Published online Feb 28, 2011. doi: 10.3748/wjg.v17.i8.1082
Revised: November 30, 2010
Accepted: December 7, 2010
Published online: February 28, 2011
AIM: To compare the efficacy and safety of paclitaxel combined with fluorouracil plus cisplatin (PCF), and oxaliplatin combined with fluorouracil plus leucovorin (FOLFOX-4) regimens for advanced gastric cancer (AGC).
METHODS: Ninety-four patients with AGC were randomly assigned to receive paclitaxel (50 mg/m2 iv) on days 1, 8 and 15, cisplatin (20 mg/m2 iv) and fluorouracil (750 mg/m2 iv) on days 1-5, or oxaliplatin (85 mg/m2 iv) and leucovorin (200 mg/m2 iv) on day 1, followed by bolus fluorouracil (400 mg/m2 iv) and fluorouracil (600 mg/m2 iv) on days 1 and 2. The primary end point was the 1-year survival time.
RESULTS: The overall response rate (ORR) of the patients was 48.0% and 45.5% to PCF and FOLFOX-4, respectively. The disease control rate (DCR) of PCF and FOLFOX-4 was 82.0% and 81.8%, respectively. The median survival times (MSTs) of the patients were 10.8 and 9.9 mo, respectively, after treatment with PCF and FOLFOX-4. The 1-year survival rate of the patients was 36.0% and 34.1%, respectively, after treatment with PCF and FOLFOX-4. No significant difference was observed in ORR, DCR, MST or 1-year survival rate between the two groups. The most common adverse events were anemia, nausea and vomiting, and grade 3/4 alopecia in PCF treatment group, and anemia, grade 1/2 neurotoxic effect and grade 3/4 neutropenia in FOLFOX-4 treatment group.
CONCLUSION: Patients with AGC have a similar response rate to PCF and FOLFOX-4 regimens with a similar survival rate. The PCF and FOLFOX-4 regimens are efficacious and tolerable as a promising therapy for AGC.
- Citation: Li XD, Shen H, Jiang JT, Zhang HZ, Zheng X, Shu YQ, Wu CP. Paclitaxel based vs oxaliplatin based regimens for advanced gastric cancer. World J Gastroenterol 2011; 17(8): 1082-1087
- URL: https://www.wjgnet.com/1007-9327/full/v17/i8/1082.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i8.1082
Gastric cancer is the second leading cause of cancer-related death worldwide, with the highest incidence in Eastern Asian and European countries[1]. The incidence of gastric cancer in Jiangsu Province of China is particularly high, and the death rate is much higher than the national average[2]. Unfortunately, most patients with advanced gastric cancer (AGC) have a miserable outcome. Even after curative gastrectomy, 60% of AGC patients develop local recurrences or distant metastasis[3-5].
Although the efficacy of palliative chemotherapy is now widely accepted[6-8], no chemotherapeutic regimen has been established as the consensus standard treatment for AGC. Among various chemotherapy regimens, paclitaxel combined with fluorouracil plus cisplatin (PCF) and oxaliplatin combined with fluorouracil plus leucovorin (FOLFOX-4) regimens are the two commonly used modalities.
It has been demonstrated that paclitaxel, an anticancer agent which binds to microtubules and induces hyperstabilization leading to cell cycle arrest and apoptosis[9,10], has a promising efficacy against gastric cancer. The response rate of gastric cancer patients to it is about 20%-25%, and the median response time of gastric cancer patients is about 7 mo after treatment with paclitaxel[11-13]. It was reported that the response rate of patients with gastric cancer to PCF regimen is 33%[14-17].
Oxaliplatin, a third-generation diaminocyclohexane platinum compound that has a wide range of antitumor activities, appears to have a better safety profile than cisplatin in terms of nausea, vomiting, nephrotoxicity, and ototoxicity[18,19]. The response rate of AGC patients to FOLFOX-4 regimen is 38%-43% and FOLFOX-4 regimen shows a manageable toxicity profile as the first-line treatment modality for AGC[20-24].
As is commonly known, there is only one best regimen at one time. No study is available comparing the efficacy and safety of PCF and FOLFOX-4 regimens. Therefore, we designed the present study to observe the therapeutic indexes of the two regimens for AGC.
