Published online Mar 14, 2012. doi: 10.3748/wjg.v18.i10.1093
Revised: November 17, 2011
Accepted: December 31, 2011
Published online: March 14, 2012
AIM: To investigate the correlation between autoimmune thyroid diseases (ATDs) and the prevalence of Cag-A positive strains of Helicobacter pylori (H. pylori) in stool samples.
METHODS: Authors investigated 112 consecutive Caucasian patients (48 females and 4 males with Graves’ disease and 54 females and 6 males with Hashimoto’s thyroiditis HT), at their first diagnosis of ATDs. Authors tested for H. pylori in stool samples using an amplified enzyme immunoassay and Cag-A in serum samples using an enzyme-linked immunoassay method (ELISA). The results were analyzed using the two-sided Fisher’s exact test and the respective odds ratio (OR) was calculated.
RESULTS: A marked correlation was found between the presence of H. pylori (P≤ 0.0001, OR 6.3) and, in particular, Cag-A positive strains (P≤ 0.005, OR 5.3) in Graves’ disease, but not in Hashimoto’s thyroiditis, where authors found only a correlation with Cag-A strains (P≤ 0.005, OR 8.73) but not when H. pylori was present.
CONCLUSION: The marked correlation between H. pylori and Cag-A, found in ATDs, could be dependent on the different expression of adhesion molecules in the gastric mucosa.
-
Citation: Bassi V, Marino G, Iengo A, Fattoruso O, Santinelli C. Autoimmune thyroid diseases and
Helicobacter pylori : The correlation is present only in Graves's disease. World J Gastroenterol 2012; 18(10): 1093-1097 - URL: https://www.wjgnet.com/1007-9327/full/v18/i10/1093.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i10.1093
Autoimmune thyroid diseases (ATDs) are represented, essentially, by Hashimoto’s thyroiditis (HT) and its variants (postpartum and sporadic thyroiditis), Graves’ disease (GD) and atrophic thyroiditis[1]. A typical marker of HT and GD is the presence of autoantibodies against thyroglobulin (TgAbs), thyroperoxidase (TPOAbs) and thyrotropin receptor (TRAbs)[2]. Both genetic and environmental factors are involved in the pathogenesis of ATDs. Some bacteria and viruses are suspected of being able to mimic the antigenic profile on the thyroid cell membrane, and play an important role in the onset of autoimmune diseases[3-6]. Helicobacter pylori (H. pylori) infection is found worldwide and has an incidence of up to 50% in the population of developed countries[7]. A cohort effect has been demonstrated for such infection, and a higher prevalence rate is found in the elderly and in males[8]. H. pylori is a gram-negative, motile bacterium, which typically colonizes and infects the gastric mucosa; the most virulent strains can usually be identified by the presence of the cytotoxin-associated gene A (Cag-A) antigen[9]. Therefore, the microorganism is responsible for gastric diseases such as gastritis, gastric/duodenal ulcers and carcinomas.
Several studies[10-12] have shown a positive correlation between the presence of H. pylori and HT, although others did not find such an association[13,14]. Moreover, we recently demonstrated a noteworthy correlation between H. pylori infection and GD, independent of hormonal status[15].
The aim of this study was to investigate the prevalence of H. pylori in ATDs and, in particular, HT, to help clarify the controversial results observed in previous studies. We detected the presence of H. pylori in fresh stool samples from our patients using an enzyme immunoassay method, and Cag-A positivity using a serological test.
We studied 112 consecutive Caucasian patients (48 females and 4 males with GD and 54 females and 6 males with HT), at their first diagnosis of ATDs, enrolled over a period of 18 mo (from October 2008 to March 2010). The mean age (± SD) of the ATDs patients was 49.7 ± 6.6 years (48.8 ± 3.9 years for GD patients and 50.2 ± 9.7 years for HT patients). The study inclusion criteria were previously reported[15]. Briefly, these criteria included the absence of other diseases, a negative anamnesis for antimicrobial drugs use for at least three months and the absence of dyspeptic symptoms (epigastric pain, nausea, heartburn) or gastric diseases. The study was approved by the ethical committee of our institution and informed consent was obtained from each patient.
