Published online Feb 28, 2011. doi: 10.3748/wjg.v17.i8.1009
Revised: November 30, 2010
Accepted: December 7, 2010
Published online: February 28, 2011
AIM: To investigate associations between ethnicity, age and sex and the risk, colon distribution and density scores of diverticular disease (DD).
METHODS: Barium enemas were examined in 1000 patients: 410 male, 590 female; 760 whites, 62 Asians, 44 black africans (BAs), and 134 other blacks (OBs). Risks and diverticula density of left-sided DD (LSDD) and right-sided-component DD (RSCDD = right-sided DD + right and left DD + Pan-DD) were compared using logistic regression.
RESULTS: Four hundred and forty-seven patients had DD (322 LSDD and 125 RSCDD). Adjusted risks: (1) LSDD: each year increase in age increased the odds by 6% (95% CI: 5-8, SE: 0.8%, P < 0.001); Asians: odds ratio (OR): 0.23 (95% CI: 0.10-0.53, SE: 0.1, P≤ 0.001) and OBs: OR: 0.25 (95% CI: 0.14-0.43, SE: 0.07, P≤ 0.001) appeared protected vs Whites; (2) RSCDD: each year increase in age increased the odds by 4% (95% CI: 2-6, SE: 1%, P < 0.001); females were 0.60 times (95% CI: 0.40-0.90, SE: 0.12, P = 0.01) less likely than males to have RSCDD; BAs were 3.51 times (95% CI: 1.70-7.24, SE: 1.30, P < 0.001) more likely than Whites to have RSCDD; and (3) DD density scores: each year increase in age increased the odds of high-density scores by 4% (95% CI: 1-6, SE: 1%, P < 0.001); RSCDD was 2.77 times (95% CI: 1.39-3.32, SE: 0.67, P < 0.001) more likely to be of high density than LSDD. No further significant differences were found in the adjusted models.
CONCLUSION: Right colonic DD might be more common and has higher diverticula density in the west than previously reported. BAs appear predisposed to DD, whereas other ethnic differences appear conserved following migration.
- Citation: Golder M, Ster IC, Babu P, Sharma A, Bayat M, Farah A. Demographic determinants of risk, colon distribution and density scores of diverticular disease. World J Gastroenterol 2011; 17(8): 1009-1017
- URL: https://www.wjgnet.com/1007-9327/full/v17/i8/1009.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i8.1009
Diverticular disease (DD) is a common disease in western societies[1]. It causes considerable acute and chronic suffering and is a financial burden to health care systems[2]. However, primary and secondary preventative treatment of DD is not possible because its cause and many aspects of its pathogenesis remain unknown. In particular, it is not known whether differences exist in the risk, colon distribution and density scores of DD between ethnic groups living in the west. Such information could aid diagnosis and point to areas of potential research into the etiology of the disease.
Such differences exist between ethnic groups living in their native countries. In Whites in the west, DD has an overall barium enema frequency of 15%-35%, affects only the left colon in 90%-99% of cases, has no sex predilection, and increases in incidence with age[3-5]. In Southeast Asia, DD has a barium enema frequency of 8%-22%([6,7], affects the right side of the colon in 70%-98% of cases[6,8], has a slight female predilection, and a peak incidence in patients aged 50-60 years[8,9]. In Sub-Saharan Africa, DD is thought to be uncommon, affects the right colon in 62%-94% of cases, affects males more often than females, and is found in patients aged 45-60 years[10-12].
Patients with left-sided DD typically present with left-sided abdominal manifestations of acute or chronic inflammation or bleeding, and the diagnosis is usually made simply on history alone, or is confirmed by the combination of endoscopic and/or radiological investigations. In contrast, complications of right colon DD may be difficult to diagnose, because of overlap between associated symptoms and signs and those of other right-sided abdominal conditions, particularly in hospitals where the disease is considered uncommon[7]. This can lead to misdiagnoses, to inappropriate operations, or to repeated and unnecessary investigations[7,13], and to an increase in suffering for the patient and cost to the hospital.
This study aimed to investigate whether associations exist between ethnicity, age and sex and the risk, colon distribution and density scores of DD, in patients undergoing barium enema examination for non-emergency gastrointestinal symptoms in London, UK.
The Ethics Committee of University Hospital Lewisham approved the study. One thousand consecutive double contrast barium enema radiographs were analyzed from 1000 patients (410 male, 590 female, mean age: 58.73 years), who presented with non-emergency gastrointestinal symptoms, over 18 mo between 2004 and 2006, at the University Hospital Lewisham, London, UK. Non-emergency cases included those with changes in bowel habits, abdominal pain, and non-massive per-rectal bleeding. Emergency cases were excluded and included those with bowel obstruction, massive colon bleeding, colon perforation, inflammatory mass, or known colon or rectal cancer. These were investigated instead by a combination of computed tomography and colonoscopy.
The London Borough of Lewisham has a population of approximately 258 000, comprising whites (65%), Black Africans (BAs) (10%), Black Caribbean and other blacks (OBs) (15%), Asians (7.5%) and other ethnic groups (2.5%). The proportions of BAs and OBs in the Lewisham population are twice the London average.