The inclusion criteria for AGC patients were (1) pathologically proved locally advanced (non-resectable) or metastatic gastric cancer; (2) age between 20 and 75 years; (3) measurable or assessable lesions by imaging studies according to the RECIST guidelines[25]; (4) no prior chemotherapy except for postoperative adjuvant chemotherapy for more than 12 mo before entry into the study; (5) Eastern Cooperative Oncology Group (ECOG) performance status 0-2; (6) adequate bone marrow functions (hemoglobin level ≥ 90 g/L, white blood cell count of 4-10 × 109/L, neutrophil count ≥ 2 × 109/L, and platelet count ≥ 100 × 109/L), hepatic function (total bilirubin ≤ 1.5 × the institutional upper limit of normal value, aspartate aminotransferase/alanine aminotransferase ≤ 2.5 × the institutional upper limit of normal value, and alkaline phosphatase ≤ 2.5 × the institutional upper limit of normal value), renal function (serum creatinine level ≤ 1.5 mg/dL and creatinine clearance ≥ 50 mL/min); and (7) estimated life expectancy of at least 3 mo and no other malignancies.
The exclusion criteria for patients included (1) pre-existing peripheral toxicity ≥ grade 2 of the National Cancer Institute Common Toxicity Criteria (NCI-CTC, Version 3.0); (2) pregnant, and breastfeeding women or women of child-bearing potential without adequate contraception; (3) concurrent or prior malignancy; (4) central nervous system metastases; (5) active infection; (6) other uncontrolled underlying medical conditions that would impair the ability of the patients to receive the planned treatment; (7) inadequate calorie and fluid intake; and (8) concurrent treatment that interfered with the study evaluation.
The study, approved by the ethics committees of all participating medical institutions, was conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice Guidelines. All patients gave their written informed consent before enrollment.
The patients were divided into PCF group and FOLFOX-4 group. Patients in the PCF group received paclitaxel (50 mg/m2 iv) for 3 h on days 1, 8 and 15, cisplatin (20 mg/m2 iv) for 2 h on days 1-5, fluorouracil (750 mg/m2 iv) for 24 h for 5 d. The treatment was repeated every 28 d for 6 cycles. Patients in the FOLFOX-4 group received oxaliplatin (85 mg/m2 iv) and leucovorin (200 mg/m2 iv) for 2 h on day 1, bolus fluorouracil (400 mg/m2 iv) and fluorouracil (600 mg/m2 iv) for 22 h on days 1 and 2. The treatment was repeated every 14 d for 12 cycles.
The dose was modified based on the hematologic parameters and the degree of non-hematologic toxicities. Physical examination, chest X-ray, complete blood test and biochemical tests were performed before each chemotherapy cycle. The toxicity was graded based on the NCI-CTC (Version 3.0).
The dose was modified for the PCF group as follows: (1) If the hepatotoxicity was grade 2, the dose of paclitaxel for the following treatment was reduced to 40 mg/m2 on days 1, 8 and 15. If the hepatotoxicity was grade 3/4, the study was discontinued; (2) If the bone marrow suppression was grade 4, the dose of paclitaxel for the following treatment was reduced to 40 mg/m2 on days 1, 8 and 15. If the bone marrow suppression was grade 4, the study was discontinued; (3) If the mucositis was grade 3/4, fluorouracil was administered from the next cycle for 3 d; and (4) If the creatinine clearance rate was 30-50 mL/min due to the nephrotoxicity, the dose of cisplatin was reduced by 50%. If the creatinine clearance rate was lower than 30 mL/min, the study was discontinued.
The dose was modified for the FOLFOX-4 group as follows: (1) If the neurotoxic effect was grade 1/2, the dose of oxaliplatin was reduced by 25%. If the neurotoxic effect was grade 3/4 or persistent, the oxaliplatin was omitted from the regimen until the neurotoxic effect was resolved to grade 1 or better; (2) If the mucositis was grade 3/4, fluorouracil was administered from the next cycle for 3 d; and (3) If grade 3/4 diarrhea, stomatitis or dermatitis occurred, the dose of fluorouracil was reduced by 25%.
