Published online Feb 14, 2011. doi: 10.3748/wjg.v17.i6.717
Revised: September 17, 2010
Accepted: September 24, 2010
Published online: February 14, 2011
AIM: To investigate the possible biological outcome and effect of glutamine depletion in neonatal mice and rodent intestinal epithelial cells.
METHODS: We developed three kinds of artificial milk with different amounts of glutamine; Complete amino acid milk (CAM), which is based on maternal mouse milk, glutamine-depleted milk (GDM), and glutamine-rich milk (GRM). GRM contains three-fold more glutamine than CAM. Eighty-seven newborn mice were divided into three groups and were fed with either of CAM, GDM, or GRM via a recently improved nipple-bottle system for seven days. After the feeding period, the mice were subjected to macroscopic and microscopic observations by immunohistochemistry for 5-bromo-2’-deoxyuridine (BrdU) and Ki-67 as markers of cell proliferation, and for cleaved-caspase-3 as a marker of apoptosis. Moreover, IEC6 rat intestinal epithelial cells were cultured in different concentrations of glutamine and were subject to a 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate cell proliferation assay, flow cytometry, and western blotting to examine the biological effect of glutamine on cell growth and apoptosis.
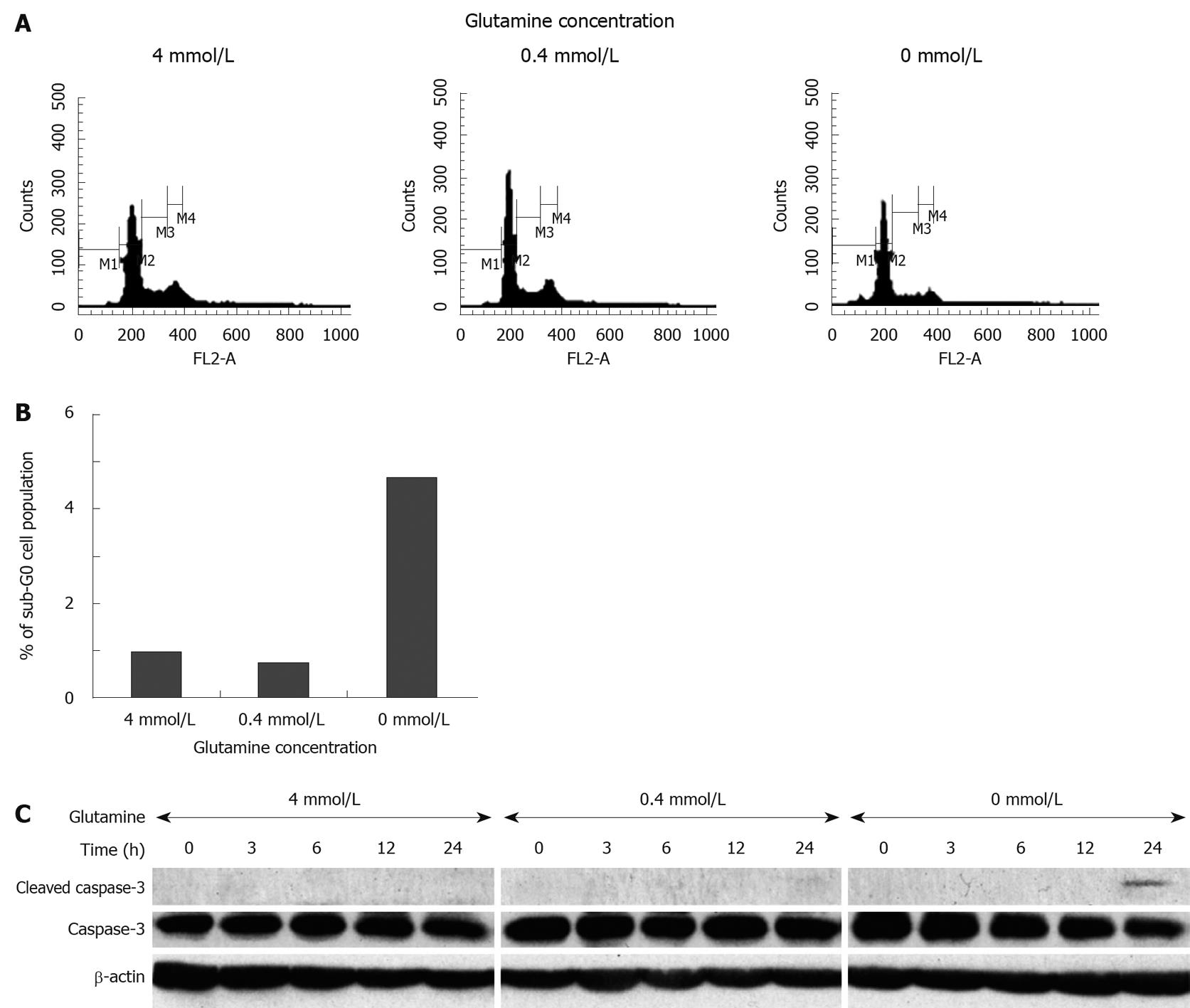
RESULTS: During the feeding period, we found colonic hemorrhage in six of 28 GDM-fed mice (21.4%), but not in the GRM-fed mice, with no differences in body weight gain between each group. Microscopic examination showed destruction of microvilli and the disappearance of glycocalyx of the intestinal wall in the colon epithelial tissues taken from GDM-fed mice. Intake of GDM reduced BrdU incorporation (the average percentage of BrdU-positive staining; GRM: 13.8%, CAM: 10.7%, GDM: 1.14%, GRM vs GDM: P < 0.001, CAM vs GDM: P < 0.001) and Ki-67 labeling index (the average percentage of Ki-67-positive staining; GRM: 24.5%, CAM: 22.4% GDM: 19.4%, GRM vs GDM: P = 0.001, CAM vs GDM: P = 0.049), suggesting that glutamine depletion inhibited cell proliferation of intestinal epithelial cells. Glutamine deprivation further caused the deformation of the nuclear membrane and the plasma membrane, accompanied by chromatin degeneration and an absence of fat droplets from the colonic epithelia, indicating that the cells underwent apoptosis. Moreover, immunohistochemical analysis revealed the appearance of cleaved caspase-3 in colonic epithelial cells of GDM-fed mice. Finally, when IEC6 rat intestinal epithelial cells were cultured without glutamine, cell proliferation was significantly suppressed after 24 h (relative cell growth; 4 mmol/L: 100.0% ± 36.1%, 0 mmol/L: 25.3% ± 25.0%, P < 0.05), with severe cellular damage. The cells underwent apoptosis, accompanied by increased cell population in sub-G0 phase (4 mmol/L: 1.68%, 0.4 mmol/L: 1.35%, 0 mmol/L: 5.21%), where dying cells are supposed to accumulate.