GD diagnosis was defined by hormonal hyperthyroidism [suppressed thyrotrophin (TSH), elevated FT3 and FT4], diffuse and high iodine capture on thyroid scintigraphy, and positive titers of TPOAbs, TgAbs and TRAbs. To eliminate possible bias between subclinical and frank primary hypothyroidism, HT diagnosis was defined by a cut-off value higher than 35 mU/mL TSH, low FT3 and FT4 values, positive titers of TPOAbs and TgAbs and hypoechogenicity pattern on echography (Table 1).
| Group | n | Sexfemale/male | Age (yr)(mean ± SD) | Smokersyes/no | Ophthalmopathyyes/no |
| Control | 100 | 90/10 | 49.0 ± 4.5 | 44/56 | - |
| GD | 52 | 48/4 | 48.8 ± 3.9 | 21/31 | 34/18 |
| HT | 60 | 54/6 | 50.2 ± 9.7 | 26/34 | 0/60 |
| ATDs | 112 | 102/10 | 49.2 ± 6.9 | 47/65 | 34/112 |
The control population was composed of 100 body mass index-, socio-economic- and inclusion criteria class-matched individuals (90 females and 10 males, mean age 49.0 ± 4.5 years, Table 1). All of these subjects showed normal TSH, FT3 and FT4 values with absent titers of TPOAbs, TgAbs and TRAbs.
The tests were performed by laboratory technicians blinded to the subject’s diagnosis. Fresh stool samples were obtained and tested using an amplified enzyme immunoassay for the detection of H. pylori antigens (Amplified IDEIA H. pylori StAR, Oxoid, United Kingdom). This test is highly specific for H. pylori antigens (sensitivity 95%, specificity 95%), with no cross-reactivity with other microorganisms. An absorbance value > 0.150 using a dual wavelength (450/620 to 650 nanometers) was considered positive for the presence of H. pylori.
Fresh serum samples were tested with the enzyme-linked immunoassay method (ELISA, Radim, Pomezia, Italy, sensitivity 93.7%, specificity 100%). Anti-Cag-A immunoglobulin-G values greater than 15 units/mL were regarded as Cag-A positive.
The relationship between the different studied groups, in terms of H. pylori and Cag-A positivity, was investigated with the two-sided Fisher’s exact test and calculation of the respective odds ratio (OR, with 95% confidence interval, using the approximation of Woolf, Instat 3.06, Graphstat Software Inc., San Diego, CA, United States). P≤ 0.05 was considered significant.
Of 112 ATDs patients, 43/52 (82%) in the GD group and 28/60 (46%) in the HT group were positive for H. pylori infection, vs 43 [43.0%, P≤ 0.0001, OR 6.3 (2.7-14.3) vs the GD group, not significant vs the HT group] of 100 controls (Table 2).
Immunoassay testing on the stool samples confirmed that the observed H. pylori positivity was dependent on ongoing H. pylori presence in the gastric mucosa and not on past infection. Furthermore, no correlation was found between the presence of H. pylori and smoking habit in the two groups of ATDs patients (data not shown).
Thirty-six (83.7%) of 43 H. pylori-positive GD patients and 25/28 (89.2%) H. pylori-positive HT patients were positive for Cag-A antigens vs 21/43 [48.8%, P≤ 0.005, OR 5.3 (1.9-14.7) vs the GD group and P≤ 0.005, OR 8.73 (2.7-33.0) vs the HT group] of infected controls.Again, considering the overall prevalence of infection by Cag-A-positive H. pylori in the studied groups of ATDs patients, the results were statistically significant, [61 of 112 or 54.4% vs the controls, 21 of 100 subjects or 21%, P≤ 0.0001 OR 4.5 (2.4-8.2), Table 2].
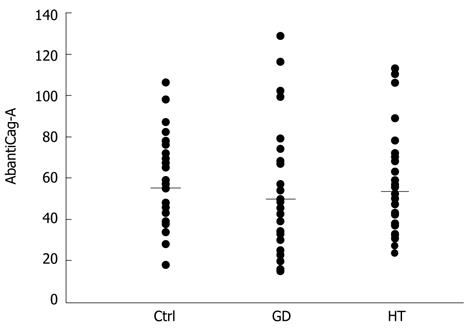
Cag-A antibody levels, expressed in mU/mL [control 56 ± 24.3 (mean ± SD), GD 50.3 ± 28.6, HT 54.1 ± 22.6], were similar among the three groups of investigated subjects (Figure 1) and did not correlate with the respective titers of TgAbs, TPOAbs or TRAbs (data not shown).