The parameters of 12-lead electrocardiogram, computed tomography (CT) scan, and levels of tumor markers (CA19-9, CA72-4, CA24-2 and carcinoembryonic antigen) were obtained from the patients within 7 d after enrollment. Hematology tests, biochemistry tests, and assessment of symptoms and signs were carried out for the patients within 3 d before enrollment and every week during the study period. CT scans were carried out and levels of tumor markers were measured before each cycle. According to the RECIST guidelines[17], responses concluded complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). To confirm the PR or CR, the levels of tumor markers were measured no less than 4 wk after the objective response was obtained. Responses were assessed by the independent review committee. The overall response rate (ORR) was defined as the sum of CR and PR rates. The disease control rate (DCR) was defined as the sum of CR, PR and SD rates. Toxic effects were evaluated according to the NCI-CTC (Version 3.0). The overall survival time (OST) was defined as the period from the date of treatment to the death of patients. The median survival time (MST) was defined as the half of OST.
Statistical analysis was performed with the SPSS software (Version 17.0, SPSS). Chi-square test was used to compare the categorical data. KaplanMeier method was used to calculate the OST. Logrank test was used to compare the OST. P < 0.05 was considered statistically significant.
From January 2003 to December 2007, 94 patients were enrolled in this study. The baseline clinical characteristics of the patients were compared between the two groups (Table 1). No significant difference was observed in any clinical characteristics between the two groups.
| Characteristics | Patients (%) | P | |
| PCF(n = 50) | FOLFOX-4(n = 44) | ||
| Gender | |||
| Female | 18 | 13 | 0.520 |
| Male | 32 | 31 | |
| Age (yr) | |||
| Median | 59 | 58 | 0.876 |
| Range | 20-74 | 20-75 | |
| Histologic type | |||
| Adenocarcinoma | 34 | 36 | 0.158 |
| Adenosquamous carcinoma | 3 | 1 | |
| Signet ring cell carcinoma | 5 | 3 | |
| Mucinous carcinoma | 7 | 3 | |
| Neuroendocrine carcinoma | 1 | 1 | |
| No. of metastatic lesion | |||
| 0-1 | 24 | 21 | 1 |
| ≥ 2 | 26 | 23 | |
| Stage | |||
| IIIb | 22 | 17 | 0.677 |
| IV | 28 | 27 | |
| Prior adjuvant chemotherapy | |||
| No | 38 | 31 | 0.642 |
| Yes | 12 | 13 | |
All the patients were evaluated for their response to PCF and FOLFOX-4 regimens and no patient was excluded from the efficacy analysis because of severe side effects.
Of the patients in PCF group, 1 achieved a CR and 23 a PR, 17 had SD, and 9 PD, with an ORR of 48%. The response rate of patients who received prior chemotherapy to PCF was 52.6% (20/38) and 33.3% (4/12), respectively, with a DCR of 82.0%.
Of the patients in FOLFOX-4 group, 1 achieved a CR and 19 a PR, and 16 had SD and 8 PD, with an ORR of 45.5%. The response rate of patients who received prior chemotherapy to FOLFOX-4 was 54.8% (17/31) and 23.1% (3/13), respectively, with a DCR of 81.8%. No significance was observed in ORR and DCR between the two groups.
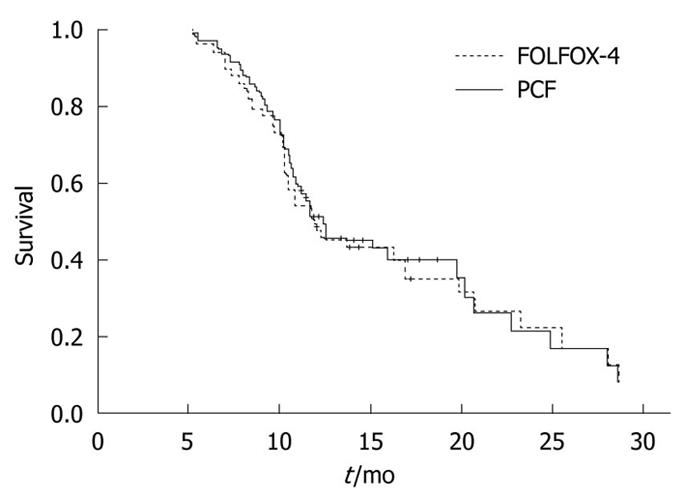
The MST and the 1-year survival rate of patients in the PCF group was 10.8 mo (95% CI: 8.9-12.7 mo) and 36.0%, respectively.
The MST and the 1-year survival rate of patients in the FOLFOX-4 group was 9.9 mo (95% CI: 8.3-11.4 mo) and 34.1%, respectively.
No significant difference was found in MST and survival rate between the two groups (Figure 1).
