Published online Feb 28, 2011. doi: 10.3748/wjg.v17.i8.1026
Revised: November 3, 2010
Accepted: November 10, 2010
Published online: February 28, 2011
AIM: To study the cell-type specific subcellular distribution of the three isoforms of nitric oxide synthase (NOS) in the rat duodenum.
METHODS: Postembedding immunoelectronmicroscopy was performed, in which primary antibodies for neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS), were visualized with protein A-gold-conjugated secondary antibodies. Stained ultrathin sections were examined and photographed with a Philips CM10 electron microscope equipped with a MEGAVIEW II camera. The specificity of the immunoreaction in all cases was assessed by omitting the primary antibodies in the labeling protocol and incubating the sections only in the protein A-gold conjugated secondary antibodies.
RESULTS: Postembedding immunoelectronmicroscopy revealed the presence of nNOS, eNOS, and iNOS immunoreactivity in the myenteric neurons, the enteric smooth muscle cells, and the endothelium of capillaries running in the vicinity of the myenteric plexus of the rat duodenum. The cell type-specific distributions of the immunogold particles labeling the three different NOS isozymes were revealed. In the control experiments, in which the primary antiserum was omitted, virtually no postembedding gold particles were observed.
CONCLUSION: This postembedding immunoelectronmicroscopic study provided the first evidence of cell-type-specific differences in the subcellular distributions of NOS isoforms.
- Citation: Talapka P, Bódi N, Battonyai I, Fekete &, Bagyánszki M. Subcellular distribution of nitric oxide synthase isoforms in the rat duodenum. World J Gastroenterol 2011; 17(8): 1026-1029
- URL: https://www.wjgnet.com/1007-9327/full/v17/i8/1026.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i8.1026
In the enteric nervous system, endogenous nitric oxide (NO) has an important role in mediating non-adrenergic, non-cholinergic (NANC) relaxation of the intestinal smooth muscle[1]. There are three genetically different isoforms of NO synthase (NOS) that account for NO production: neuronal NOS (nNOS), endothelial NOS (eNOS), and inducible NOS (iNOS)[2]. Of the three NOS isoforms, nNOS constitutes the predominant source of NO in neurons, while eNOS is the predominant source in the endothelium[3,4]. It has been demonstrated, however, that the myenteric neurons in the mouse colon express all three NOS isoforms[5], and NO synthesized by nNOS, eNOS, and iNOS regulates motility by acting as an inhibitory neurotransmitter[6,7]. NO cannot be stored in the cells; therefore, it depends on new synthesis to exert its functional properties. The subcellular compartmentalization of NOS is therefore determinative in NO production[8]. However, the tissue specificity and subcellular distribution of NOS isoforms continue to be subject to debate[9]. Accordingly, the aim of the present study was to establish the presence and subcellular distribution of NOS isoforms in the duodenum of adult rats.
All the experiments were approved by the Local Ethics Committee for Animal Research Studies at the University of Szeged. Healthy adult male Wistar rats weighing 350-400 g were used throughout the experiments. The animals were killed by cervical dislocation and segments of the duodenum were dissected, rinsed in 0.05 M phosphate buffer (PB, pH 7.4), and processed for postembedding immunohistochemistry.
The gut segments were cut along the mesentery, pinched flat, and fixed overnight at 4°C in 2% paraformaldehyde and 2% glutaraldehyde solution, buffered with 0.1 M PB. The samples were then washed and further fixed for 1 h in 1% OsO4. After fixation, the gut segments were rinsed in 0.1 M PB, dehydrated in increasing alcohol concentrations (50, 70, 96% and absolute ethanol) and acetone, and embedded in Epon epoxy resin. The Epon blocks were used to prepare ultrathin (70 nm) sections, which were mounted on Formvar-coated nickel grids and processed for immunogold labeling. All steps were performed at room temperature. Sections were preincubated in 1% bovine serum albumin solution for 30 min, incubated overnight in the primary antibodies (Table 1), followed by protein A-gold-conjugated anti-mouse (18 nm gold particles, Jackson ImmunoResearch, USA) or anti-rabbit (10 nm gold particles, Sigma-Aldrich, USA) secondary antibodies for 3 h. Sections were counterstained with uranyl acetate and lead citrate, and then examined and photographed with a Philips CM10 electron microscope equipped with a MEGAVIEW II camera. The specificity of the immunoreaction was assessed in all cases by omitting the primary antibodies from the labeling protocol and incubating the sections only in the protein A-gold-conjugated secondary antibodies. The distributions of the gold particles coding for nNOS, iNOS, and eNOS were determined in 12 ultrathin sections of the duodenum from three different animals.
