Published online Feb 21, 2011. doi: 10.3748/wjg.v17.i7.898
Revised: March 24, 2010
Accepted: March 31, 2010
Published online: February 21, 2011
AIM: To investigate the effect of Ginkgo biloba extract on the enteric neurons in the small intestine of diabetic rats.
METHODS: Fifteen Wistar rats were divided into three groups: control group (C), diabetic group (D) and diabetic-treated (DT) daily with EGb 761 extract (50 mg/kg body weight) for 120 d. The enteric neurons were identified by the myosin-V immunohistochemical technique. The neuronal density and the cell body area were also analyzed.
RESULTS: There was a significant decrease in the neuronal population (myenteric plexus P = 0.0351; submucous plexus P = 0.0217) in both plexuses of the jejunum in group D when compared to group C. With regard to the ileum, there was a significant decrease (P = 0.0117) only in the myenteric plexus. The DT group showed preservation of the neuronal population in the jejunum submucous plexus and in the myenteric plexus in the ileum. The cell body area in group D increased significantly (P = 0.0001) in the myenteric plexus of both segments studied as well as in the ileum submucosal plexus, when compared to C. The treatment reduced (P = 0.0001) the cell body area of the submucosal neurons of both segments and the jejunum myenteric neurons.
CONCLUSION: The purified Ginkgo biloba extract has a neuroprotective effect on the jejunum submucous plexus and the myenteric plexus of the ileum of diabetic rats.
-
Citation: Silva GGPD, Zanoni JN, Buttow NC. Neuroprotective action of
Ginkgo biloba on the enteric nervous system of diabetic rats. World J Gastroenterol 2011; 17(7): 898-905 - URL: https://www.wjgnet.com/1007-9327/full/v17/i7/898.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i7.898
Diabetes mellitus (DM) is a group of metabolic diseases characterized by high levels of glucose due to the lack of insulin and/or the inability of insulin to properly exercise its effects[1]. Long-term hyperglycemia induces morbid states in patients, resulting in macroangiopathy[2] complications, microangiopathy (retinopathy and nephropathy)[3] and neuropathies[4].
Neuropathy is the most common late complication in diabetic patients[5,6]. It compromises the sympathetic, parasympathetic and enteric nerves, causing a variety of abnormalities such as ulcerations of the lower limbs, sudden death by cardiac arrhythmia, gangrene, amputations, sexual dysfunction and gastrointestinal alterations[6,7].
The gastrointestinal tract is seriously affected by DM. Nearly 75% of diabetic patients may suffer with disorders such as late gastric emptying, vomiting, nausea, diarrhea, abdominal pain, swelling and constipation[8]. These disorders are usually correlated with enteric neuron lesions[9-12].
Oxidative stress plays an important role in the development and progression of diabetic neuropathy[13,14]. Hyperglycemia has been identified as the main cause in the development of oxidative stress by the production of reactive oxygen species (ROS) and reduction of endogenous antioxidants[15] due to auto-oxidation of blood glucose, excessive formation of AGEs (advanced glycation end products) and activation of the polyol pathway[13]. The excessive activation of the polyol pathway reduces the cytosolic NADPH, thus decreasing reduced glutathione (GSH), an important endogenous antioxidant. At the same time, this pathway produces an accumulation of sorbitol which causes cellular osmotic stress, also leading to oxidative stress[16].
ROS or free radicals such as superoxide anion (O2-), hydroxyl radical (OH-) or intermediate species such as hydrogen peroxide (H2O2), damage all classes of cell macromolecular components and organelles (e.g. mitochondria, endoplasmic reticulum, proteins, etc.), which can lead to cell death. These free radicals also degrade the cell membrane phospholipids through a process called lipid peroxidation[17].
The use of antioxidants has beneficial effects in the treatment of diabetic complications[17-19]. Ginkgo biloba extract, obtained from Ginkgo biloba leaves, has medicinal properties and is one of the most sold natural supplements in the world. This extract has antioxidant activity and neuroprotective effect, inhibiting cell death[20,21]. Husstedt et al[22] noticed that treatment with Ginkgo biloba reduced symmetrical polyneuropathy when they analyzed clinical and neurophysiological parameters and the hemorheologic changes in patients with diabetes.