CONCLUSION: Glutamine is an important alimentary component for the maintenance of intestinal mucosa. Glutamine deprivation can cause instability of the intestinal epithelial alignment by increased apoptosis.
- Citation: Motoki T, Naomoto Y, Hoshiba J, Shirakawa Y, Yamatsuji T, Matsuoka J, Takaoka M, Tomono Y, Fujiwara Y, Tsuchita H, Gunduz M, Nagatsuka H, Tanaka N, Fujiwara T. Glutamine depletion induces murine neonatal melena with increased apoptosis of the intestinal epithelium. World J Gastroenterol 2011; 17(6): 717-726
- URL: https://www.wjgnet.com/1007-9327/full/v17/i6/717.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i6.717
Amino acids have various roles in the living body; however, their cytobiological functions associated with cell proliferation or immune responses have not been fully elucidated. Among them, glutamine, which is the most abundant amino acid in the human body, has been recognized as a conditional essential amino acid in critical illness, stress, and injury[1-4]. Glutamine also has a key role in intermediary metabolism for rapidly dividing cells, such as enterocytes and cells of the immune system[1,5]. Indeed, the small intestine accounts for the largest uptake of glutamine of any organ, absorbing this amino acid from the lumen of the gut, as well as from the bloodstream[6]. Illness or injury can lead to a significant decrease in plasma levels of glutamine, and when this decrease is severe, it correlates with increased mortality[7,8]. Several studies have demonstrated the efficacy of either enteral or parenteral glutamine in adults and infants with a variety of conditions, such as bone marrow transplantation, critical illness, burns, trauma, surgically treated patients, and very low birth weight infants[9,10]. Thus, glutamine appears especially important for susceptible individuals who are in high stress conditions.
In the past, it was difficult to identify the roles played by these amino acids in vivo. Past studies involved the evaluation of immunological effects of amino acids by the administration of a diet rich or deficient in each amino acid (glutamine, arginine, etc.) by means of total parenteral nutrition or stomach tubing[11]. However, no such study in newborn mice has yet been reported. The lack of such a study in newborn mice is attributable to the fact that it is quite difficult to develop a method of alimentation or to prepare a diet for neonatal mice. To overcome these issues, Yajima et al[12] have recently developed artificial milk for mice with a composition very close to that of mouse maternal milk. Subsequently, artificial milk for mice with all of the proteins broken down into amino acids was developed. We expected that the use of this milk would make it possible for us to feed newborn mice with milk either deficient or enriched in certain amino acids. In addition, Hoshiba established and reported a new system for the artificial alimentation of rats and mice immediately after birth[13]. With this system, it is possible to administer certain nutrients orally to newborn mice and rats and to evaluate the effects of these nutrients directly.
In this study, we utilized the above improved special milk and feeding device to achieve the following scientific aims: (1) to explore what happens when newborn mice are fed with glutamine-devoid milk; and (2) to dissect the physiological mechanism of glutamine deprivation-oriented events.
The nursing bottle and nipple, which were made by Hoshiba[13], were used to feed the newborn mice. The nursing bottle has three tubes (a filling tube, an outlet tube, and a ventilation tube) and the nipples are made of a silicone rubber. The nipple consists of inner and outer parts, and a stopper devised to control the pressure as well as to avoid milk leakage.
Specific-pathogen-free Jcl:ICR pregnant mice were purchased from Charles River Laboratories (Yokohama, Japan). They were maintained under the following environmental conditions: lighting, 12:12-h light:dark cycle; temperature, 22 to 25°C; air changes, 12 to 14 times per hour; and humidity, 40% to 50%. Newborn pups were separated from each dam immediately after birth. They were reared with artificial milk from the nursing bottle four times per day (09:00, 12:30, 16:00, and 20:00) for seven days and sacrificed on day eight. Body weights of the pups were measured before and after feeding, and the difference between them was represented the quantity of feeding.
Artificial milk was purchased from Meiji Dairies Corporation (Odawara, Japan). The amino acid composition of the milk is shown in Table 1. The composition of the milk was made as similar as possible to mouse maternal milk. Table 2 shows the composition of mouse milk and artificial amino acid milk. The artificial amino acid milk showed an extremely high osmotic pressure compared to that of mouse maternal milk, because the proteins in the milk used in this study were in the form of amino acids with a low amount of fat. Thus, 10% lipid microsphere Intralipid (Nihon Pharmaceutical Co. Ltd., Tokyo, Japan) was added to this artificial amino acid milk at a ratio of 1:1 to yield the complete amino acid milk, thus the amount of amino acid contained in this milk was 50% of the initial amount with 16% fat, similar with that of mouse milk (22%). We named this milk complete amino acid milk (CAM). Furthermore, glutamine rich milk (GRM) had three-fold more glutamine than CAM, whereas glutamine-depleted milk (GDM) contained no glutamine.
| Amino acid | mg/100 mL of milk1 |
| Valine | 0.60 ± 0.02 |
| Leucine | 1.06 ± 0.04 |
| Isoleucine | 0.48 ± 0.04 |
| Lysine | 0.88 ± 0.06 |
| Threonine | 0.47 ± 0.02 |
| Methionine | 0.34 ± 0.04 |
| Histidine | 0.25 ± 0.03 |
| Phenylalanine | 0.51 ± 0.16 |
| Tryptophan | 0.19 ± 0.01 |
| Alanine | 0.40 ± 0.00 |
| Arginine | 0.32 ± 0.01 |
| Glutamine | 2.09 ± 0.20 |
| Proline | 0.74 ± 0.15 |
| Cystine | 0.08 ± 0.00 |
| Tyrosine | 0.56 ± 0.05 |
| Asparagine | 0.91 ± 0.07 |
| Glycine | 0.19 ± 0.00 |
| Serine | 0.53 ± 0.01 |
Resected tissues from the mice were fixed in 10% formalin, embedded in paraffin, and cut at a thickness of 5 μm. Sections were deparaffinized, rehydrated, and stained with hematoxylin and eosin. On day eight, the animals were perfused with 2% glutaraldehyde (5 mL) and fixed, followed by the removal of each organ for subsequent thin slicing and observation under an electron microscope.