Patients were divided into four groups according to self-reported ethnicity: (1) whites (n = 760) included White British (n = 732) and other white ethnic groups (n = 29); (2) Asians (n = 62) included Indian (n = 10), Chinese (n = 4), Bangladeshi (n = 3), Pakistani (n = 4) and other Asian background (n = 41); (3) BA (n = 44); and (4) OB (n = 134) included Black Caribbean (n = 80) and other black ethnicities (n = 54). An additional 20 patients of other ethnic background were excluded from the study, because this group did not have epidemiological relevance.
Double contrast barium enema is the most accurate method for the detection of diverticula[14], therefore, the period of investigation was chosen to coincide with that of a previous temporary reconfiguration of endoscopy services at the hospital, during which time the combination of barium enema and flexible sigmoidoscopy, rather than colonoscopy, was the chosen investigation for non-emergency cases. Three consultant radiologists, AF, MB and AS, assessed the density of diverticula in the sigmoid, descending, transverse, ascending and cecum segments of the colon, according to the following ranking system: no diverticula = 0; 1-5 diverticula = 1; 6-10 = 2; 11-15 = 3; 16-20 = 4; 21-25 = 5; 25+ = 6. The highest score across these segments for each patient was then ranked to either lower density (scores 1-3) or higher density (scores 4-6) for the purpose of statistical analysis.
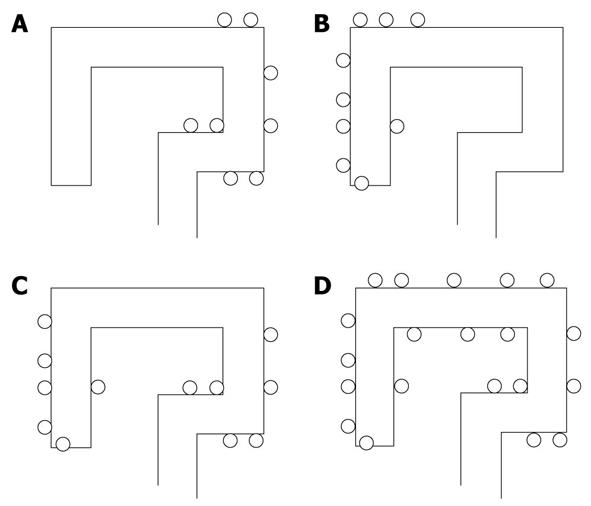
The pattern of colon distribution of diverticula was first assigned to one of four conventional categories of the disease: left-sided DD (LSDD), right-sided DD (RSDD), right and left DD (R&LDD) and Pan-DD (Figure 1). RSDD, R&LDD and Pan-DD were then grouped together as right-sided component DD (RSCDD), which included all those patients in whom DD affected the right side of the colon.
STATA 10.1 statistical package (Stata Corporation, College Station, TX, USA) was used for descriptive statistics, statistical inference and graphs. Groups, defined by sex, ethnicity and disease were compared using ANOVA or non-parametric tests. Logistic regression analysis was used to assess crude and adjusted associations between the odds of DD, DD subtypes (LSDD or RSCDD) and diverticula density, ethnicity, age and sex. Models of the risk of the disease/disease subtype/diverticula density were produced, and the Hosmer-Lemeshow test[15] was used to assess goodness of fit. Post-estimation analysis was performed to detect possible differences between non-white ethnic groups. The predefined level of type 1 error was adjusted using Bonferroni correction. For the “DD overall” analyses, this was reduced to 0.0083 (0.05 divided by 6, representing the number of all possible comparisons between the four ethnic groups), and for the disease subtype analyses to 0.004 (0.05 divided by 2 × 6). This was the first investigation of its kind, therefore, no data existed prior to the study, regarding standard deviations for the above parameters. It was therefore not possible to make sample size calculations before the study began. Budgetary constraints limited our sample size to 1000 patients.
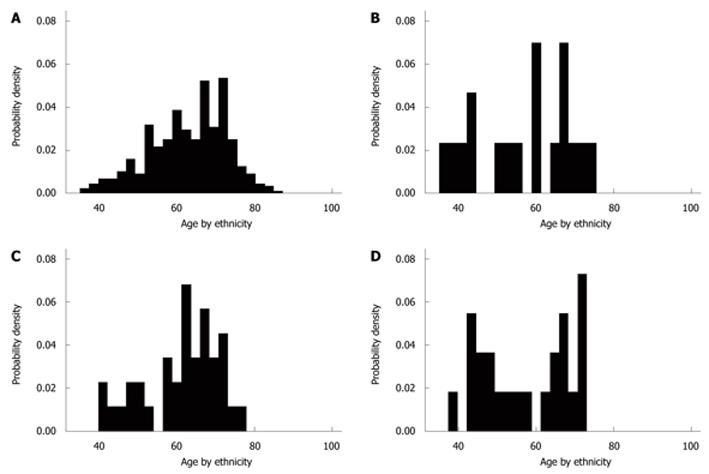
Data related to diagnosis, ethnicity and sex are summarized in Table 1. Of 1000 patients, 447 (44.7%) had DD, 322 (72%) had LSDD, and 125 (28%) had RSCDD. One hundred and ninety-one (42.7%) were male and 256 (57.3%) were female. The mean age of patients with DD was 62.28 years, which was higher than that of those without DD (55.87 years); difference 6.41 (95% CI: 5.07-7.76, P < 0.001). The mean age of patients with LSDD was 2.74 years (95% CI: 0.52-4.95) greater than that of patients with RSCDD, P = 0.016 (Bonferroni correction, P = 0.05/3 = 0.017). The age distribution in the diseased population did not exhibit normality (Figure 2). The mean ages of BAs (57.6 years) and Asians (56.8 years) were lower than that for whites (62.9 years) and OBs (61.2 years) with the disease. Nevertheless, in BA there was a bimodal age distribution, centered in younger patients (12 less than 60 years old) with a mean age of 47.83 years, and older patients (11 more than 60 years old) with a mean age of 68.18 years.