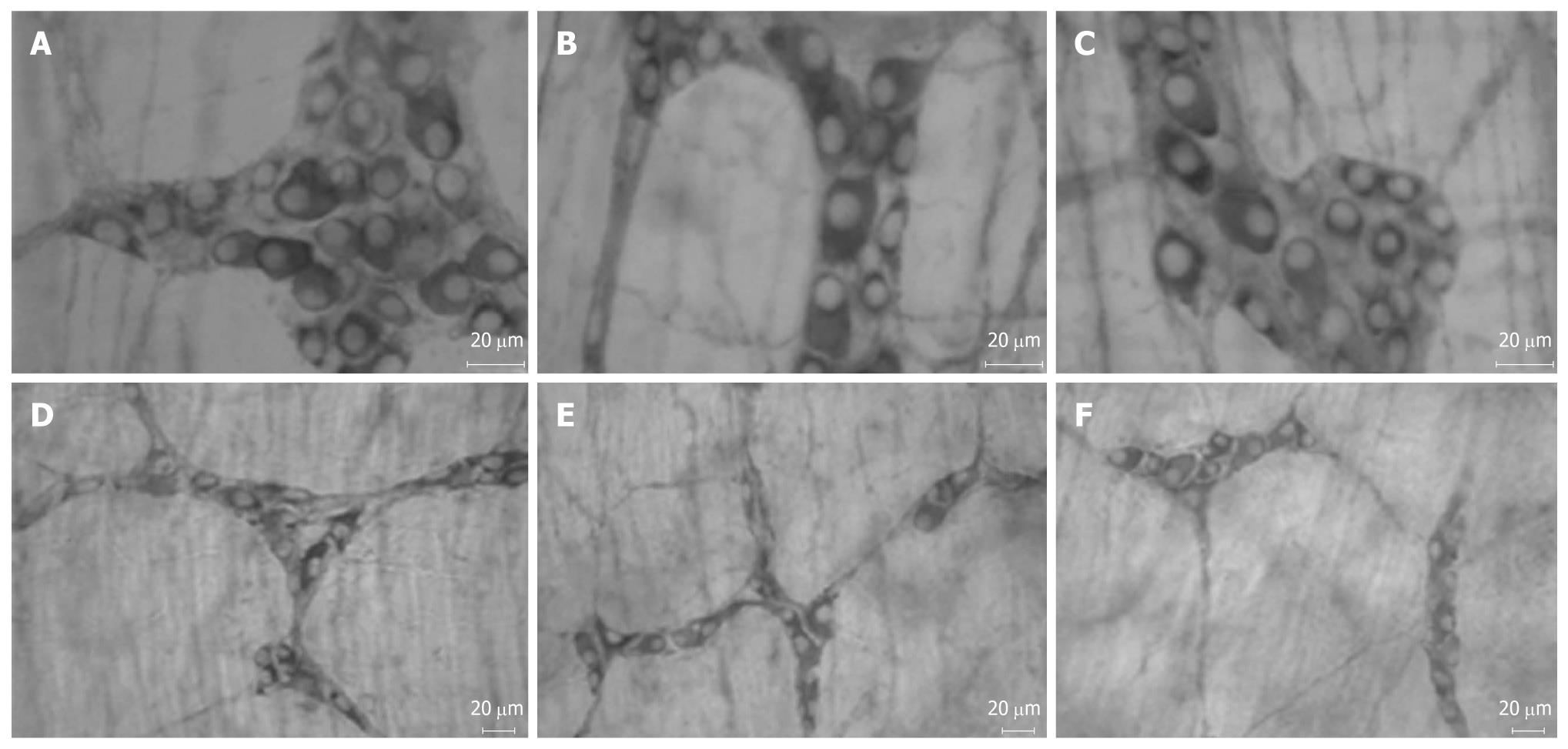
The immunohistochemical technique to identify protein myosin-V has been used to estimate the total neuronal population in different regions of the gastrointestinal tract[11,12]. This technique confers specificity in the identification of enteric neurons, because this protein is located in neuronal cytoplasm, allowing visualization of cell bodies and their projections[12].
Our aim was to analyze the effects of standardized extract of Ginkgo biloba (EGb 761) on neurons of the myenteric and submucous plexuses in the jejunum and ileum of streptozotocin-diabetic rats. To do so, a morphometric and quantitative study of enteric neurons after 120 d of treatment was carried out.
Fifteen male Wistar rats (Rattus norvegicus) were used, obtained from the Central Vivarium of the Universidade Estadual de Maringá (UEM). The animal procedures described in this work were conducted in accordance with the ethical principles of the Brazilian Academy in Animal Experimentation (COBEA) and approved by the Ethics Committee in Animal Experimentation of UEM.
The weight of animals at the beginning of the experiment was 400 g, corresponding to an approximate age of 150 d. The animals were kept for 120 d in groups of five per box in a room with a light cycle of 12/12 h (7:00 to 19:00) and at constant room temperature of 21-22°C. They were fed with Nuvilab standard diet and water ad libitum.
The animals were divided into 3 experimental groups, each group comprised of 5 animals: control group (C) (normoglycemic); diabetic group (D); diabetic-treated with Ginkgo biloba extract (EGb 761) group (DT).
To induce diabetes the rats in groups D and DT were weighed and fasted for 16 h. Then, they were injected intravenously with streptozotocin (Sigma, St. Louis, MO, USA) at a dose of 35 mg/kg of body weight.
Blood glucose levels were determined after 7 d by the glucose oxidase method to confirm the disease onset. Only animals with blood glucose higher than 200 mg/dL were kept in groups D and DT.
Besides their normal diet, the DT group animals were treated daily by gavage with the Gingko biloba (EGb 761) extract (Tebonin, Altana Pharma, Jaguariúna, São Paulo, Brazil) at a dose of 50 mg/kg of body weight throughout the experiment.
At the end of the 120-d trial period, all animals were anesthetized intraperitoneally with thiopental (40 mg/kg body weight) (Abbott Laboratories, Chicago, IL, USA). Blood was collected through cardiac puncture to assess the glycemia. After a laparotomy, the jejunum and ileum segments were collected. These segments were washed with 0.9% saline solution, the ends tied up and inflated with a fixative solution [periodate-lysine-paraformaldehyde (10 mmol/L sodium periodate, 75 mmol/L lysine, and 1% paraformaldehyde in 37 mmol/L phosphate buffer, pH 7.4)]. They were kept in vials containing the same solution for one and half hours. Thirty minutes later, two small holes were made near each end, and the fixative content was drained.
In order to improve the antibody tissue permeability, fragments of the jejunum and ileum were dehydrated in increasing series of alcohols (50%, 70%, 80%, 90%, 95%, 100% I, 100% II), cleared in xylol and rehydrated in decreasing series of alcohol up to 70%.