To detect cells synthesizing DNA, 5-bromo-2’-deoxyuridine (BrdU) was injected (100 mg/kg i.p.) 1 h before sacrificing the animals. The organs were then placed in 4% paraformaldehyde for 48 h prior to paraffin embedding. BrdU incorporation in colon sections was determined by immunohistochemical staining. Briefly, sections were deparaffinized with xylene (Mallinckrodt Baker, Paris, Kentucky) and taken through a graded series of alcohol/water mixtures to rehydrate the tissue. To retrieve the antigen, sections were incubated with 2 mol/L HCl for 30 min and then incubated with 0.1 mol/L Na2VO4O7 at room temperature for 30 s. Sections were exposed to rat anti-BrdU monoclonal antibodies (OBT, Oxford, UK) for 60 min at room temperature. Peroxidase-conjugated anti-mouse IgG1 antibody (DAKO, Carpinteria, CA) was then applied, and 3,3’-diaminobenzidine chromogen was added as the peroxidase substrate. A light counterstain of modified Mayer’s hematoxylin (Muto Pure Chemicals, Tokyo, Japan) was then applied so that unlabeled nuclei could be easily identified. Immunostaining for Ki-67 and cleaved caspase-3, the biological markers for cell proliferation and apoptosis, respectively, used a rabbit anti-Ki-67 monoclonal antibody (Ylem, Rome, Italy) and a rabbit anti-cleaved caspase-3 polyclonal antibody (Cell Signaling Technology, Beverly, MA, USA), respectively.
There was no commercially available mouse intestinal epithelial cell line; therefore, we used IEC6 rat intestinal epithelial cells, which were obtained from Dainippon Pharmaceutical Co. Ltd. (Tokyo, Japan), and maintained in dulbecco’s modified eagle medium (DMEM) with 10% FBS and 4 mmol/L L-glutamine for 24 h. Cells were incubated in DMEM with 0, 0.4, or 4 mmol/L L-glutamine without FBS for the indicated time periods (0, 3, 6, 12, 24 and 48 h).
The number of surviving cells was measured by the 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate (WST-1) assay (Roche, Indianapolis, USA) for the indicated time periods after plating.
Cells were lysed in 1 × sodium dodecyl sulfate (SDS) sample buffer (62.5 mmol/L Tris-HCl (pH 6.8 at 25°C), 2% w/v SDS, 10% glycerol, 50 mmol/L dithiothreitol, and 0.01% w/v bromophenol blue or phenol red) and centrifuged at 4°C. The sample viscosity was reduced by pipetting. The samples were then boiled for 5 min and cooled on ice. The samples were separated by SDS-poly-acrylamide gel electrophoresis on 12% polyacrylamide gels, transferred onto a polyvinylidene difluoride membrane, immunoblotted with Rabbit anti-caspase-3 polyclonal antibody (Upstate Cell Signaling Solutions, Charlottesville, VA) and Rabbit anti-cleaved caspase-3 polyclonal antibody (Millipore corporation, Bedford, MA) followed by anti-rabbit immunoglobulin G-horseradish peroxidase (GE, Piscataway, NJ). Immunoreactive proteins were visualized using an Enhanced ChemiLuminescence kit according to the manufacturer’s protocol (GE, Piscataway, NJ).
Cell cycle analysis was performed by propidium iodide (PI) staining. Briefly, IEC6 cells were first seeded into 10-cm dishes at a cell density of 5 × 105. After further culture for 48 h and 96 h, the cells were trypsinized and harvested in phosphate buffered saline (PBS) followed by resuspension in PBS at 1-2 × 106/mL. Finally, the cells were stained with 0.5 mL of PI staining solution (3.8 mmol/L sodium citrate, 50 mg/mL PI in PBS) for 1 h at room temperature to analyze cell cycle distribution by FACSCalibur (Becton Dickinson Immunocytometry Systems, San Jose, CA) excitation at 488-nm. The DNA-linked red fluorescence (PI) was measured through a 600-nm wavelength filter. This experiment was performed three times.
For in vivo experiments, the significance of differences between the control and test values was determined by Tukey’s test using JMP 6.0.3 software (SAS Institute, Cary, NC). P < 0.05 was considered statistically significant.
Newborn mice were assigned to three groups (GRM, CAM, and GDM) and were fed four times a day according to the above-mentioned schedule. During the observation periods, the mice gained weight regardless of the amount of glutamine, and there were no significant differences between the groups (data not shown).
We measured the glutamine concentration in serum taken from each mouse fed with different types of milk. As expected, the GRM-mouse serum contained the highest amount of glutamine (7.53% of total amino acids), while the GDM-mouse serum had the lowest amount of glutamine (4.92% of total amino acids) in circulation. The CAM-mice maintained less glutamine in serum (5.74% of total amino acids) than the dam-reared mice (6.44%). These data suggested that the amount of glutamine in the milk fed to the pups did affect the concentration of glutamine circulating in the animal body.