| Ethnicity sex DD site | Whites | Asians | OBs | BAs | Sub-totals by sex | Totals | |||||
| M | F | M | F | M | F | M | F | M | F | ||
| A. LSDD | 114 | 176 | 3 | 4 | 5 | 12 | 2 | 6 | 126 | 196 | 322 |
| B. RSDD | 6 | 4 | 4 | 1 | 2 | 4 | 4 | 1 | 16 | 10 | 26 |
| C. R&LDD | 13 | 10 | 1 | 0 | 1 | 1 | 4 | 1 | 19 | 12 | 31 |
| D. Pan-DD | 20 | 26 | 3 | 2 | 7 | 5 | 2 | 3 | 32 | 36 | 68 |
| No disease | 150 | 241 | 24 | 20 | 34 | 63 | 11 | 10 | 219 | 334 | 553 |
| Sub-totals by sex (all) | 303 | 457 | 35 | 27 | 49 | 85 | 23 | 21 | 410 | 590 | 1000 |
| Sub-totals by ethnicity (all) | 760 | 62 | 134 | 44 | 410 | 590 | 1000 | ||||
| Sub-totals by sex (DD overall) | 153 | 216 | 11 | 7 | 15 | 22 | 12 | 11 | 191 | 256 | 447 |
| Sub-totals by ethnicity (DD overall) | 369 | 18 | 27 | 23 | 191 | 256 | 447 | ||||
| Right-sided component (B+C+D) | 39 | 40 | 8 | 3 | 10 | 10 | 10 | 5 | 67 | 58 | 125 |
| (RSCDD) | 79 | 11 | 20 | 15 | 67 | 58 | 125 | ||||
Age and ethnicity, but not sex, were associated with the risk of DD (Table 2). A 1-year increase in the age of an individual increased the odds of having the disease by 6% (4%-7%), (P < 0.001), irrespective of ethnicity. Univariate analysis showed that Asians were 0.43 times (95% CI: 0.25-0.76, P = 0.004), and OBs were 0.40 times (95% CI: 0.27-0.61 P < 0.001) less likely than whites to have DD. BAs were 2.87-fold (95% CI: 1.42-5.77, P = 0.003) more likely to acquire the disease compared with OBs. There was a trend towards BA being more likely than Asians to develop the disease [odds ratio (OR): 2.68, 95% CI: 1.2-5.99, P = 0.017], but this was not significant when the pre-defined level dropped to 0.004. No significant differences were found between BAs and whites or between OBs and Asians at the studied sample size. In the adjusted models, sex was dropped because it had no confounding effect. Statistical significance was preserved for OR between OBs and whites (P < 0.001) and between BAs and OBs (P = 0.002), but the odds in Asians increased to 0.49 of that in whites (P = 0.018).