H. pylori infection is found world wide and affects up to 50% of the population of developed countries, such as Italy, and the most virulent strains are identified by the presence of Cag-A antigens[9]. Recently, a significant correlation was shown between the Cag-A carrier H. pylori strains and GD, independent of the hormonal status of the investigated patients[15]. Moreover, other studies have investigated the association between such microorganisms and HT, however, the results are controversial. Some investigations point to a noteworthy correlation[10,11,12], others do not[13,14]. The use of different techniques to assess H. pylori infection could explain these conflicting conclusions. For instance, serological detection of H. pylori antibodies can not discriminate between past and ongoing infection. Conversely, the 13C-urea breath test and immunoassay test on fresh stool samples can only detect ongoing H. pylori infection, therefore these tests are currently considered the preferred not-invasive methods of investigation[7]. Moreover, the presence of similar antigenic sites for Cag-A and TPO could cause false positive results in the Abs titers against H. pylori, leading to a bias in group selection of the enrolled patients[16]. In addition, the different grade of thyroid function in HT patients, such as subclinical or frank hypothyroidism, could be a misleading factor.
Our results, using a stool antigen test, confirmed that a correlation was present between H. pylori and hyperthyroid GD patients, but this correlation was not seen in hypothyroid HT patients.
In accordance with the guidelines[7], we did not perform further invasive exams, such as gastroscopy, in consideration of the age (usually under 45 years old in the investigated patients) and the absence of digestive symptoms in H. pylori-positive patients.
Several factors could be considered to explain the different results regarding H. pylori prevalence in GD and HT. Usually, the onset and/or progression of ATDs are dependent on different autoimmune mechanisms. Cellular autoimmunity with the TH1 profile of CD4 + T helper precursor cells is predominant in HT, whereas humoral autoimmunity (production of TRAbs or TSH-receptor blocking antibodies) with the TH2 profile is prevalent in GD and atrophic thyroiditis[17]. These different activated profiles in ATDs induce the expression of different panels of cytokines, such as interleukin (IL)-4, IL-5, IL-6 and IL-10 in GD and IL-2, interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) in HT[17]. Also, the opposite thyroid function, i.e., hyperthyroidism in GD vs hypothyroidism in HT, could be another factor leading to the controversial results on H. pylori prevalence in GD and HT patients.
In our study, both GD and HT show a comparable elevated prevalence of Cag-A positive strains in H. pylori-positive patients, in agreement with previous observations in TH patients[11].
The involved factors could operate through a common pathway, such as the glycoconjugates-mediated adhesion of H. pylori to the gastric mucosa, which represents a crucial step in the establishment of successful infection. H. pylori glycan receptors include fucosylated ABO blood group antigens[18,19] and glycans with charged groups, such as sialic acid[20]or sulfate[21], and neolacto core chains[22] Two different H. pylori adhesins have been characterized on the basis of their interactions with the receptors: the blood group antigen-binding adhesin (BabA) is specific for H type-1 and Lewis b antigens, admitting terminal blood groups A and B glycan determinants, whereas the sialic acid binding adhesin (SabA) recognizes the Sialyl-Lewis a and Sialyl-Lewis x antigens[20,23].
In particular, the potential effect of the suggested factors, such as hyperthyroidism or the production of cytokines induced by humoral immunity, could modify the profile of the adhesion molecules expressed on the gastric mucosa, increasing H. pylori binding in GD and selecting the Cag-A positive strains in ATDs.
Regarding the pathogenetic role of HP in the onset of ATDs, it has been postulated that viral and bacterial infections could play a noteworthy role. Usually, elevated levels of antibodies against some bacteria have been found in GD patients[4-6] and, conversely, an antigen structure, such as TSH-binding protein, is described in many gram-positive and gram-negative bacteria[24]. Moreover, Cag-A positive H. pylori strains show some nucleotide sequence similarity to TPO sequence[25]. A positive linear regression between H. pylori-Abs titers and microsomal autoantibodies[9] and a significant reduction in these antibodies after H. pylori eradication have been demonstrated[26]. Therefore, cross-reactivity of the antibodies produced against thyroid antigen structures during H. pylori infections could potentially induce a biological effect[27], in a similar way to that of H. pylori which triggers the onset of autoantibodies against the H+K+-ATPase in the gastric autoimmunity[28-29]. Moreover, the increased prevalence of H. pylori in GD, on first diagnosis, and the observation that, usually, H. pylori infection starts during childhood[30], suggest that the bacterium could be present before the onset of the autoimmune disease. Larizza et al[31]
proposed that H. pylori infection can induce and/or worsen the course of GD in susceptible young patients, carrying the human leukocyte DRB1*0301 antigen. The authors also suggested that H. pylori eradication could prevent GD in these “at high risk” children.