No patient was excluded from the efficacy analysis because of severe side effects. Grade 3/4 neutropenia occurred in 8% and 10% of patients in the PCF and FOLFOX-4 groups, respectively. Anemia occurred in 60% and 57% of patients in the PCF and FOLFOX-4 groups, respectively. Nausea and vomiting occurred in 12.0% and 9% of patients in the PCF and FOLFOX-4 groups, respectively.
Grade 3/4 alopecia occurred in 10.0% and 0% of patients in the PCF group and FOLFOX-4 group, respectively (P < 0.05). Grade 1/2 neurotoxic effect was observed in 6% and 17% of patients in the PCF group and FOLFOX-4 group, respectively (P < 0.05).
Diarrhea, hepatic or renal toxicities and oral mucosa ulcer were relatively infrequent and slight.
In China, gastric cancer patients are usually diagnosed at a relative advanced stage with metastasis to other organs[26]. Although a number of treatment modalities for gastric cancer such as surgical resection combined with chemotherapy are available[27,28], no current regimen can be considered a standard therapy for AGC, thus new therapeutic strategies are required to achieve a better clinical efficacy with an acceptable toxicity profile. PCF and FOLFOX-4 regimens are commonly used as the first-line therapy for AGC. The efficacies of the two regimens against AGC are similar with different adverse events.
Taxane is one of the three milestones of anti-cancer drugs, used in the 1990’. It was reported that the ORR of AGC patients to PCF is 34%-52%[13]. The survival time of most AGC patients does not exceed 12 mo after PCF therapy with taxane or 5-FU plus cisplatin[11,12]. Kim et al[14] reported that the MST of AGC patients is 13.2 mo. The results of this study are consistent with the reported findings[11,12]. Overall, the efficacy of PCF against AGC is stable.
Oxalipaltin has been used in treatment of advanced colorectal carcinoma. It has been shown that the response rate of patients with advanced colorectal carcinoma to oxaliplatin in combination with fluorouracil is 36%-58%[29-31]. Vita et al[32] revealed that the ORR of patients with advanced colorectal carcinoma to oxaliplatin is 38% with a TTP of 7.1 mo and an OST of 11.2 mo. The results of the current study indicate that a biweekly FOLFOX-4 regimen can significantly improve the symptoms of AGC patients. The decreased ORR observed in our study might be related to the selected gastric cancer patients at IIIb or IV stage. Considering the smaller sample size and the modified doses in the present study, further study is warranted to confirm the results.
It was reported that the toxic rate of FOLFOX-4 regimen for grade 3/4 neutropenia, and nausea and vomiting is 7.6%-34% and 4%-18.1%, respectively[13], which is consistent with our results. In the present study, the neurotoxic rate of FOLFOX-4 regimen for diarrhea and oral mucosa ulcer was low, which might be due to the low PS score. The reported neurotoxic rate of PCF regimen for grade 3/4 neutropenia is 22%-86%[12,33], which is also consistent with our results. Alopecia occurred more frequently in PCF group than in FOLFOX-4 group, and vice versa. Overall, these regimens may not only prolong the survival time but also for improve the life quality of gastric cancer patients.
In summary, both PCF and FOLFOX-4 regimens can be used in treatment of AGC. Further study is warranted to confirm the results of this study.
Gastric cancer is the second most common cause of cancer-related death globally. Its incidence is the highest in Eastern Asian and European countries. Unfortunately, most gastric cancer patients are at the advanced stage when they are diagnosed. The outcome of patients with advanced gastric cancer (AGC) is poor. Chemotherapy is often used for AGC and its efficacy is now widely accepted. However, no standard combination of chemical drugs has been established. Among the different combinations, paclitaxel combined with fluorouracil plus cisplatin (PCF) and oxaliplatin combined with fluorouracil plus leucovorin (FOLFOX-4) regimens are the two commonly used modalities. There is only one best regimen at one time. No study comparing the efficacy and safety of PCF and FOLFOX-4 regimens is available at present. Therefore, we designed the present phase-2 study to observe the therapeutic indexes of the two regimens for AGC.
No current regimen can be considered as a standard therapy for AGC. PCF and FOLFOX-4 regimens are the commonly used first-line therapy for AGC. The overall survival rate (ORR) of AGC patients is 34%-52% after PCF therapy. The survival time of most AGC patients after PCF therapy with taxane, or 5-FU plus cisplatin does not exceed 12 mo. It was reported that the response rate of AGC patients to FOLXOF-4 regimen is 36%-58%. Both PCF and FOLFOX-4 regimens can be used in treatment of AGC.