| Univariate analysis | Adjusted analysis-final models | |||
| OR/proportional odds (SE) (95% CI) | P value | OR/proportional odds (SE) (95% CI) | P value | |
| Disease vs no disease | ||||
| Asian (vs white) | 0.43 (0.13) (0.25, 0.76) | 0.004 | 0.49 (0.15) (0.27, 0.87) | 0.018 |
| OB (vs white) | 0.40 (0.08) (0.27, 0.61) | < 0.001 | 0.43 (0.09) (0.28, 0.65) | < 0.001 |
| BA (vs white) | 1.16 (0.36) (0.63, 2.13) | 0.63 | 1.39 (0.45) (0.74, 2.62) | 0.31 |
| Sex (F vs M) | 0.88 (0.11) (0.68, 1.13) | 0.32 | - | - |
| Age (yr) (proportional odds) | 1.06 (0.007) (1.04, 1.07) | < 0.001 | 1.06 (0.007) (1.04, 1.07) | < 0.001 |
| Post estimation | ||||
| BA (vs Asian) | 2.68 (1.10) (1.20, 5.99) | 0.017 | 2.83 (1.21) (1.22, 6.56) | 0.015 |
| BA (vs OB) | 2.87 (1.03) (1.42, 5.77) | 0.003 | 3.22 (1.21) (1.55, 6.71) | 0.002 |
| OB (vs asian) | 0.93 (0.32) (0.44, 1.74) | 0.84 | 0.87 (0.31) (0.45, 1.79) | 0.70 |
| LSDD vs no disease | ||||
| Asian (vs white) | 0.21 (0.09) (0.1, 0.48) | < 0.001 | 0.23 (0.1) (0.10, 0.53) | 0.001 |
| OB (vs white) | 0.24 (0.06) (0.14, 0.40) | < 0.001 | 0.25 (0.07) (0.14, 0.43) | < 0.001 |
| BA (vs white) | 0.51 (0.22) (0.22, 1.18) | 0.12 | 0.62 (0.27) (0.26, 1.46) | 0.27 |
| Sex (F vs M) | 1.05 (0.15) (0.79, 1.39) | 0.75 | - | - |
| Age (yr) (proportional odds) | 1.06 (0.008) (1.05, 1.08) | < 0.001 | 1.06 (0.008) (1.05, 1.08) | < 0.001 |
| Post estimation | ||||
| BA (vs asian) | 2.39 (1.39) (0.77, 7.49) | 0.13 | 2.71 (1.62) (0.83, 8.71) | 0.10 |
| BA (vs OB) | 1.10 (0.53) (0.43, 2.85) | 0.84 | 2.48 (1.26) (0.91, 6.72) | 0.075 |
| OB (vs Asian) | 2.17 (1.07) (0.83, 5.70) | 0.11 | 1.09 (0.54) (0.41, 2.89) | 0.88 |
| RSCDD vs no disease | ||||
| Asian (vs white) | 1.24 (0.44) (0.61, 2.50)) | 0.55 | 1.23 (0.45) (0.60, 2.54) | 0.57 |
| OB (vs white) | 1.02 (0.28) (0.60, 1.75) | 0.94 | 1.07 (0.30) (0.62, 1.85) | 0.82 |
| BA (vs white) | 3.54 (1.27) (1.75, 7.16) | < 0.001 | 3.51 (1.30) (1.70, 7.24) | 0.001 |
| Sex (F vs M) | 0.57 (0.11) (0.38, 0.84) | 0.004 | 0.60 (0.12) (0.40, 0.90) | 0.01 |
| Age (yr) (proportional odds) | 1.04 (0.01) (1.02, 1.06) | < 0.001 | 1.04 (0.01) (1.02, 1.06) | < 0.001 |
| Post estimation | ||||
| BA (vs asian) | 2.86 (1.36) (1.12, 7.28) | 0.028 | 2.85 (1.39) (1.10, 7.40) | 0.03 |
| BA (vs OB) | 3.46 (1.45) (1.53, 7.86) | 0.003 | 3.30 (1.41) (1.42, 7.62) | 0.005 |
| OtB (vs asian) | 0.82 (0.34) (0.36, 1.87) | 0.64 | 0.87 (0.37) (0.36, 1.87) | 0.73 |
Age, but not sex, was a predictor for LSDD, with the percentage increase in odds per year the same as for DD overall (Table 2). Asians and OBs appeared protected against left-sided disease compared with whites, even after adjusting for age, with the odds of LSDD being 23% (95% CI: 10-53) and 25% (95% CI: 14-43, P≤ 0.001) of that in whites, respectively, (Table 2). The differences between other groups were not statistically significant, at the studied sample size.
Sex was a predictor of RSCDD (Table 2). The odds of women having RSCDD was about 40% less than that in men; an effect that was conserved after adjusting for age and ethnicity (OR: 0.60, 95% CI: 0.40-0.90, P = 0.01). Age was a strong predictor, with the adjusted analysis (unchanged from the crude estimate) finding a 4% (95% CI: 2-6, P < 0.001) increase in the odds of RSCDD, per year of age. Univariate analysis showed that BAs were 3.54 times (95% CI: 1.75-7.16, P < 0.001) more likely than whites to have RSCDD (Table 2); a ratio that was relatively unchanged after adjusting for age and sex (OR: 3.51, 95% CI: 1.70-7.24, P = 0.001). BAs were 3.46-fold (95% CI: 1.53-7.86, P < 0.003) more likely to be diagnosed with RSCDD than OBs in the crude analysis, but the difference became non-significant following adjustments for age and sex. BAs were more likely to develop RSCDD than Asians, but this trend was not significant at the studied sample size.
In the analysis of colon segments, Pan-DD had comparatively higher density scores [median (range)]: Pan-DD (sigmoid) 5 (0-6), (descending) 3 (0-6), (transverse) 3 (0-6), (ascending) 2 (0-6), (cecum) 0 (0-6); RSDD (transverse) 0 (0-2), (ascending) 1 (0-2), (cecum) 1 (0-3); R+LDD (sigmoid) 2 (0-6), (descending) 1 (0-3), (ascending) 2 (0-4), (cecum) 1 (0-2); LSDD (sigmoid) 3 (0-6), (descending) 0 (0-6), (transverse) 0 (0-4).