| Primary antibody | Clone | Host | Company | Working dilution |
| nNOS | Monoclonal IgG1, NOS-B1 | Mouse | Sigma-Aldrich (USA) | 0.180555556 |
| eNOS | Monoclonal IgG1 clone 3 | Mouse | Transduction Laboratories (Canada) | 0.215277778 |
| iNOS | Polyclonal IgG | Rabbit | Santa Cruz Biotechnology (USA) | 0.388888889 |
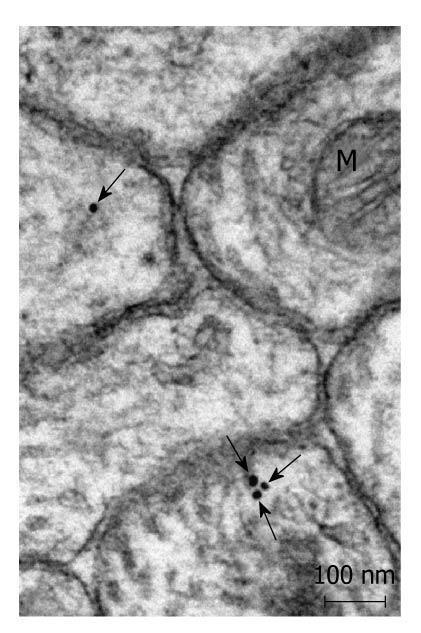
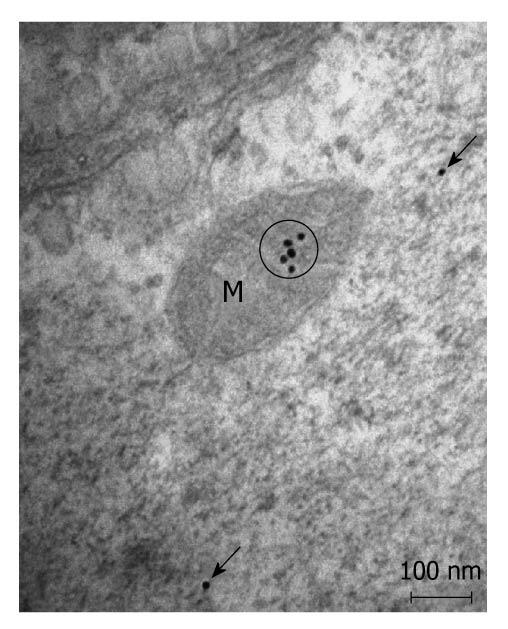
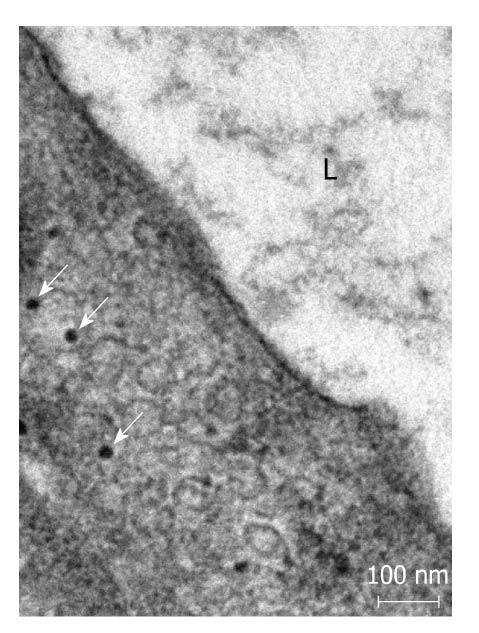
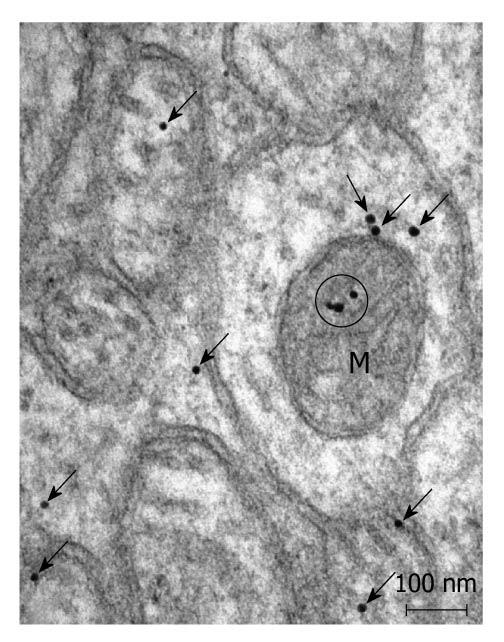
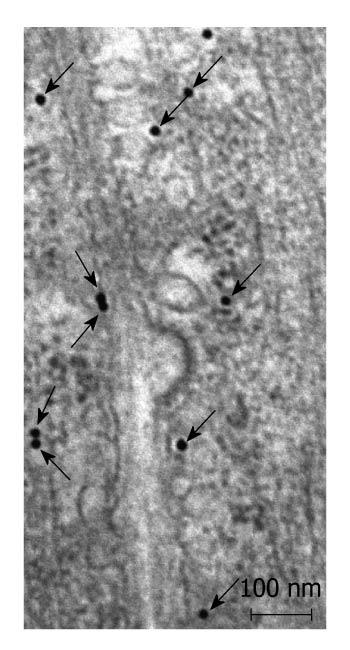
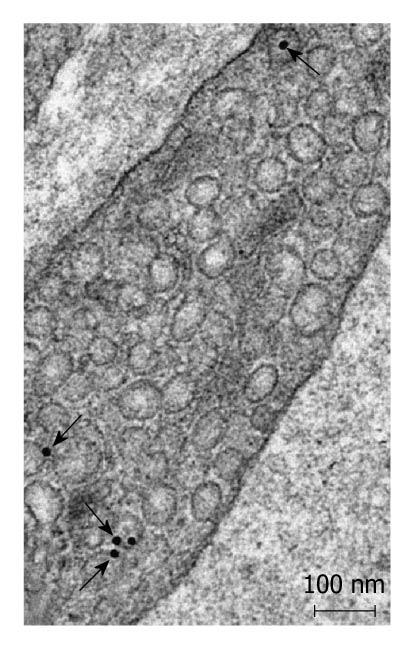
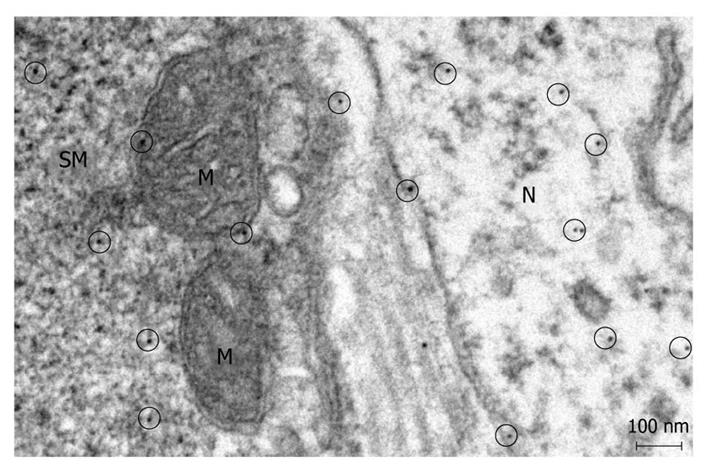
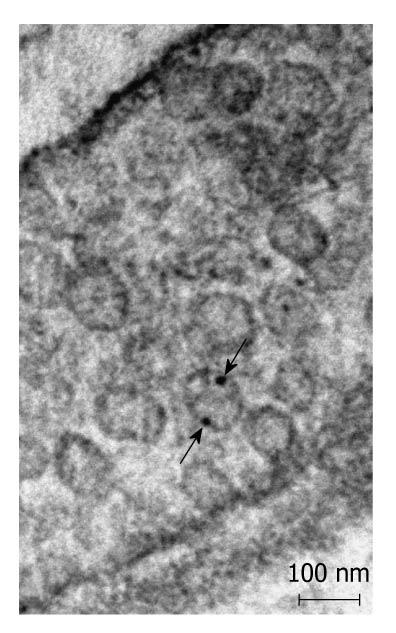
Postembedding immunoelectronmicroscopy revealed the presence of nNOS (Figures 1-3), eNOS (Figures 4-6) and iNOS (Figures 7 and 8) immunoreactivity in myenteric neurons, in enteric smooth muscle cells, and in the endothelium of the capillaries running in the vicinity of the myenteric plexus of the rat duodenum. The cell type-specific distributions of the immunogold particles labeling the three different NOS isozymes were revealed. Gold particles labeling nNOS (Figure 3), eNOS (Figure 6), or iNOS (Figure 8) in the local endothelium were localized almost exclusively to vesicle membranes or the interior of vesicles. The majority of the gold particles labeling nNOS in enteric muscle cells (Figure 2), or eNOS in nerve terminals (Figure 4), accumulated in the mitochondria. The gold particles labeling nNOS in myenteric neurons (Figure 1) or eNOS in enteric smooth muscle cells (Figure 5) were dispersed above the structureless cytoplasm. iNOS was evenly distributed in enteric muscle cells and also in myenteric neurons (Figure 7). In the control experiments in which the primary antiserum was omitted, virtually no postembedding gold particles were observed (not shown).