Conversely, hyperthyroid GD patients could just be more susceptible to H. pylori infection, and the presence of the microorganism could represent an epiphenomenon, not involved in the onset of the autoimmune disease.
In conclusion, we report an increased H. pylori prevalence only in hyperthyroid GD patients, but not in hypothyroid HT patients, although the strains involved in both GD and HT are, prevalently, carriers of Cag-A antigens. These results suggest the execution of screening for H. pylori in ATDs patients, taking into account either the presence of virulent strains in autoimmune diseases and the increased H. pylori prevalence in GD. Therefore, a possible role of H. pylori infection might be postulated for GD, but further studies are needed to confirm such a hypothesis.
We thank Dr. Giovanna Allevato and Dr. Serena Leone for careful reading of the manuscript.
Autoimmune thyroid diseases (ATDs) are represented, essentially, by Hashimoto’s thyroiditis (HT) and its variants (postpartum and sporadic thyroiditis) and Graves’ disease (GD). Helicobacter pylori (H. pylori) infection is found worldwide with an incidence of up to 50% in the population of developed countries and a possible correlation has been suggested between the bacterium and ATDs.
A wide range of diseases are correlated with the presence of H. pylori and a possible pathogenetic role is suspected.
A noteworthy correlation between H. pylori and GD, but not with HT, has been demonstrated. In contrast, the prevalence of Cag-A expression was increased in both ATDs.
Screening for H. pylori in ATDs patients is suggested, taking into account either the presence of virulent strains in autoimmune diseases and the increased H. pylori prevalence in GD.
The manuscript is well written and the methods are adequate. The results justify the conclusions drawn.
Peer reviewers: Majid Assadi, MD, Associate Professor, The Persian Gulf Biomedical Sciences Institute, Bushehr University of Medical Sciences, Boostan 19 Alley, Sangi Street, Bushehr 7514763448, Iran; Mario M D’Elios, Professor, University of Florence, viale Morgagni 85, Florence 50134, Italy
S- Editor Gou SX L- Editor Webster JR E- Editor Zhang DN
| 1. | Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med. 2003;348:2646-2655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 550] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 2. | Delemarre FG, Simons PJ, Drexhage HA. Histomorphological aspects of the development of thyroid autoimmune diseases: consequences for our understanding of endocrine ophthalmopathy. Thyroid. 1996;6:369-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Rapoport B, McLachlan SM. Thyroid autoimmunity. J Clin Invest. 2001;108:1253-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Valtonen VV, Ruutu P, Varis K, Ranki M, Malkamaki M, Makela P. Serological evidence for the role of bacterial infections in the pathogenesis of thyroid diseases. Acta Med Scand. 1986;219:105-111. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Joasoo A, Robertson P, Murray IPC. Viral antibodies and thyrotoxicosis. Lancet. 1975;2:125-131. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Tomer Y, Davies TF. Infection, thyroid disease, and autoimmunity. Endocr Rev. 1993;14:107-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 79] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1352] [Article Influence: 75.1] [Reference Citation Analysis (1)] |
| 8. | Ponzetto A, Pellicano R, Morgando A, Cirillo D, Marchiaro G, Curti F, Rizzetto M. Seroprevalence of Helicobacter pylori infection among blood donors in Torino, Italy. Minerva Gastroenterol Dietol. 2001;47:3-7. [PubMed] |
| 9. | Atherton JC. H. pylori virulence factors. Br Med Bull. 1998;54:105-120. [PubMed] |
| 10. | de Luis DA, Varela C, de La Calle H, Cantón R, de Argila CM, San Roman AL, Boixeda D. Helicobacter pylori infection is markedly increased in patients with autoimmune atrophic thyroiditis. J Clin Gastroenterol. 1998;26:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Figura N, Di Cairano G, Lorè F, Guarino E, Gragnoli A, Cataldo D, Giannace R, Vaira D, Bianciardi L, Kristodhullu S. The infection by Helicobacter pylori strains expressing CagA is highly prevalent in women with autoimmune thyroid disorders. J Physiol Pharmacol. 1999;50:817-826. [PubMed] |