Disease subtype (LSDD and RSCDD) and age were strong predictors of density. The odds of a higher score in RSCDD was 1.91 times that found in LSDD (95% CI: 1.25-2.91 P = 0.003), and after adjustment for age, this increased to 2.77 (95% CI: 1.39-3.32, P < 0.001) (Table 3). In the final model, each year increase in age yielded a 4% (95% CI: 1-6, P < 0.001) increase in the odds of having a higher density score. There was not enough evidence to support an association between diverticula density and sex or ethnicity, in either the crude or final models, at the sample size studied.
| Diverticula density scores | Predictors | Univariate analysis | Adjusted analysis - Final model | ||
| OR (SE) (95% CI) | P value | OR (SE) (95% CI) | P value | ||
| Higher vs lower | Disease type: RSCDD vs LSDD | 1.91 (0.41) (1.25, 2.91) | 0.003 | 2.77 (0.67) (1.39, 3.32) | 0.001 |
| Asian (vs white) | 0.59 (0.32) (0.21, 1.70) | 0.33 | 0.47(0.27) (0.16, 1.43) | 0.18 | |
| OB (vs white) | 0.57 (0.22) (0.27, 1.22) | 0.15 | 0.41(0.17) (0.18, 0.92) | 0.03 | |
| BA (vs white) | 0.43 (0.22) (0.16, 1.18) | 0.1 | 0.30(0.17) (0.10, 0.88) | 0.03 | |
| Sex (F vs M) | 0.98 (0.19) (0.67, 1.45) | 0.92 | - | - | |
| Age (proportional odds) | 1.032 (0.01) (1.01, 1.05) | 0.002 | 1.04 (0.01) (1.01, 1.06) | < 0.001 | |
| Post-estimation | |||||
| BA (vs Asian) | 0.72 (0.52) (0.17, 3.02) | 0.66 | 0.73(0.47) (0.21, 2.60) | 0.63 | |
| BA (vs OB) | 0.96 (0.62) (0.27, 3.40) | 0.95 | 0.64 (0.48) (0.15, 2.79) | 0.55 | |
| OB (vs Asian) | 0.75 (0.47) (0.22, 2.56) | 0.65 | 0.87(0.58) (0.24, 3.21) | 0.84 | |
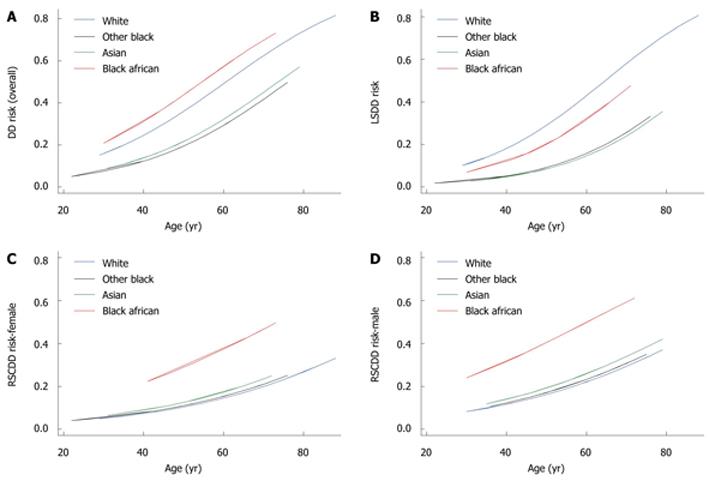
The tests for Hosmer-Lemeshow statistics were consistent with a good fit of the models to the data; with P = 0.66 for DD overall, and P = 0.65, P = 0.71 and P = 0.55 for LSDD, RSCDD and for diverticula density, respectively. Using the adjusted estimates from Tables 2 and 3, the predicted risks for disease overall, disease subtype and higher density (HD), (RDD/RLSDD/RRSCDD/RHD, respectively) were computed by:
log [RDD/(1-RDD)] = -0.10 - 0.71 ×IAS - 0.84 ×IOB + 0.33 ×IBA + 0.05 × (age - 58.73)
log [RLSDD/(1-RLSDD)] = -0.36 - 1.48 ×IAS - 1.39 ×IOB - 0.48 ×IBA + 0.06 × (age - 58.73)
log [RRSCDD/(1-RRSCDD)] = -0.31 + 0.21 ×IAS - 0.06 ×IOB + 1.26 ×IBA + 0.04 × (age - 58.73) - 0.51 ×IFEMALE
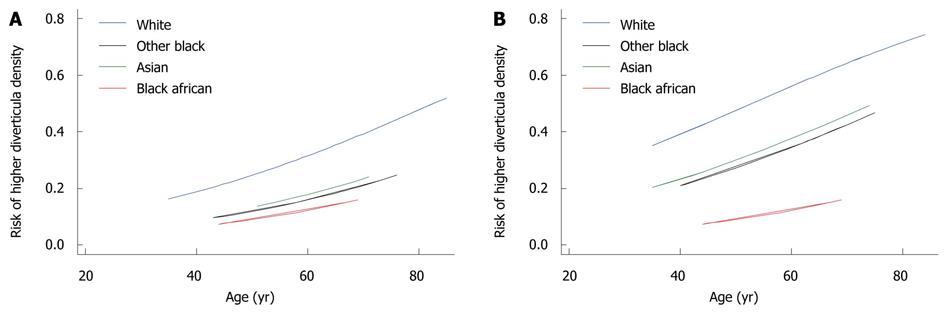
log [RHD/(1-RHD)] = -0.70 - 0.75 ×IAS - 0.89 ×IOB + 1.20 ×IBA + 0.034 × (age - 62.27) + 1.02 ×IRSCDD
The symbols IAS/IOB/IBA/IFEMALE were indicators that took the value 1 if an individual belonged to the AS/OB/BA ethnic group or was female, and 0 if otherwise. The linear combination on the right side of each expression was the linear predictor.