The dissection procedures were performed by cutting transversely the cylindrical segments of the jejunum and ileum, which were then opened longitudinally at the mesenteric insertion in order to obtain rectangular pieces. The procedure was carried out under a stereoscopy microscope and samples handled with watchmaker tweezers to obtain myenteric plexus membrane whole mounts. The mucosa and submucosal tunica were removed from the myenteric plexus, while the external muscular layer was kept. The mucosa was removed from the submucosal plexus with the aid of a wooden spatula.
The myenteric and submucous plexuses were stained by the anti-myosin-V immunohistochemical technique as described by Buttow et al[23]. The final concentration of antibody was 0.89 mg/mL. The dilution used was 1:1000 (v/v). The membranes were first immersed in a blocking solution of 0.1 mol/L PBS containing 2% bovine serum albumin (BSA) and 0.5% Triton X-100 and normal goat serum at a ratio of 1:50 (v/v) for 3 h. The material was incubated with primary antibody for 48 h at room temperature (RT); this was performed in a solution of 0.1 mol/L PBS containing 1% BSA and 0.1% Triton X-100 and normal goat serum in the proportion of 1:50 (v/v). After the incubation, the material was washed twice for 15 min with PBS solution 0.1 mol/L and Triton X-100 0.1% and then also washed twice in PBS 0.1 mol/L and Tween 20 at a concentration of 0.05% for 15 min. The whole-mounts were then incubated with anti-rabbit secondary antibody produced in goat, peroxidase-conjugated [ImmunoPure® Goat Anti-Rabbit IgG, (Fc), Peroxidase Conjugated, brand Pierce] in a blocking solution containing 0.1 mol/LPBS, 1% BSA and 0.05% Tween 20 for 24 h at RT. Normal goat serum at 1:50 (v/v) was also added to this blocking solution. The material was washed 4 times for 15 min in a solution of 0.1 mol/L PBS containing 0.05% Tween 20. The membranes were developed with the use of a diaminobenzidine solution (Sigma, St. Louis, MO, USA) for approximately 10 min at a concentration of 0.14 mg/mL. After developing, the material was mounted on histological slides with glycerol-gel (containing 50% glycerol, 0.07 g/mL gelatin in PBS, and 2 μL/mL phenol). The slides were then placed in refrigerator (4°C), in order to slowly dry the whole-mounts.
Enteric neurons were counted on a BX 40 Olympus microscope under a 40 × lens. Forty microscopy fields, randomly selected, were counted for each preparation. The area of each field was 0.229 mm2. The results were expressed in number of neurons per cm2.
Images of the ganglia were taken and then measured with the aid of the image analysis software Image Pro-Plus 3.0.1 (Media Cybernetics, Silver Spring, MD, USA) to study the area of neurons in different groups. The area (μm2) of 100 cell bodies per animal was measured, for a total of 500 neurons (5 animals per group). Neurons were classified into the class interval of 10 μm2, and the percentage of each group was calculated for each interval.
To compare the parameters of the studied groups we used analysis of variance (ANOVA). When there was a significant difference we used Tukey’s test. For this study we used the Prism software version 3.0. Results were considered significant when P < 0.05. The results were shown as mean ± SE, n indicating the number of samples in each group.
Streptozotocin caused diabetic syndrome onset in animal groups D and DT, as evidenced by the significant increase in blood glucose, as well as a significant reduction in body weight, when compared to group C (Table 1). Other typical symptoms of the disease (polyuria, polydipsia and polyphagia) were observed during the experimental period.
| Group | Final weight (g) | Blood glucose (mg/dL) |
| C | 445.6 ± 63.04 | 78.97 ± 5.12 |
| D | 264.6 ± 22.88 | 253 ± 64.97 |
| DT | 308 ± 19.27 | 322 ± 20.42 |
There was a significant reduction (P < 0.05) in the neuronal density of myenteric neurons in the jejunum in group D when compared to C (Table 2). There was no significant difference in the DT group when compared to groups C and D. The neuronal density of submucosal neurons decreased significantly (P < 0.05) in group D when compared to C. No significant difference in the neuronal density was observed when group DT was compared to C (Table 2).
| Group | Myenteric plexus | Submucous plexus | ||
| Neuronal density (cm2) | Mean area of cell body (μm2) | Neuronal density (cm2) | Mean area of cell body (μm2) | |
| C | 15 884 ± 712.0 | 234.2 ± 88.10 | 12 602 ± 233.8 | 230.6 ± 62.89 |
| D | 13 483 ± 617.9 | 245.6 ± 77.19 | 11 383 ± 159.6 | 235.4 ± 67.99 |
| DT | 14 426 ± 301.2 | 218.2 ± 72.10 | 12 682 ± 353.4 | 216.2 ± 62.03 |
The neuronal density of myenteric neurons in the ileum decreased significantly (P < 0.05) in group D when compared to C (Table 3). No significant difference was seen when comparing group DT to C. There was no significant reduction in the neuronal density in the ileum submucous plexus when the three groups were compared (Table 3).