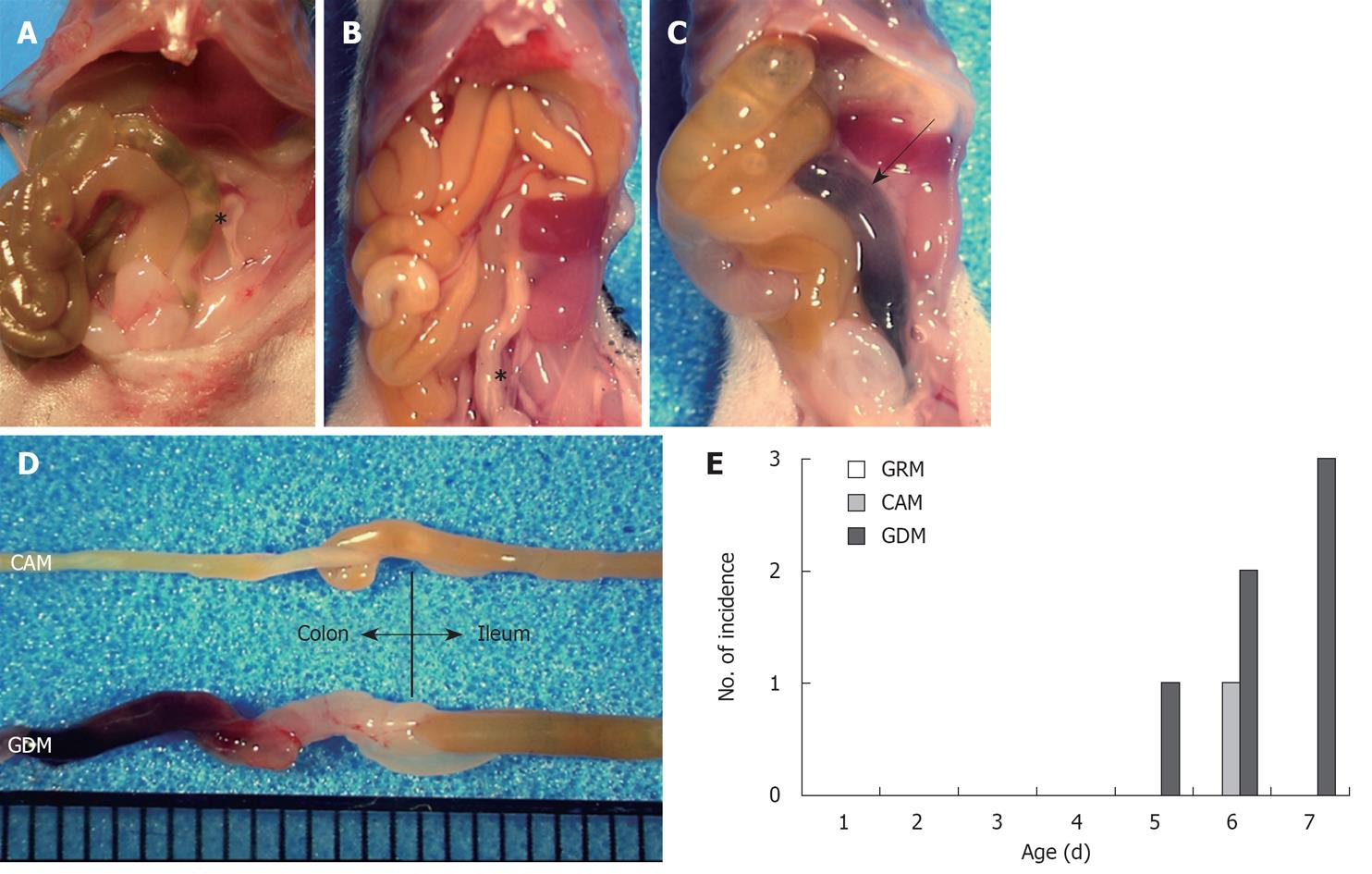
When the mice were sacrificed on day eight, we found a pool of blood in the colons of the GDM-fed mice accompanied by melena (Figure 1A-C). In addition, the entire bowel of these mice showed a massive edematous change, accompanied by wall thickening, redness, and dilatation of the small intestine (Figure 1D). No macroscopic changes were observed in mice fed with glutamine-containing milk, except for one mouse fed with CAM (Figure 1A-D). These events were observed in six of 28 GDM-fed mice (21.4%) and one CAM-fed mouse (3.3%), and started on days five to seven (one mouse on day five, four mice including the mouse fed with CAM, on day six, and two mice on day seven) (Figure 1E). No mice fed with GRM developed melena during the observation period. The incidence of melena was significantly higher in the GDM group compared to the other groups (Figure 1E), suggesting that glutamine depletion must affect the maintenance of the neonatal gastrointestinal tract.
Histological observation by hematoxylin-eosin staining revealed the destruction of the villous structure, wall thickening with edematous dilatation of the submucosal layer, and inflammatory cell infiltration around the hemorrhage site of the colons in the mice fed without glutamine (Figure 2A). In addition, a reduction of goblet cells was noted in this group, suggesting a disorder of cell maturation. Interestingly, the intestinal mucosa of the GRM-fed mice were higher than those of the CAM-fed mice (Figure 2A).
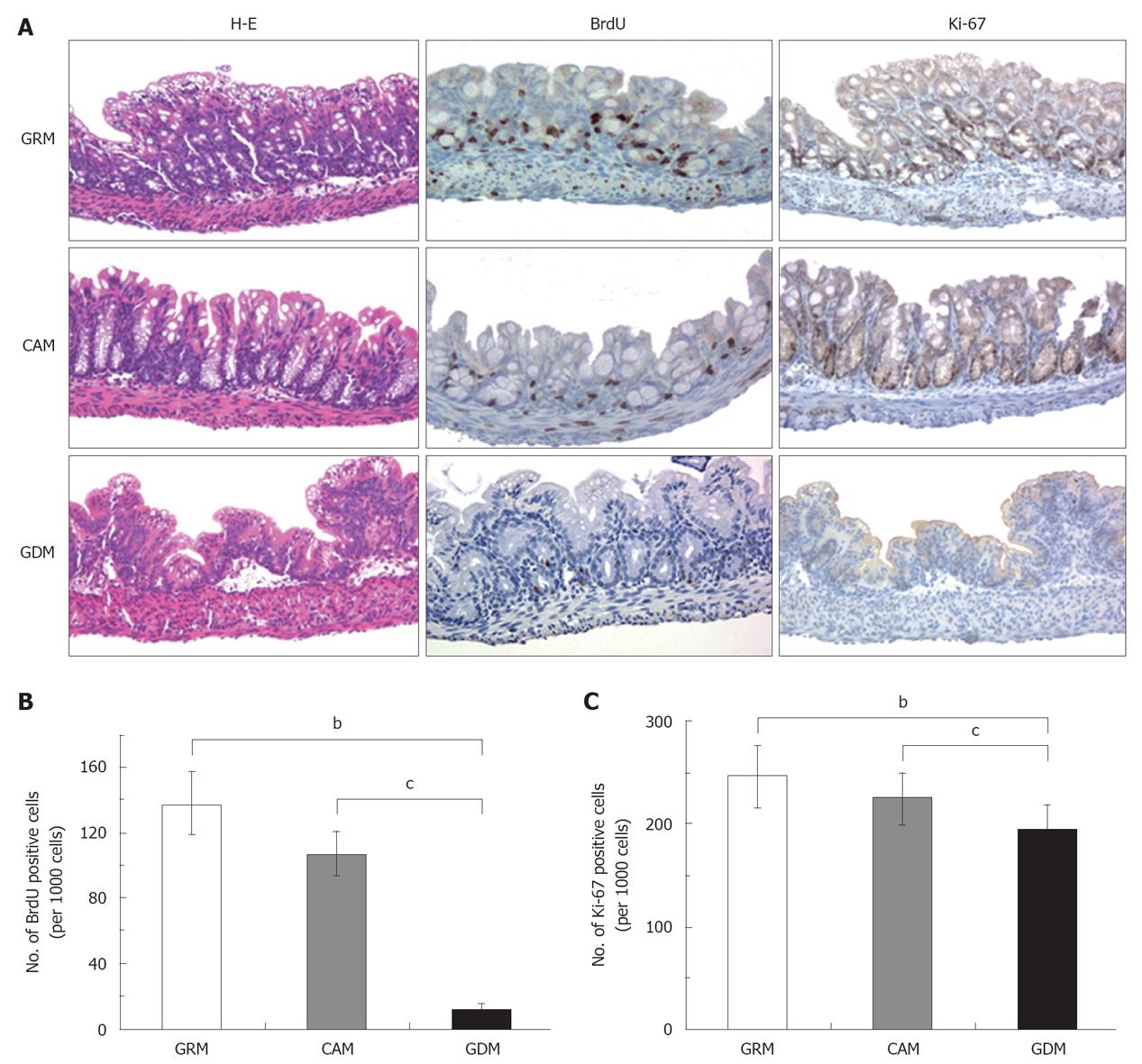