The free terms for disease risk RDD (e.g -0.10) represented the log odds of having that condition, where age was set to the average of 58.73 years of the population in the study and the ethnic group was set to white. For RRSCDD, the free term had a similar connotation but was in addition set to males. The free term for higher density RHD (e.g. -0.70) represented the log odds of acquiring a higher density amongst white individuals of 62.27 years of age (mean age in LSDD/RSCDD patients), with the LSDD disease type. The generic risks of disease and higher density were calculated by: R = [exp (linear predictor)/1 + (linear predictor)].
The predicted curves for risk of DD overall, DD subtypes and higher density scores are given in Figures 3 and 4.
We investigated demographic factors associated with the risk, colon distribution and density scores of DD in patients who underwent a barium enema examination for non-emergency gastrointestinal symptoms in London, UK. There were significant differences in the risk and colon distribution of DD between certain ethnic groups, and diverticula density was predicted by colon distribution and age. The results suggest that RSCDD, which comprises Pan-DD (54%), RSDD (21%) and R&LDD (25%), is significantly more common than previous studies in the west, and is most frequent in BAs. The prevalence of DD overall (47%) was high compared with previous studies in the west (15%-35%)[3-5] and supports previous reports that the prevalence of DD may be increasing with time[16,17]. Age, but not sex, was found to be a strong predictor of DD, with the mean age of patients with the disease comparable to that found in previous studies[5,18], and the chance of having the disease increased by 6% per year, irrespective of sex or ethnicity. Whites and BAs had a similar high predisposition to DD, while Asians and OBs appeared relatively protected.
These results suggest the risk of DD in BAs in London is higher than that of their indigenous counterparts, even if one accounts for an increase in prevalence of DD following urbanization in Africa[10,12,19]. Our results appear to contradict two previous studies of African migrants in Europe, which found significantly lower rates of admissions with complications of the disease, compared with native whites[16,20]. They reported that the rate was highest in younger migrants[16], and that it increased with time following settlement, possibly due to acculturation to a western diet[20]. However, the low rates of admission in those studies could have been related to low rates of acute complications that required admission amongst Africans with DD, compared with other ethnic groups, and not to an actual low prevalence of the disease. Furthermore, the reported relative high number of admissions in younger blacks could have been due to a higher proportion of blacks in the younger, compared with the older local populations. Such an explanation is supported by the current study, which found that, although there was a peak frequency of DD in young BAs, the predictive risk of the disease increased in a uniform manner with age.
It is possible that our study also suffered bias towards symptomatic patients, because it was an observational study, rather than a population study. It is therefore not possible to draw definitive conclusions from our results on actual population incidence of the disease. However, our study did have an advantage over previous studies, in that it determined with accuracy the proportion of the cases that had the disease. Furthermore, our study design did allow comparisons to be made with previous studies of DD, which all studied patients with symptomatic gastrointestinal disease, and which all included patients with symptomatic and asymptomatic DD. It also had the advantage of providing information on a cohort of patients that was typical of that seen in a standard gastrointestinal outpatient clinic. Any study that includes only asymptomatic patients or those with specific symptoms would appear artificial and could introduce bias towards certain ethnic groups and/or subtypes of the disease.
Our results suggest that the comparatively low risk of DD in Asians[6,7] is conserved in those who live outside Asia, even though more recent reports have suggested that DD is increasing in frequency in Asia with time[8,9]. Our results support those of a previous study that showed the admission rate in migrant Asians to be half that of native Europeans[20]. There have been no previous reports on the prevalence of DD in OB groups, and it remains unclear why they should be protected against the disease.
LSDD was more frequent than RSCDD, but it accounted for a lower proportion of cases compared with previous studies in the west[3,5]. Age, but not sex, was a strong predictor, with the odds of LSDD increasing by 6% per annum. Whites appeared predisposed, with Asians and OBs significantly protected. The finding that Asians are relatively protected against LSDD suggests that the typical colon distribution of the disease in Asians is conserved following population migration. There were no significant differences in predisposition between BAs and other ethnic groups, at the studied sample size.
RSCDD accounted for 28% of all cases of DD, a far higher proportion than reported previously in the west (10%-17%)[3,5], even though the sex and age mixes were comparable. This trend was probably due to predominance of Pan-DD in the largest ethnic group, whites. BA ethnicity was a strong predictor of RSCDD. BAs were significantly more likely than whites and OBs to develop RSCDD, and appeared equally predisposed to each of the three subtypes of RSCDD. There was a trend towards BAs being more likely than Asians to develop RSCDD, but this was not statistically significant, at the sample size studied.