No study comparing the efficacy and safety of PCF and FOLFOX-4 regimens. Therefore, we designed the present phase II study to observe the therapeutic indexes of the two regimens for AGC. The results of this study show that the two regimens are benefit not only for survival but also for quality of life patients after resection of colorectal cancer.
PCF regimen can be used for those who need to do fine jobs, and FOLFOX-4 regimen can be used for those who care more about their appearance. The current study may help patients to take more consideration about various demands in daily life.
The present study provides important data about PCF and FOLFOX-4 regimens for gastric cancer and the article is well written.
Peer reviewer: Georgina Que, MD, Department of Surgery, Mayo Clinic, 200 First Street Southwest, Rochester, MN 55905, United States
S- Editor Sun H L- Editor Wang XL E- Editor Zheng XM
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. |
| 2. | Shen X, Zhang J, Yan Y, Yang Y, Fu G, Pu Y. Analysis and estimates of the attributable risk for environmental and genetic risk factors in gastric cancer in a Chinese population. J Toxicol Environ Health A. 2009;72:759-766. |
| 3. | Wilke H, Preusser P, Fink U, Achterrath W, Mayer HJ, Stahl M, Lenaz L, Meyer J, Siewert JR, Gerlings H. New developments in the treatment of gastric carcinoma. Cancer Treat Res. 1991;55:363-373. |
| 4. | Rivera F, Vega-Villegas ME, López-Brea MF. Chemotherapy of advanced gastric cancer. Cancer Treat Rev. 2007;33:315-324. |
| 5. | Macdonald JS. Treatment of localized gastric cancer. Semin Oncol. 2004;31:566-573. |
| 6. | Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72:37-41. |
| 7. | Pyrhönen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995;71:587-591. |
| 8. | Glimelius B, Ekström K, Hoffman K, Graf W, Sjödén PO, Haglund U, Svensson C, Enander LK, Linné T, Sellström H. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163-168. |
| 9. | Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665-667. |
| 10. | Ganansia-Leymarie V, Bischoff P, Bergerat JP, Holl V. Signal transduction pathways of taxanes-induced apoptosis. Curr Med Chem Anticancer Agents. 2003;3:291-306. |
| 11. | Ajani JA, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E. Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group. J Clin Oncol. 2007;25:3210-3216. |
| 12. | Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991-4997. |
| 13. | Im CK, Jeung HC, Rha SY, Yoo NC, Noh SH, Roh JK, Chung HC. A phase II study of paclitaxel combined with infusional 5-fluorouracil and low-dose leucovorin for advanced gastric cancer. Cancer Chemother Pharmacol. 2008;61:315-321. |
| 14. | Kim YH, Shin SW, Kim BS, Kim JH, Kim JG, Mok YJ, Kim CS, Rhyu HS, Hyun JH, Kim JS. Paclitaxel, 5-fluorouracil, and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma. Cancer. 1999;85:295-301. |
| 15. | Murad AM, Petroianu A, Guimaraes RC, Aragao BC, Cabral LO, Scalabrini-Neto AO. Phase II trial of the combination of paclitaxel and 5-fluorouracil in the treatment of advanced gastric cancer: a novel, safe, and effective regimen. Am J Clin Oncol. 1999;22:580-586. |
| 16. | Roth AD, Maibach R, Martinelli G, Fazio N, Aapro MS, Pagani O, Morant R, Borner MM, Herrmann R, Honegger H. Docetaxel (Taxotere)-cisplatin (TC): an effective drug combination in gastric carcinoma. Swiss Group for Clinical Cancer Research (SAKK), and the European Institute of Oncology (EIO). Ann Oncol. 2000;11:301-306. |
| 17. | Shin SJ, Chun SH, Kim KO, Kim MK, Lee KH, Hyun MS, Bae SH, Ryoo HM, Do YR, Kwon KY. The efficacy of paclitaxel and cisplatin combination chemotherapy for the treatment of metastatic or recurrent gastric cancer: a multicenter phase II study. Korean J Intern Med. 2005;20:135-140. |