| Group | Myenteric plexus | Submucous plexus | ||
| Neuronal density (cm²) | Mean area of cell body (μm²) | Neuronal density (cm²) | Mean area of cell body (μm²) | |
| C | 16 522 ± 625.5 | 232.7 ± 82.97 | 11 657 ± 403.9 | 210.0 ± 59.18 |
| D | 14 568 ± 424.7 | 251.4 ± 98.23 | 11 275 ± 281.9 | 231.3 ± 74.37 |
| DT | 16 884 ± 366.1 | 239.3 ± 81.19 | 11 943 ± 299.3 | 204.5 ± 57.36 |
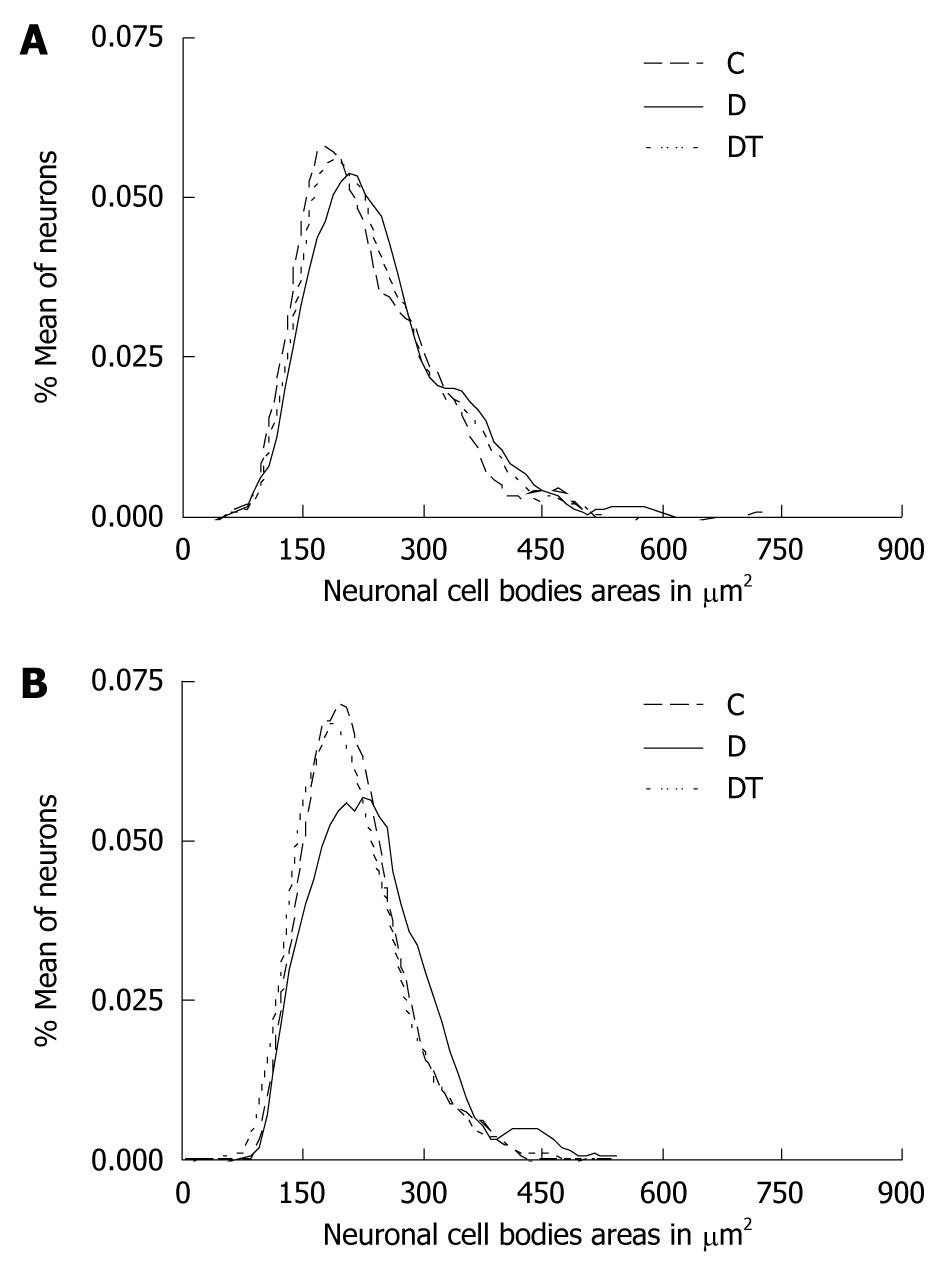
The results obtained with the measurements of 500 neurons per studied group were distributed according to the relative frequency of areas of neuronal cell bodies at intervals of 10 μm2 (Figures 1 and 2). The cell body area in the jejunum ranged between 81.33 and 538.9 μm2 for animals in group C; between 119.9 and 588.9 μm2 in group D; and between 101.0 and 609.2 μm2 in group DT. There were no significant differences in the mean areas of the jejunum myenteric neurons when comparing groups C and D. However, there was a significant reduction in the mean area (P < 0.05) of the DT group when compared to the other two groups (Table 2). The cell body area in the submucosal neurons in the jejunum ranged between 106.1 and 474.4 μm2 in group C, between 102.3 to 523.4 μm2 in group D and between 91.73 to 401.1 μm2 in group DT. There were no significant differences between the mean cell body areas in groups C and D (P > 0.05). However, there was a significant reduction (P < 0.05) in group DT when compared to groups C and D (Table 2).
The cell body area of myenteric neurons in the ileum ranged between 97.70 and 725.7 μm2 in group C, between 101.5 and 595.5 μm2 in group D, and between 96.32 and 512.9 μm2 in group DT. There was a significant increase (P < 0.05) in group D when compared to C. No significant difference was observed when comparing group DT to groups C or D (Table 3). As for the ileum submucous plexus, the area ranged between 89.54 and 426.2 μm2, between 99.52 and 534.0 μm2 in group D, and between 72.77 and 435.0 μm2 in group DT. There was a significant increase in the mean cell body area in group D (P < 0.05) when compared to C. The DT group showed no significant difference in mean cell body area when compared to group C (Table 3). In the submucous plexus, reduction in neuronal profile area was greater than in the myenteric plexus; the values in the submucous plexus just below those of the control group.