We then assessed cell proliferation in colonic epithelial cells by immunostaining for BrdU and Ki-67, which are markers for DNA synthesis and cell proliferation, respectively (Figure 2A). BrdU incorporation was significantly decreased in colonic epithelial cells of the GDM-fed mice compared to those of CAM-mice and GRM-mice (average percentage of BrdU-positive staining per 1000 cells; GRM: 13.8%, CAM: 10.7%, GDM: 1.14%, Tukey test; GRM vs GDM, P < 0.001; CAM vs GDM, P < 0.001) (Figure 2B). Ki-67-positive staining was also significantly decreased in the colonic epithelia of the GDM-fed mice (average percentage of Ki-67-positive staining; GRM: 24.5%, CAM: 22.4%, GDM: 19.4%, Tukey test; GRM vs GDM, P = 0.001; CAM vs GDM, P = 0.049) (Figure 2C). These data indicated that glutamine deprivation strongly diminished cell growth of the intestinal epithelium.
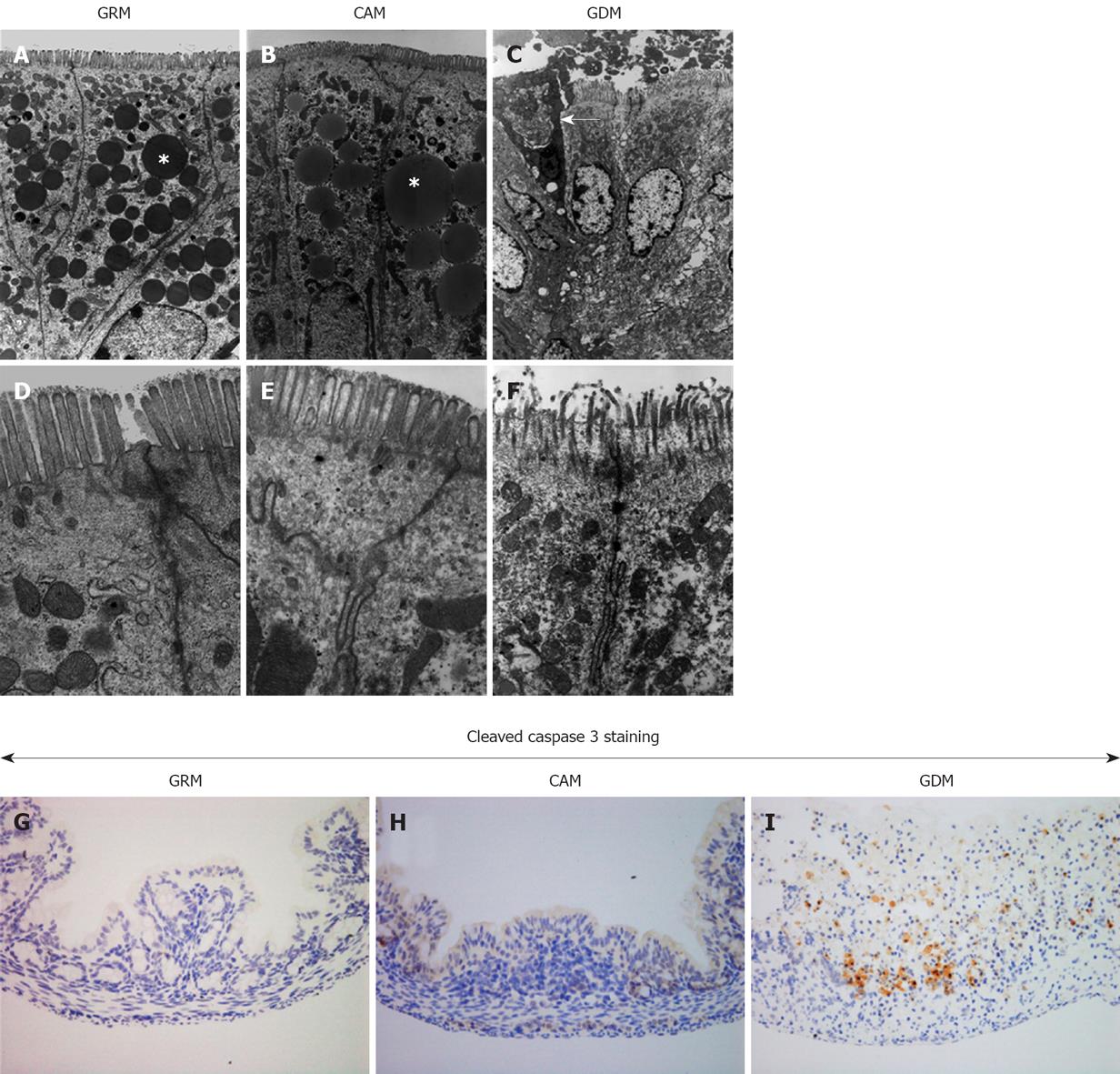
We further examined the damaged colonic mucosa under the electron microscope. Figure 3A-F shows representative pictures of colonic epithelia from each group. Glutamine deprivation caused deformation of the nuclear membrane and the plasma membrane, accompanied by the destruction of microvilli and the disappearance of glycocalyx, resulting in nuclear deformation and chromatin degeneration. Fat droplets, which were seen in the colons of the mice fed with the glutamine-containing milk, were absent in the GDM Group. Furthermore, a partial loss of colonic epithelial cells was noted in the GDM Group, indicating that glutamine deprivation promotes cell death. To confirm glutamine deprivation-induced cell death, cleavage of caspase-3 was assessed by immunohistochemistry. As shown in Figure 3G-I, there was a remarkable number of cleaved caspase-3-positive stained cells in the glutamine-deprived mucosa, suggesting that a lack of glutamine induces colonic epithelial cell death, possibly through the process of apoptosis.
