Lab Clin Med. 1997;130:576-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 164] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Kawamori T, Lubet R, Steele VE, Kelloff GJ, Kaskey RB, Rao CV, Reddy BS. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;59:597-601. [PubMed] |
| 10. | Lee KW, Kim JH, Lee HJ, Surh YJ. Curcumin inhibits phorbol ester-induced up-regulation of cyclooxygenase-2 and matrix metalloproteinase-9 by blocking ERK1/2 phosphorylation and NF-kappaB transcriptional activity in MCF10A human breast epithelial cells. Antioxid Redox Signal. 2005;7:1612-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Lev-Ari S, Vexler A, Starr A, Ashkenazy-Voghera M, Greif J, Aderka D, Ben-Yosef R. Curcumin augments gemcitabine cytotoxic effect on pancreatic adenocarcinoma cell lines. Cancer Invest. 2007;25:411-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Zhang F, Altorki NK, Mestre JR, Subbaramaiah K, Dannenberg AJ. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis. 1999;20:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 201] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Goel A, Boland CR, Chauhan DP. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett. 2001;172:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 256] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 14. | Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, Howells L. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013-6020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 468] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 15. | Ravindranath V, Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology. 1980;16:259-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 290] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15:1867-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1167] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 17. | Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76:1590-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 811] [Cited by in RCA: 783] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 18. | Somparn P, Phisalaphong C, Nakornchai S, Unchern S, Morales NP. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol Pharm Bull. 2007;30:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 237] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 19. | Shao J, Lee SB, Guo H, Evers BM, Sheng H. Prostaglandin E2 stimulates the growth of colon cancer cells via induction of amphiregulin. Cancer Res. 2003;63:5218-5223. [PubMed] |
| 20. | Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61:1058-1064. [PubMed] |
| 21. | Du B, Jiang L, Xia Q, Zhong L. Synergistic inhibitory effects of curcumin and 5-fluorouracil on the growth of the human colon cancer cell line HT-29. Chemotherapy. 2006;52:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Changtam C, de Koning HP, Ibrahim H, Sajid MS, Gould MK, Suksamrarn A. Curcuminoid analogs with potent activity against Trypanosoma and Leishmania species. Eur J Med Chem. 2010;45:941-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 23. | Chen MF, Chen LT, Boyce HW. Effect of 5-fluorouracil on methotrexate transport and cytotoxicity in HT29 colon adenocarcinoma cells. Cancer Lett. 1995;88:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5258] [Cited by in RCA: 5701] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 25. | Janssen A, Maier TJ, Schiffmann S, Coste O, Seegel M, Geisslinger G, Grösch S. Evidence of COX-2 independent induction of apoptosis and cell cycle block in human colon carcinoma cells after S- or R-ibuprofen treatment. Eur J Pharmacol. 2006;540:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Williams CS, Luongo C, Radhika A, Zhang T, Lamps LW, Nanney LB, Beauchamp RD, DuBois RN. Elevated cyclooxygenase-2 levels in Min mouse adenomas. Gastroenterology. 1996;111:1134-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 208] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Lev-Ari S, Strier L, Kazanov D, Madar-Shapiro L, Dvory-Sobol H, Pinchuk I, Marian B, Lichtenberg D, Arber N. Celecoxib and curcumin synergistically inhibit the growth of colorectal cancer cells. Clin Cancer Res. 2005;11:6738-6744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Shpitz B, Giladi N, Sagiv E, Lev-Ari S, Liberman E, Kazanov D, Arber N. Celecoxib and curcumin additively inhibit the growth of colorectal cancer in a rat model. Digestion. 2006;74:140-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1186] [Cited by in RCA: 1193] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 30. | Wahlström B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol (Copenh). 1978;43:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 361] [Article Influence: 7.7] [Reference Citation Analysis (0)] |