The present postembedding immunoelectronmicroscopic study has provided the first evidence of cell-type-specific differences in the subcellular distributions of NOS isoforms in myenteric neurons, enteric muscle cells, and the endothelium of local capillaries in the rat duodenum. We confirmed previous results[5] that all three NOS isoforms are present in myenteric neurons. However, the subcellular locations that we verified here for the NOS isoforms in the different cellular elements of the duodenal wall of rat contrasted with results of previous localization studies, where nNOS and iNOS were considered to be cytosolic[3] and to be eNOS membrane-associated[4]. To date, there has been no explanation as to why the three different NOS isoforms are expressed in myenteric neurons, enteric muscle cells, and the capillary endothelium. It appears, conceivable, however, that, in consequence of the differences in compartmentalization, the different NOS isoforms are not simultaneously active in NO synthesis in these cells. Hence, the presence of three NOS isoforms with similar functions in the same cells might reflect a functional plasticity, in which one NOS isoform can replace another under different pathophysiological conditions. The rearrangement of the subcellular NOS compartments under pathological conditions will therefore be the subject of our further investigations.
Despite being a simple molecule, nitric oxide (NO) has fundamental roles in the fields of neuroscience, physiology and immunology. In the intestine, NO has an important role in the gastrointestinal motility.
The presence of the three nitric oxide synthase (NOS) isoforms in the same cells might reflect a functional plasticity.
The present study provided the first direct evidence of cell-type-specific differences in the subcellular distributions of NOS isoforms in the rat duodenum.
The presence of three NOS isoforms with similar functions in the same cells might reflects a functional plasticity, in which one NOS isoform can replace another under different pathophysiological conditions. Further research will focus on the potential rearrangement of the subcellular NOS compartments under pathological conditions. The regulatory elements of this rearrangement might offer attractive targets for therapeutic approaches.
There are three genetically different isoforms of NOS, which account for NO production: neuronal, endothelial, and inducible NOS.
NO is a highly active molecule; therefore, the subcellular compartmentalization of the three NOS is determinative in NO production. Using postembedding immunoelectronmicroscopy it has been demonstrated that neurons, smooth muscle cells, and also endothelial cells in the gut wall express all three NOS isoforms.
Peer reviewer: Guangcun Huang, MD, PhD, Department of Center for Clinical and Translational Research, The Research Institute at Nationwide Children’s Hospital, 700 Childrens Drive, Center for Clinical and Translational Research, Research II, WA 2112, Columbus, OH 43205, United States
S- Editor Sun H L- Editor Stewart GJ E- Editor Zheng XM
| 1. | Mizuta Y, Takahashi T, Owyang C. Nitrergic regulation of colonic transit in rats. Am J Physiol. 1999;277:G275-G279. |
| 2. | Bredt DS, Glatt CE, Hwang PM, Fotuhi M, Dawson TM, Snyder SH. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991;7:615-624. |
| 3. | Marletta MA. Nitric oxide synthase: aspects concerning structure and catalysis. Cell. 1994;78:927-930. |
| 4. | Sessa WC, Barber CM, Lynch KR. Mutation of N-myristoylation site converts endothelial cell nitric oxide synthase from a membrane to a cytosolic protein. Circ Res. 1993;72:921-924. |
| 5. | Vannucchi MG, Corsani L, Bani D, Faussone-Pellegrini MS. Myenteric neurons and interstitial cells of Cajal of mouse colon express several nitric oxide synthase isoforms. Neurosci Lett. 2002;326:191-195. |
| 6. | Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Van Maercke YM, Herman AG. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990;345:346-347. |
| 8. | Oess S, Icking A, Fulton D, Govers R, Müller-Esterl W. Subcellular targeting and trafficking of nitric oxide synthases. Biochem J. 2006;396:401-409. |
| 9. | Lacza Z, Snipes JA, Zhang J, Horváth EM, Figueroa JP, Szabó C, Busija DW. Mitochondrial nitric oxide synthase is not eNOS, nNOS or iNOS. Free Radic Biol Med. 2003;35:1217-1228. |