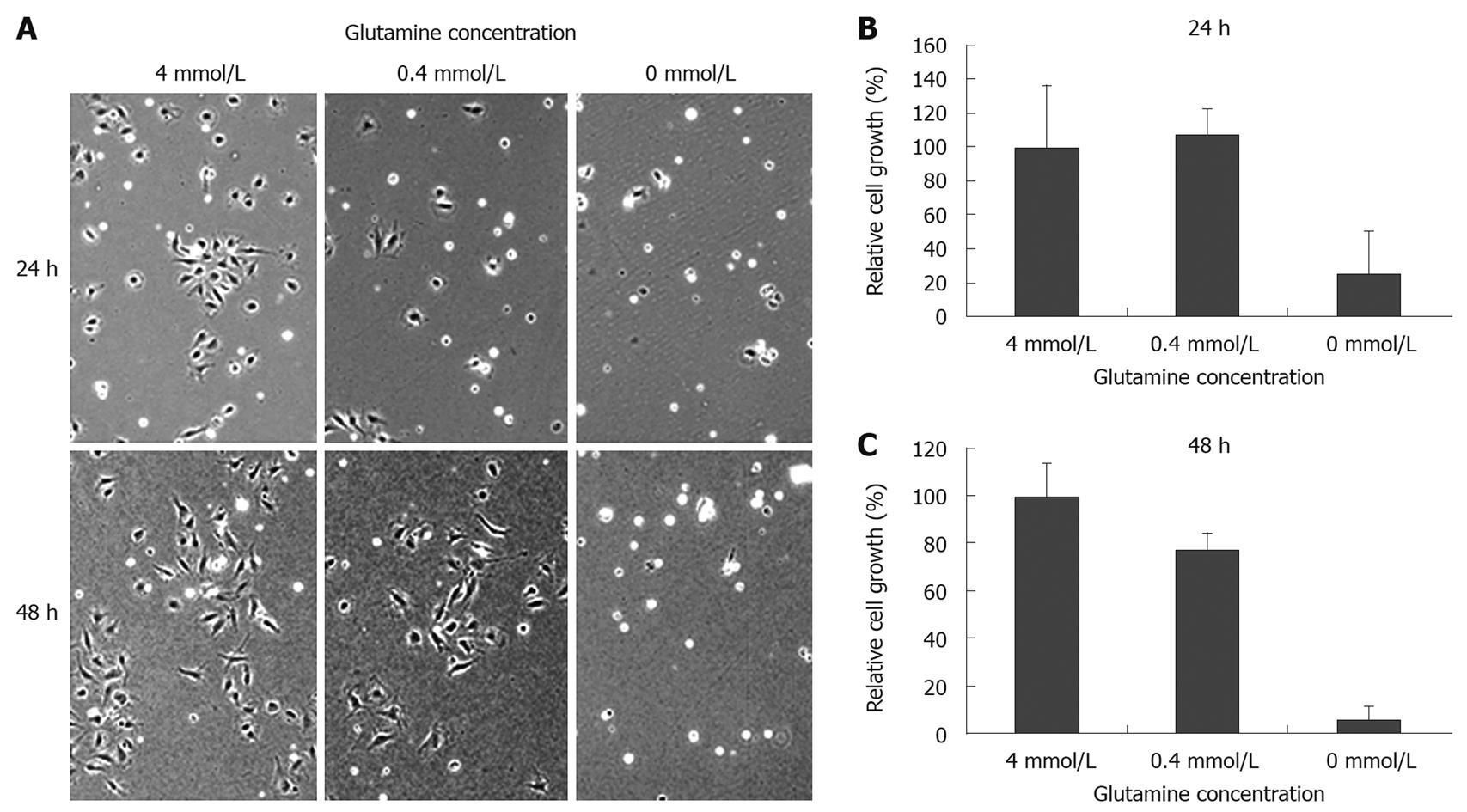
To clarify the effect of glutamine depletion on intestinal epithelial cell growth, IEC6 rat intestinal epithelial cells were cultured in the presence of varying concentrations of glutamine. Microscopic observation showed that glutamine depletion damaged cell morphology and reduced cell density (Figure 4A). As shown in Figure 4B and C, glutamine depleted conditions significantly suppressed IEC6 cell growth after 24 h (4 mmol/L: 100.0 ± 36.1, 0 mmol/L: 25.3 ± 25.0, P < 0.05), while there was no apparent difference between 4 mmol/L and 0.4 mmol/L after 24 h (4 mmol/L: 100.0 ± 36.1, 0.4 mmol/L: 107.4 ± 15.4, P = 0.7614), or after 48 h (4 mmol/L: 100.0 ± 14.0, 0.4 mmol/L: 77.4 ± 6.9, P = 0.0659). We further assessed the inhibitory effect on cell proliferation by cell cycle analysis, using flow cytometry (Figure 5A and B). We did not observe significant cell growth arrest at the G1 or G2 phase; however, an increased cell population at the sub-G0 phase was observed within 48 h of glutamine depletion (4 mmol/L: 1.68%, 0.4 mmol/L: 1.35%, 0 mmol/L: 5.21%, Figure 5B), which is consistent with the observation in the animal model that glutamine-depletion seemed to be lethal to intestinal epithelial cells.