Our findings suggest that the BA predisposition to right colon involvement[10-12] is conserved following population migration. This, together with the fact that this distribution of DD in Africa is conserved even in those regions where the incidence of the disease has increased to western levels[19] suggests that there could be specific genetic and or environmental factors involved in the pathogenesis of DD in BAs, and that such environmental factors could be conserved following population migration. Important information on possible genetic and environmental causes of the disease may come from follow-up investigations into whether the patterns of DD found in this study are exhibited by specific generations of migrants.
Evidence suggests that dietary fiber may be protective against DD[21] but exposure to red meat[4,22] and other unidentified toxins[10,12], inflammation[23], as well as genetic factors[24] may also be involved. It is probable that an imbalance between protective and promoting factors leads to abnormalities in colonic nerve innervation and connective tissue turnover found in DD[25] and to the abnormalities in colon motility[26] and subsequent diverticula formation[27].
RSCDD and age were found to be strong independent predictors of the higher density form of the disease in the final models, whereas sex and ethnic background were not. It may be argued that because patients with RSCDD were on average twice as likely to have a higher density score compared with those with LSDD, this contradicts previous anecdotal reports that the density of RSDD is less than that of LSDD. However, our results suggest that the relative contribution made by Pan-DD, which accounted for 54% of the total RSCDD, and which had significantly higher median density scores compared with the other disease subtypes, was the reason for this apparent discrepancy.
However, it is not known whether differences in the distribution or density of diverticula affect the risk of acute or chronic complications of the disease. One study has estimated that acute complications occur in 15%-20% for predominantly LSDD in the west[28] and another cited 1.0%-4.5% for predominantly RSDD in Southeast Asia, and that patients with only one or two diverticula are least likely to experience acute symptoms[8]. The proportion of patients diagnosed with DD on barium enema, who then go on to experience a chronic bleed or symptoms of irritable colon, is also unknown, although one uncontrolled study has suggested the latter may be as high as 55%[29]. Nevertheless, our results suggest that one should maintain a high index of suspicion of RSCDD as a potential diagnosis, both in outpatient and emergency settings.
However, such a diagnosis and its management may not be straight forward. There is considerable overlap between symptoms and signs of RSDD and those of other abdominal conditions including acute appendicitis[30], irritable bowel syndrome[26] and colon cancer[30]. Bleeding from RSDD can also be difficult to manage, because it may mimic upper gastrointestinal bleeding due to the presence of melena, and is less likely to respond to non-surgical treatment, compared with left-sided disease[31].
Overall, our results suggest that right-colonic DD is more common and has higher density scores in the west than previously reported. BAs appear predisposed to DD, while other ethnic differences appear conserved following migration. Within clinical practice, such knowledge is likely to aid diagnosis and management, and within research, to point to areas of potential future investigation.
Diverticular disease (DD) is common in western societies, causes considerable suffering and is a financial burden to hospitals. Differences exist in the incidence and colon distribution of DD between indigenous populations worldwide, but it is unknown if these are conserved following ethnic migration.
Indigenous Asians and Africans appear protected against DD compared with whites in the west. Right-colonic DD is more frequent in Asia and Africa than in the west. The etiology of DD and the reasons for variations in its colon distribution and severity remain unknown.
Right-colonic DD is more common in the west than previously reported and is most frequent in black africans (BAs). BAs appear predisposed to DD, while other ethnic differences appear conserved following migration. The severity of DD is determined by its colon distribution and patient age.
Within clinical practice, our findings are likely to aid diagnosis and management, and within research, to point to areas of potential future investigation.
The density scores were used as an indication of the numbers of diverticula present within the respective segments of the colon, and were calculated by diverticula counts on barium enema examinations.
This manuscript presents data on the incidence of diverticulosis in 1000 consecutive barium enema evaluations performed in London, UK. The development of diverticulosis as a result of immigration to the west is an interesting topic and one that has been reported little in contemporary literature.