The distribution of the relative frequency of areas of cell bodies in the jejunum showed a displacement curve to the right in the myenteric plexus; thus showing a higher relative frequency of neurons at about 160 μm2 in both plexuses (Figure 1). There was a similarity in the curves of groups C and DT in both plexuses in the ileum (Figure 2). Group D showed a displacement to the right in both plexuses.
Streptozotocin (STZ) is widely used in experimental animal models to induce DM. Its cellular action includes irreversible changes in genetic material causing lethal alterations in the metabolism of β cells[24]. There is a reduction in overall myenteric plexus neuron population in animal models with chronic STZ-diabetes[11,12,25,26]. There are no studies of changes caused by diabetes in the overall neuronal population of the submucous plexus. Our study showed that the 120-d treatment with purified Ginkgo biloba extract (EGb 761) has a neuroprotective effect on the ileum myenteric plexus and on the jejunum submucous plexus of STZ-diabetic rats.
Characteristic diabetic symptoms (polydipsia, polyuria and polyphagia) were observed in animals of D and DT groups. These data support the experimental model of streptozotocin-induced diabetes[27-29]. The immunohistochemical technique, anti-myosin-V (Figures 3 and 4), was used to assess the effect of Ginkgo biloba extract (EGb 761) on the enteric neuronal population. The protein myosin-V is present in cell bodies and projections of enteric neurons[30] and is being used as a pan-neuronal marker.
The reduction of the myenteric neuron density in the jejunum was 15.12% in group D when compared to C (P < 0.05). The submucosal neuron density was 9.61% lower in group D when compared to C (P < 0.05). A reduction of 11.83% in myenteric neuron density was observed in the ileum in group D when compared to C (P < 0.05). The submucosal neuron density in the ileum was similar among the three groups. Several authors report the reduction of myenteric neuron density in rats with STZ-diabetes in different regions of the gastrointestinal tract, including the cecum[31], ileum[11,26], jejunum[25] and proximal colon[12]. There are no studies in the submucosal plexus of the total neuronal population in STZ-diabetes models. Pereira et al[26] reported a 24% reduction in the number of myosin-V myenteric neurons in the ileum (after 120 d) of diabetic rats when compared to non-diabetic ones. De Freitas et al[25] observed a 37.9% neuronal loss of myosin-V myenteric neurons in the jejunum of diabetic rats when compared to non-diabetic animals, also after 120 d. These studies used 90-d-old animals at the beginning of the experiment and our study was carried out with 150-d-old rats, which may have contributed to the neuronal loss variation due to age.
The degenerative changes that affect the enteric nervous system seen in DM are due to metabolic disorders. High oxidative stress, resulting from the imbalance between ROS production and neutralization, is a well established mechanism of diabetic neuropathy pathogenesis and other complications[32,33]. The levels of endogenous and exogenous antioxidants are reduced in this condition. New studies have confirmed the destruction of endogenous antioxidants in peripheral nerves and the increased production of free radicals in the vasa nervorum[4].
Ginkgo biloba extract is widely used for its neuroprotective and antioxidant activity in several cardiovascular and neurologic disorders[34,35]. The Ginkgo biloba extract (EGb 761) was given at a daily dose of 50 mg/kg body weight for 120 d in this experiment. This standardized extract contains 24% flavonoid glycosides (quercetin, kaempferol, isorhamnetin) and 6% terpene lactones (ginkgolides, bilobalides). The EGb 761 extract components eliminate free radicals such as the hydroxyl radical and the superoxide anion[36]. Quercetin is a powerful antioxidant within the flavonoid family due to its molecular configuration which is capable of eliminating free radicals[37].
The myenteric neuronal density in the jejunum in the DT group was 9.17% lower when compared to C, though this reduction is not significant. On the other hand, the submucosal neuronal density in DT had very similar values to those of group C. The treatment with EGb 671 resulted in the preservation of the neuronal population in the ileum, represented by very similar values to those of the control group (Table 2), thus demonstrating a neuroprotective effect on this complex. The submucosal neuronal density in this segment was similar in all three groups. The Ginkgo biloba extract reduces the oxidative stress in diabetic rats by increasing the activity of antioxidant enzymes[38]. Wu et al[39] reported that this extract may be vital to postpone diabetic cataract, since their studies showed that, besides inhibiting aldose reductase activity, Ginkgo biloba also inhibits apoptosis induced by high glucose levels by reducing the Bax/Bcl2 ratio. This high ratio harms the mitochondria which release apoptosis-inducing proteins, such as the apoptosis-inducing factor, leading to the activation of caspase-3 via caspase 9. The myenteric plexus neuroprotection, seen only in the ileum, is similar to results in aging models[40] where 120-d treatment of rats with the same dose of Ginkgo biloba extract was more efficient in the ileum myenteric plexus than in the jejunum.
Few studies have been carried out in the submucous plexus due to the difficulty of dissection. Some authors have reported changes in neuronal subpopulations through the neurotransmitter immunoreactivity. Belai et al[41] observed an increase in VIP and neuropeptide Y immunoreactivity when analyzing the submucous plexus in the ileum of STZ-diabetic rats aged 8 and 16 wk. They also observed a reduction in calcitonin gene-related peptide (CGRP) immunoreactivity. However, no change in substance P immunoreactivity or dopamine beta hydroxylase was seen. VIP-ergic neurons of diabetic rats show increased immunoreactivity in the jejunum[42] and ileum[43] submucous plexus.