| 12. | Papamichael KX, Papaioannou G, Karga H, Roussos A, Mantzaris GJ. Helicobacter pylori infection and endocrine disorders: is there a link? World J Gastroenterol. 2009;15:2701-2707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Tomasi PA, Dore MP, Fanciulli G, Sanciu F, Realdi G, Delitala G. Is there anything to the reported association between Helicobacter pylori infection and autoimmune thyroiditis? Dig Dis Sci. 2005;50:385-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Franceschi F, Satta MA, Mentella MC, Penland R, Candelli M, Grillo RL, Leo D, Fini L, Nista EC, Cazzato IA. Helicobacter pylori infection in patients with Hashimoto's thyroiditis. Helicobacter. 2004;9:369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Bassi V, Santinelli C, Iengo A, Romano C. Identification of a correlation between Helicobacter pylori infection and Graves' disease. Helicobacter. 2010;15:558-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Johansen HK, Nørgaard A, Andersen LP, Jensen P, Nielsen H, Høiby N. Cross-reactive antigens shared by Pseudomonas aeruginosa, Helicobacter pylori, Campylobacter jejuni, and Haemophilus influenzae may cause false-positive titers of antibody to H. pylori. Clin Diagn Lab Immunol. 1995;2:149-155. [PubMed] |
| 17. | Roura-Mir C, Catálfamo M, Sospedra M, Alcalde L, Pujol-Borrell R, Jaraquemada D. Single-cell analysis of intrathyroidal lymphocytes shows differential cytokine expression in Hashimoto's and Graves' disease. Eur J Immunol. 1997;27:3290-3302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Aspholm-Hurtig M, Dailide G, Lahmann M, Kalia A, Ilver D, Roche N, Vikström S, Sjöström R, Lindén S, Bäckström A. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science. 2004;305:519-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 303] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 19. | Borén T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 805] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 20. | Mahdavi J, Sondén B, Hurtig M, Olfat FO, Forsberg L, Roche N, Angstrom J, Larsson T, Teneberg S, Karlsson KA. Helicobacter pylori SabAadhesin in persistent infection and chronic inflammation. Science. 2002;297:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 665] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 21. | Guzman-Murillo MA, Ruiz-Bustos E, Ho B, Ascencio F. Involvement of the heparansulphate-binding proteins of Helicobacter pylori in its adherence to HeLa S3 and Kato III cell lines. J Med Microbiol. 2001;50:320-329. [PubMed] |
| 22. | Miller-Podraza H, Weikkolainen K, Larsson T, Linde P, Helin J, Natunen J, Karlsson KA. Helicobacter pylori binding to new glycans based on N-acetyllactosamine. Glycobiology. 2009;19:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Ilver D, Arnqvist A, Ogren J, Frick IM, Kersulyte D, Incecik ET, Berg DE, Covacci A, Engstrand L, Borén T. Helicobacter pylori adhesin binding fucosylatedhisto-blood group antigens revealed by retagging. Science. 1998;279:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 842] [Article Influence: 31.2] [Reference Citation Analysis (1)] |
| 24. | Byfield PG, Davies SC, Copping S, Barclay FE, Borriello SP. Thyrotrophin (TSH)-binding proteins in bacteria and their creoss reaction with autoantibodies against the human TSH receptor. J Endocrinol. 1989;121:571-577. |
| 25. | Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2635] [Cited by in RCA: 2587] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 26. | Bertalot G, Montresor G, Tampieri M, Spasiano A, Pedroni M, Milanesi B, Favret M, Manca N, Negrini R. Decrease in thyroid autoantibodies after eradication of Helicobacter pylori infection. Clin Endocrinol (. Oxf). 2004;61:650-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Ko GH, Park HB, Shin MK, Park CK, Lee JH, Youn HS, Cho MJ, Lee WK, Rhee KH. Monoclonal antibodies against Helicobacter pylori cross-react with human tissue. Helicobacter. 1997;2:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | D'Elios MM, Appelmelk BJ, Amedei A, Bergman MP, Del Prete G. Gastric autoimmunity: the role of Helicobacter pylori and molecular mimicry. Trends Mol Med. 2004;10:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Bergman MP, D'Elios MM. Cytotoxic T cells in H. pylori-related gastric autoimmunity and gastric lymphoma. J Biomed Biotechnol. 2010;2010:104918. [PubMed] |
| 30. | Nabwera HM, Nguyen-van-Tam JS, Logan RFA. Prevalence of Helicobacter pylori infection in Kenyan schoolchildren aged 3–15 years and risk factors for infection. Eur J Gastroenterol Hepatol. 2000;12:483-487. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Larizza D, Calcaterra V, Martinetti M, Negrini R, De Silvestri A, Cisternino M, Iannone AM, Solcia E. Helicobacter pylori infection and autoimmune thyroid disease in young patients: the disadvantage of carrying the human leukocyte antigen-DRB1*0301 allele. J Clin Endocrinol Metab. 2006;91:176-179. [PubMed] |