Peer reviewer: Patrick O’Dwyer, MB, BCh, BAO, FRCS (1), MCh, FRCS (Glasg), University Department of Surgery, Western Infirmary, Glasgow, G11 6NT, United Kingdom
S- Editor Sun H L- Editor Kerr C E- Editor Ma WH
| 1. | Campbell WB, Lee EJ, Van de Sijpe K, Gooding J, Cooper MJ. A 25-year study of emergency surgical admissions. Ann R Coll Surg Engl. 2002;84:273-277. |
| 2. | Etzioni DA, Mack TM, Beart RW Jr, Kaiser AM. Diverticulitis in the United States: 1998-2005: changing patterns of disease and treatment. Ann Surg. 2009;249:210-217. |
| 3. | Blachut K, Paradowski L, Garcarek J. Prevalence and distribution of the colonic diverticulosis. Review of 417 cases from Lower Silesia in Poland. Rom J Gastroenterol. 2004;13:281-285. |
| 4. | Manousos ON, Truelove SC, Lumsden K. Transit times of food in patients with diverticulosis or irritable colon syndrome and normal subjects. Br Med J. 1967;3:760-762. |
| 5. | Koehler R. The incidence of colonic diverticulosis in finland and sweden. Acta Chir Scand. 1963;126:148-155. |
| 6. | Munakata A, Nakaji S, Takami H, Nakajima H, Iwane S, Tuchida S. Epidemiological evaluation of colonic diverticulosis and dietary fiber in Japan. Tohoku J Exp Med. 1993;171:145-151. |
| 7. | Chan CC, Lo KK, Chung EC, Lo SS, Hon TY. Colonic diverticulosis in Hong Kong: distribution pattern and clinical significance. Clin Radiol. 1998;53:842-844. |
| 8. | Miura S, Kodaira S, Shatari T, Nishioka M, Hosoda Y, Hisa TK. Recent trends in diverticulosis of the right colon in Japan: retrospective review in a regional hospital. Dis Colon Rectum. 2000;43:1383-1389. |
| 9. | Fong SS, Tan EY, Foo A, Sim R, Cheong DM. The changing trend of diverticular disease in a developing Nation. Colorectal Dis. 2011;13:312-316. |
| 10. | Ihekwaba FN. Diverticular disease of the colon in black Africa. J R Coll Surg Edinb. 1992;37:107-109. |
| 11. | Madiba TE, Mokoena T. Pattern of diverticular disease among Africans. East Afr Med J. 1994;71:644-646. |
| 13. | Castronovo G, Ciulla A, Tomasello G, Damiani S, Maiorana AM. Diverticular disease of right colon. Clinical variants and personal experience. Chir Ital. 2006;58:213-217. |
| 14. | Irvine EJ, O'Connor J, Frost RA, Shorvon P, Somers S, Stevenson GW, Hunt RH. Prospective comparison of double contrast barium enema plus flexible sigmoidoscopy v colonoscopy in rectal bleeding: barium enema v colonoscopy in rectal bleeding. Gut. 1988;29:1188-1193. |
| 15. | Hosmer DW, Lemeshow S. Applied Logistic Regression. John Wiley & Sons, USA, 2 ed. 2000;. |
| 16. | Jeyarajah S, Papagrigoriadis S. Diverticular disease increases and effects younger ages: an epidemiological study of 10-year trends. Int J Colorectal Dis. 2008;23:619-627. |
| 17. | Kang JY, Hoare J, Tinto A, Subramanian S, Ellis C, Majeed A, Melville D, Maxwell JD. Diverticular disease of the colon--on the rise: a study of hospital admissions in England between 1989/1990 and 1999/2000. Aliment Pharmacol Ther. 2003;17:1189-1195. |
| 18. | Debray C, Hardouin JP, Besancon F, Raimbault J. [Incidence of colic diverticulosis according to age. Statistical study from 500 barium enemas]. Sem Hop. 1961;37:1743-1745. |
| 19. | Mensah Y, Dakubo J, Asiamah S, Naaeder S. Outcome of barium enema in patients with colorectal symptoms. Ghana Med J. 2008;42:113-116. |
| 20. | Hjern F, Johansson C, Mellgren A, Baxter NN, Hjern A. Diverticular disease and migration--the influence of acculturation to a Western lifestyle on diverticular disease. Aliment Pharmacol Ther. 2006;23:797-805. |
| 21. | Aldoori WH, Giovannucci EL, Rockett HR, Sampson L, Rimm EB, Willett WC. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J Nutr. 1998;128:714-719. |
| 22. | Lin OS, Soon MS, Wu SS, Chen YY, Hwang KL, Triadafilopoulos G. Dietary habits and right-sided colonic diverticulosis. Dis Colon Rectum. 2000;43:1412-1418. |
| 23. | Simpson J, Sundler F, Humes DJ, Jenkins D, Scholefield JH, Spiller RC. Post inflammatory damage to the enteric nervous system in diverticular disease and its relationship to symptoms. Neurogastroenterol Motil. 2009;21:847-858. |
| 24. | Deshpande AV, Oliver M, Yin M, Goh TH, Hutson JM. Severe colonic diverticulitis in an adolescent with Williams syndrome. J Paediatr Child Health. 2005;41:687-688. |
| 25. | Golder M, Burleigh DE, Ghali L, Feakins RM, Lunniss PJ, Williams NS, Navsaria HA. Longitudinal muscle shows abnormal relaxation responses to nitric oxide and contains altered levels of NOS1 and elastin in uncomplicated diverticular disease. Colorectal Dis. 2007;9:218-228. |
| 26. | Sugihara K, Muto T, Morioka Y. Motility study in right sided diverticular disease of the colon. Gut. 1983;24:1130-1134. |
| 27. | Havia T, Manner R. The irritable colon syndrome. A follow-up study with special reference to the development of diverticula. Acta Chir Scand. 1971;137:569-572. |
| 28. | Parks TG. Natural history of diverticular disease of the colon. Clin Gastroenterol. 1975;4:53-69. |
| 29. | Simpson J, Neal KR, Scholefield JH, Spiller RC. Patterns of pain in diverticular disease and the influence of acute diverticulitis. Eur J Gastroenterol Hepatol. 2003;15:1005-1010. |
| 30. | Yang HR, Huang HH, Wang YC, Hsieh CH, Chung PK, Jeng LB, Chen RJ. Management of right colon diverticulitis: a 10-year experience. World J Surg. 2006;30:1929-1934. |