The mean cell body areas of myenteric neurons in the jejunum were similar in groups C and D. These results are similar to those observed by De Freitas et al[25], who did not observe an increase in the mean area of the cell body of immunoreactive myosin-V neurons in the jejunum of diabetic rats when compared to non-diabetic rats. The mean areas of cell bodies of submucosal neurons in the jejunum were similar in groups C and D. Studies on morphometric changes in the submucosal plexus caused by diabetic syndrome report an increase in the mean area of the cell body of neuronal subpopulations. Defani et al[42] observed an increase in the mean area of the cell body of submucous VIP-ergic neurons in the jejunum. The technique used to stain the total population showed no change in the mean area of submucosal neurons in the jejunum. The mean area of the cell body of myenteric neurons in the ileum was 7.44% (P < 0.05) higher in group D than in group C in our study. This increase was also observed by Zanoni et al[11] and Pereira et al[26] in Wistar rats after a 120-d experimental period. The mean area of the body cell of submucosal neurons in the ileum showed a statistically significant increase of 9.2% (P < 0.05) in group D when compared to C. Zanoni et al[43] reported an increase in the mean area of the body cell of submucous VIP-ergic neurons in the ileum.
The increase in the neuronal cell body area in rats with chronic diabetes may be the result of neuronal edema[11]. The aldose reductase hyperactivity observed in diabetes is associated with increased levels of sorbitol[44] which increases the intracellular osmolarity, resulting in edema and neuronal lesions[43].
The EGb 761 treatment induced a reduction of 6.8% in the mean area of the cell body in the jejunum myenteric neurons in DT when compared to C (P < 0.05). The mean area of the cell body of submucosal neurons decreased 6.2% in group DT when compared to C (P < 0.05). The mean area of the cell body of myenteric and submucosal neurons in the ileum in DT was reduced to values similar to group C. Schneider et al[40] observed that the EGb 761 treatment reduced the mean area of myenteric neuronal cell bodies in the jejunum and ileum of aging rats. However, studies by Perez et al[45] in the large intestine treated with EGb 761 at a dose of 50 mg/kg of body weight observed that the EGb 761 extract promotes an increase in the mean area of myenteric neurons in rats in the aging process. These results show that the response to the use of antioxidants such as the Gingko biloba extract may be different according to the segment evaluated.
This study showed that treatment with Ginkgo biloba extract reduced the area of the cell body of myenteric and submucosal neurons in the jejunum and ileum of diabetic-treated rats (group DT) when compared to non-treated diabetic rats (group D). However, the reduction in the mean area of the cell body of myenteric neurons in the ileum was not significant. The inhibitory action of Ginkgo biloba on aldose redutase[19] enzyme activity may be responsible for the reduction in the mean area of neuronal cell bodies observed in rats treated with EGb 761 (DT group).
In conclusion, our results show that the 50 mg/kg of body weight dose of standardized Ginkgo biloba extract (EGb761) has a neuroprotective effect on the ileum myenteric plexus and on the jejunum submucous plexus of STZ-diabetic rats.
Gingko biloba extract possesses various biological activities and has been shown to be useful in diabetes treatment. Oxidative stress has been known to play an important role in the development and progression of diabetes mellitus (DM), and reactive oxygen species (ROS) production is a direct consequence of hyperglycemia. Chronic hyperglycemia in diabetes is involved in direct neuronal damage caused by intracellular glucose which leads to altered neurotransmitter functions and reduced motor activity. Oxygen free radicals are also thought to play an important role in the diabetic and hypoxic condition of cells. Success of Ginkgo biloba application is determined by its main active substances, flavonoids (flavone glycosides, primarily composed of quercetin) and terpenoids (ginkgolides and bilobalides). Ginkgo biloba can improve hemodynamics, scavenge ROS, suppress platelet-activating factor (PAF) and relax vascular smooth muscle.
Gastrointestinal (GI) afflictions are not normally life threatening but do profoundly affect quality of life. Diabetic patients experience a wide range of GI discomforts including nausea, vomiting, heartburn, diarrhea, constipation, abdominal pain and fecal incontinence. The high morbidity, high socioeconomic costs and lack of specific treatments are key factors that define the relevance of DM for human health and the importance of research on neuronal protective agents. Some studies provide a strong case for the application of Ginkgo biloba in diabetic nephropathy therapy.
Ginkgo biloba has been ascertained to be protective against DM. However, there has been little in the literature reporting on the protective effects of Ginkgo biloba on the enteric nervous system of the small intestine of streptozotocin-induced diabetic rats in vivo.
This study indicated that standardized extract of Ginkgo biloba (EGb 761) could improve antioxidant ability and protect the enteric nervous system of the small intestine of streptozotocin-induced diabetic rats in vivo. These biological activities have considerable potential in diabetes mellitus treatment.
The authors investigated the effect of Ginkgo biloba extract on the enteric neurons on the small intestine of diabetic rats. They found purified Ginkgo biloba extract has a neuroprotective effect on the jejunum submucous plexus and the myenteric plexus of the ileum of diabetic rats. This is a well written paper.