Finally, we determined whether glutamine depletion-mediated cell death in cultured IEC6 cells occurs due to induction of apoptosis. Cells cultured with a complete depletion of glutamine showed a time-dependent decrease in caspase-3 expression, accompanied by cleavage of caspase-3 within 24 h (Figure 5C). These data support our finding in the animal study that a lack of glutamine affects the maintenance of intestinal epithelial cells, suppresses cell growth and induces apoptosis, resulting in melena.
Until recently, it was quite difficult to study the physiological and cytobiological effects of amino acids in vivo, especially for neonatal animals, because of the lack of methods of alimentation or of preparing a diet for neonatal mice. In this study, using a totally new milk feeding system and amino acid milk, we successfully observed the physiological effects of glutamine depletion in newborn mice. Interestingly, a significantly high incidence of colonic hemorrhage occurred in mice fed with GDM, compared to the CAM or GRM groups. We had one CAM-fed mouse that had a similar colonic hemorrhage on day six. This mouse might have had a genetic/physiological susceptibility to the event. It could also have been caused by the fact that the amount of amino acid contained in the CAM was 50% of the ordinary amount because emulsified fat was added to the artificial amino acid milk at a ratio of 1:1 to yield CAM. The proteins in the milk used in this study were in the form of amino acids, resulting in a very high osmotic pressure with a low amount of fat (Table 2). If the artificial amino acid milk is applied directly to newborn mice without adding fat, which is a major nutrient for them, a fat deficiency might occur. Therefore, the melena that occurred in the CAM-fed mouse may be explained by an insufficient intake of glutamine. Meanwhile, no animal developed colonic hemorrhage in the GRM Group; thus, we assume that the glutamine level in this milk was high enough to maintain the intestinal epithelium.
Other macroscopic findings in the hemorrhagic intestines of the GDM-fed mice were the apparent inflammatory changes of the entire intestine, with intestinal wall thickening by edema. Microscopic observations supported these findings, with infiltration of inflammatory cells in and around destroyed colonic mucosa at the site of the hemorrhage. On the other hand, the height of the colonic mucosa of GRM-fed mice was well conserved and was higher than that of the CAM mice. In addition, as more glutamine was administered, more positive-staining cells for BrdU and Ki-67 appeared, suggesting that glutamine is a critical nutrient for the proliferation of intestinal cells. Moreover, both electron microscopic observations and immunohistochemistry for cleaved caspase-3 reflected an increased incidence of apoptosis induced by glutamine depletion. However, no exact intracellular mechanism has been identified for the destruction of the nuclear membrane and microvilli, or the disappearance of glycocalyx, which were induced by GDM. Clarifying the molecular/biophysical mechanism of this glutamine depletion-mediated event will be crucial to understanding the intrinsic functions of glutamine or other amino acids.
Experimental data from cultured IEC6 cells supported the findings of the animal experiments, with the reduced cell proliferation, accumulation of a cell population in the sub-G0 phase, and the cleavage of caspase-3 under glutamine-depleted culture conditions. According to these results, glutamine depletion induces cell death of colonic mucosa, presumably due to an acute induction of apoptosis, followed by the destruction of mucosa maintenance, which eventually leads to colonic hemorrhage.
In the present study, it was remarkable that colonic hemorrhage could be induced simply by removing one amino acid, glutamine. Glutamine has attracted close attention as an amino acid nutrient and as an immunopotentiating factor for intestinal cells[14-16]. However, no case of intestinal hemorrhage induced by glutamine deficiency has been reported to date. Although glutamine is a non-essential amino acid and can be produced in the living body, neonatal mice are actively growing and are very dependent on external alimentation due to a heavier consumption of nutrients, including glutamine, compared to adults[17]. It seems likely that the consumption of glutamine by these neonatal mice might exceed the amount of glutamine pooled and formed in the living body, leading to the emergent status of glutamine deficiency.
In terms of molecular/biological effects of glutamine, there are outstanding several issues that remain to be clarified. One of our interests is to explore the possible involvement of certain signaling pathways. It has recently been unveiled that amino acids are involved in the control of the mTOR signaling pathway[18]. We have also demonstrated previously that leucine activates the mTOR signaling pathway in a hepatocellular carcinoma cell line[19]. It has also been shown that leucine and arginine activate the mTOR signaling pathway in a small bowel epithelial cell line derived from rats[20]. We also found that glutamine regulates the activation of this pathway in the same cell line[21]. Therefore, future studies should focus on the mechanism for the induction of colonic hemorrhage and how glutamine is involved in the intracellular signaling pathway, such as in mTOR[22-24].
In conclusion, we found that feeding neonatal mice with GDM induced a high incidence of colonic hemorrhage within a week, and that this was due to an induction of epithelial cell death. Further investigation is necessary to explore the biological mechanism.
Glutamine, the most abundant amino acid in the human body, is conditionally essential, especially for susceptible individuals who are in high stress conditions. A lack of glutamine correlates with increased mortality.
The small intestine accounts for the largest uptake of glutamine of any organ, absorbing this amino acid from the lumen of the gut, as well as from the bloodstream. Past studies involved the evaluation of immunological effects of amino acids by the administration of a diet rich or deficient in each amino acid (glutamine, arginine, etc.) by means of total parenteral nutrition or stomach tubing. However, no such study in newborn mice has yet been reported. The lack of such a study in newborn mice is attributable to the difficulty in developing a method of alimentation or preparing a diet for neonatal mice.
The authors developed three kinds of artificial milk with different amounts of glutamine. Using these amino acid milks and a recently improved nipple-bottle feeding system, they successfully fed the newborn mice with the new amino acid milk and observed colonic hemorrhage in mice fed without glutamine. It was remarkable that colonic hemorrhage could be induced simply by removing one amino acid, glutamine. In addition, glutamine deprivation can cause instability of intestinal epithelial alignment by increased apoptosis.
No exact intracellular mechanism has been identified for the destruction of the nuclear membrane and microvilli or the disappearance of glycocalyx, which were induced by glutamine depletion. Thus, we must explore the mechanism of the induction of colonic hemorrhage and investigate glutamine’s involvement in the intracellular signaling pathway, to better understand the intrinsic functions of glutamine and other amino acids.