| 18. | Di Francesco AM, Ruggiero A, Riccardi R. Cellular and molecular aspects of drugs of the future: oxaliplatin. Cell Mol Life Sci. 2002;59:1914-1927. |
| 19. | Eriguchi M, Nonaka Y, Yanagie H, Yoshizaki I, Takeda Y, Sekiguchi M. A molecular biological study of anti-tumor mechanisms of an anti-cancer agent Oxaliplatin against established human gastric cancer cell lines. Biomed Pharmacother. 2003;57:412-415. |
| 20. | Louvet C, André T, Tigaud JM, Gamelin E, Douillard JY, Brunet R, François E, Jacob JH, Levoir D, Taamma A. Phase II study of oxaliplatin, fluorouracil, and folinic acid in locally advanced or metastatic gastric cancer patients. J Clin Oncol. 2002;20:4543-4548. |
| 21. | Al-Batran SE, Atmaca A, Hegewisch-Becker S, Jaeger D, Hahnfeld S, Rummel MJ, Seipelt G, Rost A, Orth J, Knuth A. Phase II trial of biweekly infusional fluorouracil, folinic acid, and oxaliplatin in patients with advanced gastric cancer. J Clin Oncol. 2004;22:658-663. |
| 22. | Chao Y, Yeh KH, Chang CJ, Chen LT, Chao TY, Wu MF, Chang CS, Chang JY, Chung CY, Kao WY. Phase II study of weekly oxaliplatin and 24-h infusion of high-dose 5-fluorouracil and folinic acid in the treatment of advanced gastric cancer. Br J Cancer. 2004;91:453-458. |
| 23. | Lordick F, Lorenzen S, Stollfuss J, Vehling-Kaiser U, Kullmann F, Hentrich M, Zumschlinge R, Dietzfelbinger H, Thoedtmann J, Hennig M. Phase II study of weekly oxaliplatin plus infusional fluorouracil and folinic acid (FUFOX regimen) as first-line treatment in metastatic gastric cancer. Br J Cancer. 2005;93:190-194. |
| 24. | Cavanna L, Artioli F, Codignola C, Lazzaro A, Rizzi A, Gamboni A, Rota L, Rodinò C, Boni F, Iop A. Oxaliplatin in combination with 5-fluorouracil (5-FU) and leucovorin (LV) in patients with metastatic gastric cancer (MGC). Am J Clin Oncol. 2006;29:371-375. |
| 25. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. |
| 26. | Chen L, Tian H, Chen J, He ZG, Tao SF, Lokesh G, Peng SY. Surgical management of gastric stump cancer: a report of 37 cases. J Zhejiang Univ Sci B. 2005;6:38-42. |
| 27. | Kollmannsberger C, Budach W, Stahl M, Schleucher N, Hehr T, Wilke H, Schleicher J, Vanhoefer U, Jehle EC, Oechsle K. Adjuvant chemoradiation using 5-fluorouracil/folinic acid/cisplatin with or without paclitaxel and radiation in patients with completely resected high-risk gastric cancer: two cooperative phase II studies of the AIO/ARO/ACO. Ann Oncol. 2005;16:1326-1333. |
| 28. | Lin WL, Li DG, Chen Q, Lu HM. Clinical and experimental study of oxaliplatin in treating human gastric carcinoma. World J Gastroenterol. 2004;10:2911-2915. |
| 29. | Lévi F, Misset JL, Brienza S, Adam R, Metzger G, Itzakhi M, Caussanel JP, Kunstlinger F, Lecouturier S, Descorps-Declère A. A chronopharmacologic phase II clinical trial with 5-fluorouracil, folinic acid, and oxaliplatin using an ambulatory multichannel programmable pump. High antitumor effectiveness against metastatic colorectal cancer. Cancer. 1992;69:893-900. |
| 30. | Lévi FA, Zidani R, Vannetzel JM, Perpoint B, Focan C, Faggiuolo R, Chollet P, Garufi C, Itzhaki M, Dogliotti L. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. J Natl Cancer Inst. 1994;86:1608-1617. |
| 31. | de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947. |
| 32. | De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, Infusino S, Damiano V, Simeone E, Diadema MR, Lieto E. A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer. 2005;92:1644-1649. |
| 33. | Lorenzen S, Hentrich M, Haberl C, Heinemann V, Schuster T, Seroneit T, Roethling N, Peschel C, Lordick F. Split-dose docetaxel, cisplatin and leucovorin/fluorouracil as first-line therapy in advanced gastric cancer and adenocarcinoma of the gastroesophageal junction: results of a phase II trial. Ann Oncol. 2007;18:1673-1679. |