Peer reviewer: Claudio Daniel Gonzalez, MD, Professor of Pharmacology, Department of Pharmacology, CEMIC University Hospital, Buenos Aires, Argentina
S- Editor Tian L L- Editor Logan S E- Editor Zheng XM
| 1. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31 Suppl 1:S55-S60. [Cited in This Article: ] |
| 2. | Bortolotto LA. [Modifications of structural and functional properties of large arteries in diabetes mellitus]. Arq Bras Endocrinol Metabol. 2007;51:176-184. [Cited in This Article: ] |
| 3. | Aguiar LG, Villela NR, Bouskela E. [Microcirculation in diabetes: implications for chronic complications and treatment of the disease]. Arq Bras Endocrinol Metabol. 2007;51:204-211. [Cited in This Article: ] |
| 4. | Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta. 2009;1792:931-940. [Cited in This Article: ] |
| 5. | Rang HP, Dali MM, Ritter JM. O pâncreas endócrino e o controle da glicemia. 4th ed. Rio de Janeiro: Guanabara Koogan 2000; 318-329. [Cited in This Article: ] |
| 6. | Boucek P. Advanced Diabetic Neuropathy: A Point of no Return? Rev Diabet Stud. 2006;3:143-150. [Cited in This Article: ] |
| 7. | Vinik AI, Mehrabyan A. Diabetic neuropathies. Med Clin North Am. 2004;88:947-999, xi. [Cited in This Article: ] |
| 8. | Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil. 2007;19:951-960. [Cited in This Article: ] |
| 9. | Pasricha PJ, Pehlivanov ND, Gomez G, Vittal H, Lurken MS, Farrugia G. Changes in the gastric enteric nervous system and muscle: a case report on two patients with diabetic gastroparesis. BMC Gastroenterol. 2008;8:21. [Cited in This Article: ] |
| 10. | Belai A, Lincoln J, Milner P, Burnstock G. Progressive changes in adrenergic, serotonergic, and peptidergic nerves in proximal colon of streptozotocin-diabetic rats. Gastroenterology. 1988;95:1234-1241. [Cited in This Article: ] |
| 11. | Zanoni JN, Buttow NC, Bazotte RB, Miranda Neto MH. Evaluation of the population of NADPH-diaphorase-stained and myosin-V myenteric neurons in the ileum of chronically streptozotocin-diabetic rats treated with ascorbic acid. Auton Neurosci. 2003;104:32-38. [Cited in This Article: ] |
| 12. | Tashima CM, Tronchini EA, Pereira RV, Bazotte RB, Zanoni JN. Diabetic rats supplemented with L-glutamine: a study of immunoreactive myosin-V myenteric neurons and the proximal colonic mucosa. Dig Dis Sci. 2007;52:1233-1241. [Cited in This Article: ] |
| 13. | Figueroa-Romero C, Sadidi M, Feldman EL. Mechanisms of disease: the oxidative stress theory of diabetic neuropathy. Rev Endocr Metab Disord. 2008;9:301-314. [Cited in This Article: ] |
| 14. | Ramakrishna V, Jailkhani R. Evaluation of oxidative stress in Insulin Dependent Diabetes Mellitus (IDDM) patients. Diagn Pathol. 2007;2:22. [Cited in This Article: ] |
| 15. | Bonnefont-Rousselot D. Glucose and reactive oxygen species. Curr Opin Clin Nutr Metab Care. 2002;5:561-568. [Cited in This Article: ] |
| 16. | Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25:612-628. [Cited in This Article: ] |
| 17. | Shirpoor A, Ansari MH, Salami S, Pakdel FG, Rasmi Y. Effect of vitamin E on oxidative stress status in small intestine of diabetic rat. World J Gastroenterol. 2007;13:4340-4344. [Cited in This Article: ] |
| 18. | Aksoy N, Vural H, Sabuncu T, Arslan O, Aksoy S. Beneficial effects of vitamins C and E against oxidative stress in diabetic rats. Nutr Res. 2005;25:625-630. [Cited in This Article: ] |
| 19. | Head KA. Natural therapies for ocular disorders, part two: cataracts and glaucoma. Altern Med Rev. 2001;6:141-166. [Cited in This Article: ] |
| 20. | Calapai G, Crupi A, Firenzuoli F, Marciano MC, Squadrito F, Inferrera G, Parisi A, Rizzo A, Crisafulli C, Fiore A. Neuroprotective effects of Ginkgo biloba extract in brain ischemia are mediated by inhibition of nitric oxide synthesis. Life Sci. 2000;67:2673-2683. [Cited in This Article: ] |
| 21. | Maclennan KM, Darlington CL, Smith PF. The CNS effects of Ginkgo biloba extracts and ginkgolide B. Prog Neurobiol. 2002;67:235-257. [Cited in This Article: ] |
| 22. | Husstedt IW, Grotemeyer KH, Evers S, Staschewski F, Wertelewski R. Progression of distal symmetric polyneuropathy during diabetes mellitus: clinical, neurophysiological, haemorheological changes and self-rating scales of patients. Eur Neurol. 1997;37:90-94. [Cited in This Article: ] |
| 23. | Buttow NC, Santin M, Macedo LC, Neres Teixeira AC, Novakowski GC, Bolonheis Armelin TR, Assmann K. Study of the myenteric and submucous plexuses after BAC treatment in the intestine of rats. Biocell. 2004;28:135-142. [Cited in This Article: ] |
| 24. | Delfino VDA, Figueiredo JF, Matsuo T, Favero ME, Matni AM, Mocelin AJ. Streptozotocin-induced diabetes mellitus: long-term comparison of two drug administration routes. J Bras Nefrol. 2002;24:31-36. [Cited in This Article: ] |