The authors have described that glutamine depletion induces neonatal mice melena and apoptosis of intestinal epithelium in vivo and in vitro. The authors obtained good data allowing the conclusion that glutamine depletion induces neonatal mice melena and apoptosis of intestinal epithelium, using appropriate methods. An animal model was established for glutamine deprivation-induced destruction of the intestinal epithelium. It would also be meaningful to study the effects of glutamine-deprivation on human intestinal function.
Peer reviewer: Julio Mayol, MD, PhD, Department of Digestive surgery, Hospital Clinico San Carlos, MARTIN-LAGOS S/n, Madrid, 28040, Spain
S- Editor Sun H L- Editor Stewart GJ E- Editor Lin YP
| 1. | D'Souza R, Powell-Tuck J. Glutamine supplements in the critically ill. J R Soc Med. 2004;97:425-427. [Cited in This Article: ] |
| 2. | Novak F, Heyland DK, Avenell A, Drover JW, Su X. Glutamine supplementation in serious illness: a systematic review of the evidence. Crit Care Med. 2002;30:2022-2029. [Cited in This Article: ] |
| 3. | Scheltinga MR, Young LS, Benfell K, Bye RL, Ziegler TR, Santos AA, Antin JH, Schloerb PR, Wilmore DW. Glutamine-enriched intravenous feedings attenuate extracellular fluid expansion after a standard stress. Ann Surg. 1991;214:385-393; discussion 393-395. [Cited in This Article: ] |
| 4. | Thompson SW, McClure BG, Tubman TR. A randomized, controlled trial of parenteral glutamine in ill, very low birth-weight neonates. J Pediatr Gastroenterol Nutr. 2003;37:550-553. [Cited in This Article: ] |
| 5. | Griffiths RD, Jones C, Palmer TE. Six-month outcome of critically ill patients given glutamine-supplemented parenteral nutrition. Nutrition. 1997;13:295-302. [Cited in This Article: ] |
| 6. | Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1-105. [Cited in This Article: ] |
| 7. | Becker RM, Wu G, Galanko JA, Chen W, Maynor AR, Bose CL, Rhoads JM. Reduced serum amino acid concentrations in infants with necrotizing enterocolitis. J Pediatr. 2000;137:785-793. [Cited in This Article: ] |
| 8. | Biolo G, Zorat F, Antonione R, Ciocchi B. Muscle glutamine depletion in the intensive care unit. Int J Biochem Cell Biol. 2005;37:2169-2179. [Cited in This Article: ] |
| 9. | Avenell A. Hot topics in parenteral nutrition. Current evidence and ongoing trials on the use of glutamine in critically-ill patients and patients undergoing surgery. Proc Nutr Soc. 2009;68:261-268. [Cited in This Article: ] |
| 10. | Klimberg VS, Souba WW, Dolson DJ, Salloum RM, Hautamaki RD, Plumley DA, Mendenhall WM, Bova FJ, Khan SR, Hackett RL. Prophylactic glutamine protects the intestinal mucosa from radiation injury. Cancer. 1990;66:62-68. [Cited in This Article: ] |
| 11. | Manhart N, Vierlinger K, Spittler A, Bergmeister H, Sautner T, Roth E. Oral feeding with glutamine prevents lymphocyte and glutathione depletion of Peyer's patches in endotoxemic mice. Ann Surg. 2001;234:92-97. [Cited in This Article: ] |
| 12. | Yajima M, Kanno T, Yajima T. A chemically derived milk substitute that is compatible with mouse milk for artificial rearing of mouse pups. Exp Anim. 2006;55:391-397. [Cited in This Article: ] |
| 13. | Hoshiba J. Method for hand-feeding mouse pups with nursing bottles. Contemp Top Lab Anim Sci. 2004;43:50-53. [Cited in This Article: ] |
| 14. | Yagi M, Sakamoto K, Hasebe K, Ito H, Onishi I, Tani T, Hashimoto T, Shimizu K, Miwa K. Effect of a glutamine-enriched diet on small bowel allograft during immunosuppressive therapy. Nutrition. 1997;13:778-782. [Cited in This Article: ] |
| 15. | Marion R, Coëffier MM, Gargala G, Ducrotté P, Déchelotte PP. Glutamine and CXC chemokines IL-8, Mig, IP-10 and I-TAC in human intestinal epithelial cells. Clin Nutr. 2004;23:579-585. [Cited in This Article: ] |
| 16. | Dupont B, Dupont C, Justum AM, Piquet MA, Reimund JM. Enteral nutrition in adult Crohn's disease: present status and perspectives. Mol Nutr Food Res. 2008;52:875-884. [Cited in This Article: ] |
| 17. | Pierro A, Eaton S. Metabolism and nutrition in the surgical neonate. Semin Pediatr Surg. 2008;17:276-284. [Cited in This Article: ] |
| 18. | Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273:14484-14494. [Cited in This Article: ] |
| 19. | Shigemitsu K, Tsujishita Y, Miyake H, Hidayat S, Tanaka N, Hara K, Yonezawa K. Structural requirement of leucine for activation of p70 S6 kinase. FEBS Lett. 1999;447:303-306. [Cited in This Article: ] |
| 20. | Ban H, Shigemitsu K, Yamatsuji T, Haisa M, Nakajo T, Takaoka M, Nobuhisa T, Gunduz M, Tanaka N, Naomoto Y. Arginine and Leucine regulate p70 S6 kinase and 4E-BP1 in intestinal epithelial cells. Int J Mol Med. 2004;13:537-543. [Cited in This Article: ] |
| 21. | Nakajo T, Yamatsuji T, Ban H, Shigemitsu K, Haisa M, Motoki T, Noma K, Nobuhisa T, Matsuoka J, Gunduz M. Glutamine is a key regulator for amino acid-controlled cell growth through the mTOR signaling pathway in rat intestinal epithelial cells. Biochem Biophys Res Commun. 2005;326:174-180. [Cited in This Article: ] |
| 22. | Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521-534. [Cited in This Article: ] |
| 23. | Sakiyama T, Musch MW, Ropeleski MJ, Tsubouchi H, Chang EB. Glutamine increases autophagy under Basal and stressed conditions in intestinal epithelial cells. Gastroenterology. 2009;136:924-932. [Cited in This Article: ] |
| 24. | Fumarola C, La Monica S, Guidotti GG. Amino acid signaling through the mammalian target of rapamycin (mTOR) pathway: Role of glutamine and of cell shrinkage. J Cell Physiol. 2005;204:155-165. [Cited in This Article: ] |