| 25. | De Freitas P, Natali MR, Pereira RV, Miranda Neto MH, Zanoni JN. Myenteric neurons and intestinal mucosa of diabetic rats after ascorbic acid supplementation. World J Gastroenterol. 2008;14:6518-6524. [Cited in This Article: ] |
| 26. | Pereira RV, de Miranda-Neto MH, da Silva Souza ID, Zanoni JN. Vitamin E supplementation in rats with experimental diabetes mellitus: analysis of myosin-V and nNOS immunoreactive myenteric neurons from terminal ileum. J Mol Histol. 2008;39:595-603. [Cited in This Article: ] |
| 27. | Romano EB, Miranda-Neto MH, Cardoso RCS. Preliminary investigation about the effects of streptozotocin-induced chronic diabetes on the nerve cell number and size of myenteric ganglia in rat colon. Rev Chil Anat. 1996;14:139-145. [Cited in This Article: ] |
| 28. | Büttow NC, Miranda Neto MH, Bazotte RB. Morphological and quantitative study of the myenteric plexus of the duodenum of streptozotocin-induced diabetic rats. Arq Gastroenterol. 1997;34:34-42. [Cited in This Article: ] |
| 29. | Hernandes L, Bazotte RB, Gama P, Miranda-Neto MH. Streptozotocin-induced diabetes duration is important to determine changes in the number and basophily of myenteric neurons. Arq Neuropsiquiatr. 2000;58:1035-1039. [Cited in This Article: ] |
| 30. | Drengk AC, Kajiwara JK, Garcia SB, Carmo VS, Larson RE, Zucoloto S, Espreafico EM. Immunolocalisation of myosin-V in the enteric nervous system of the rat. J Auton Nerv Syst. 2000;78:109-112. [Cited in This Article: ] |
| 31. | Zanoni JN, de Miranda Neto MH, Bazotte RB, de Souza RR. Morphological and quantitative analysis of the neurons of the myenteric plexus of the cecum of streptozotocin-induced diabetic rats. Arq Neuropsiquiatr. 1997;55:696-702. [Cited in This Article: ] |
| 32. | Van Dam PS, Van Asbeck BS, Erkelens DW, Marx JJ, Gispen WH, Bravenboer B. The role of oxidative stress in neuropathy and other diabetic complications. Diabetes Metab Rev. 1995;11:181-192. [Cited in This Article: ] |
| 33. | Figueroa-Romero C, Sadidi M, Feldman EL. Mechanisms of disease: the oxidative stress theory of diabetic neuropathy. Rev Endocr Metab Disord. 2008;9:301-314. [Cited in This Article: ] |
| 34. | Diamond BJ, Shiflett SC, Feiwel N, Matheis RJ, Noskin O, Richards JA, Schoenberger NE. Ginkgo biloba extract: mechanisms and clinical indications. Arch Phys Med Rehabil. 2000;81:668-678. [Cited in This Article: ] |
| 35. | Defeudis FV. Bilobalide and neuroprotection. Pharmacol Res. 2002;46:565-568. [Cited in This Article: ] |
| 36. | Ni Y, Zhao B, Hou J, Xin W. Preventive effect of Ginkgo biloba extract on apoptosis in rat cerebellar neuronal cells induced by hydroxyl radicals. Neurosci Lett. 1996;214:115-118. [Cited in This Article: ] |
| 37. | Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325-337. [Cited in This Article: ] |
| 38. | Lu Q, Yin XX, Wang JY, Gao YY, Pan YM. Effects of Ginkgo biloba on prevention of development of experimental diabetic nephropathy in rats. Acta Pharmacol Sin. 2007;28:818-828. [Cited in This Article: ] |
| 39. | Wu ZM, Yin XX, Ji L, Gao YY, Pan YM, Lu Q, Wang JY. Ginkgo biloba extract prevents against apoptosis induced by high glucose in human lens epithelial cells. Acta Pharmacol Sin. 2008;29:1042-1050. [Cited in This Article: ] |
| 40. | Schneider LC, Perez GG, Banzi SR, Zanoni JN, Natali MR, Buttow NC. Evaluation of the effect of Ginkgo biloba extract (EGb 761) on the myenteric plexus of the small intestine of Wistar rats. J Gastroenterol. 2007;42:624-630. [Cited in This Article: ] |
| 41. | Belai A, Burnstock G. Changes in adrenergic and peptidergic nerves in the submucous plexus of streptozocin-diabetic rat ileum. Gastroenterology. 1990;98:1427-1436. [Cited in This Article: ] |
| 42. | Defani MA, Zanoni JN, Natali MR, Bazotte RB, de Miranda-Neto MH. Effect of acetyl-L-carnitine on VIP-ergic neurons in the jejunum submucous plexus of diabetic rats. Arq Neuropsiquiatr. 2003;61:962-967. [Cited in This Article: ] |
| 43. | Zanoni JN, Hernandes L, Bazotte RB, Miranda Neto MH. Terminal ileum submucous plexus: Study of the VIP-ergic neurons of diabetic rats treated with ascorbic acid. Arq Neuropsiquiatr. 2002;60:32-37. [Cited in This Article: ] |
| 44. | Obrosova IG. Increased sorbitol pathway activity generates oxidative stress in tissue sites for diabetic complications. Antioxid Redox Signal. 2005;7:1543-1552. [Cited in This Article: ] |
| 45. | Perez GG, Schneider LC, Buttow NC. Ginkgo biloba (EGb 761) extract: effects on the myenteric plexus of the large intestine in Wistar rats. Dig Dis Sci. 2009;54:232-237. [Cited in This Article: ] |