INTRODUCTION
Inflammation and coagulation are two crucial systems in mammals. They constantly influence each other and are constantly in balance. In particular, inflammatory processes can promote coagulation which, in turn, can also sustain inflammation. The inter-dependence of the two processes is confirmed by clinical settings where the inherited or acquired deficiency of natural anticoagulants is associated with an increase in inflammatory processes[1].
This observation is particularly relevant in acute inflammatory diseases, such as sepsis[1], but it also seems to be very important in chronic inflammatory conditions, such as inflammatory bowel disease (IBD).
Patients with Crohn’s disease (CD) and ulcerative colitis (UC) have an increased risk of thromboembolic events[2], which appears to be more frequent when IBD is in an active phase[3-5] and is affecting the whole colon[3,6,7]. However, it is worth noting that, in a large study, one-third of thromboembolic complications occurred during disease quiescence, supporting the hypothesis of a greater prothrombotic tendency in IBD, independent of disease activity[3].
The incidence of thromboembolic events in patients with IBD has been reported to be 1%-8%[4,8,9]. Patients with IBD have a 3-fold increased risk for deep vein thrombosis and pulmonary embolism compared with the general population[8,10,11]. In addition, IBD patients experience more thromboembolic events at a younger age than the general population or patients affected by rheumatoid arthritis or celiac disease[4,8].
Finally, indirect evidence that vascular thrombosis may be involved in the pathogenesis of IBD was provided by an epidemiological study performed on a large cohort of subjects with hemophilia or von Willebrand’s disease[3,12]. In this population, in which more than 9000 patients were included (6000 patients with hemophilia and more than 3000 with von Willebrand’s disease), IBD occurred less frequently than expected, and it was suggested that inherited hemorrhagic disorders might be protective against IBD[3].
Most available reports tried to explain the increased thromboembolic risk in IBD by analyzing different components of the coagulation cascade, such as serological/phenotypical markers and genetic pro-thrombotic mutations/polymorphisms. Several studies exist on major pro-thrombotic genetic predispositions and IBD. Most published data demonstrate that there is no difference in the prevalence of Factor V Leiden between IBD patients and healthy controls[3,13-15], as well as PT gene G20210A mutation[3,15-17]. Polymorphisms of Methylenetetrahydrofolate reductase, the enzyme involved in the re-methylation pathway of homocysteine metabolism, have been found to have discordant results in IBD patients compared to controls[3,15,18]. Other studies looking at the prevalence of Antithrombin III deficiency[17,19], Protein C[20] and Protein S deficiencies[21] in IBD have been contradictory or equivocal[22,23], suggesting that these factors, although possibly related to IBD pathogenesis, are not genetically related to IBD[23]. Finally, the inherited Val34Leu factor XIII polymorphism, which is protective against thrombosis, has been evaluated in IBD patients[3,24]. Available data demonstrated no significant difference in the prevalence of this polymorphism in IBD patients with respect to the general population[25].
Taken together, the information on genetic factors does not explain the greater risk of venous thrombosis in CD and UC[26,27]. On the contrary, a pathogenesis-oriented approach suggests that the coagulation abnormalities occurring in IBD are very likely the result of the biological and biochemical effects exerted by the activation of the inflammatory machinery (cells, cytokines, etc.) in these disorders. Furthermore, activation of the coagulation cascade can in turn sustain activation of inflammatory reactions, promoting the vicious circle between chronic inflammation and thrombosis.
In this review, we will firstly describe single quantitative abnormalities of coagulation factors observed in IBD and cellular components closely involved in the coagulation pathway. We will then describe the mechanisms by which these abnormalities interfere with intestinal mucosa homeostasis. Finally, the possible therapeutic implications emerging from the unraveling of coagulation abnormalities associated with IBD pathogenesis will also be briefly presented and critically reviewed
QUANTITATIVE ALTERATIONS OF HEMOSTATIC FACTORS IN IBD
Coagulation is a complex system, which can be schematically divided into different pathways, referred to as “intrinsic”, “extrinsic” and “common” pathways[28]. An important role is also played by the fibrinolysis system, which controls clot dissolution, and the family of serine protease inhibitors, which inhibits many coagulation enzymes. We will use this classification to better summarize findings concerning the linkage between the coagulation system and inflammation associated with IBD.
The extrinsic pathway
The extrinsic pathway is initiated by tissue damage that exposes tissue factor (TF) to blood, causing the formation of TF/FVIIa, which circulates at low levels in the bloodstream. It is thought that high factor VIIa activity is associated with an increased risk of ischemic myocardial events in men over 40 years of age[8,29] It is the main determinant of the laboratory assay referred to as prothrombin time (PT). No definitive data are available on changes of the extrinsic pathway in IBD. Most of the data available report no significant difference in PT among active UC and/or CD and control patients[3,8,30]. Other studies reported different findings showing that PT values and platelet levels are predictors of CD activity index in female patients[2]. Levels of factor VIIa seem to be higher in active IBD compared to controls[3,31,32]. This finding suggests the existence of a pro-thrombotic tendency in IBD patients, arising from activation of the extrinsic pathway.
The intrinsic pathway
Activation of the “intrinsic” pathway of coagulation leads to formation of factor Xa (FXa)[28]. This process stems from previous activation of Factor IX and FVIII, with formation of the tenase complex, that is FVIIIa-factor IXa on the membrane of platelets and endothelial cells[28]. As FVIII strongly accelerates the formation of FXa, recent studies showed that inherited high levels of FVIII (> 140%) can be considered a risk factor for venous thromboembolic disorders[33]. No significant difference in APTT value or FVIII level was observed in IBD patients compared to controls[8,34]. However, higher APTT levels were found in other reports, although this finding may be the expression of mere consumption of some coagulation factors upon their activation[2]. Other investigations found that factor XIa and XIIa levels, were higher in active IBD patients compared to patients in the quiescent phase[8,35]. This finding may be in agreement with studies showing that higher levels of FXIa and FXIIa may be considered coagulation markers associated with increased risk for thromboembolic disorders[36-38].
The common pathway
FXa thus occupies a central position in the coagulation cascade as a convergence point between the intrinsic and extrinsic pathway. In fact, in the presence of its cofactor FVa, FXa converts prothrombin to thrombin[28]. The common pathway is considered the main determinant of both PT and APTT assays. In observational studies, FXa and FVa levels were significantly elevated in active IBD patients compared to those in patients with disease remission[3,8,39]. The protease-mediated stimulus for inflammatory reactions, particularly for FXa and thrombin, is mediated by cleavage of membrane cleavable receptors and will be discussed below.
The thrombin-generating system
Markers of the thrombin generating system directly involve zymogen prothrombin but also other side-products of prothrombin cleavage such as prothrombin fragment 1+2 (F1+2) and the thrombin-antithrombin complex (TAT). Prothrombin levels in active IBD patients were significantly higher than those in patients with inactive disease or control patients[8,30,32,39-41]. The same observation was also made for F1+2 and TAT, suggesting that thrombin generation might be an early event in IBD[8,40,42,43].
Factor XIII
Coagulation factor XIII is a plasma transglutaminase involved in the crosslinking of fibrin, the last step of the coagulation cascade and a connective tissue factor contributing to the wound healing process. It circulates as a heterotetrameric molecule consisting of two identical proenzyme subunits (factor XIIIA) and two carrier protein subunits (factor XIIIS).
A study by Chiarantini et al[30] reported decreased factor XIII (FXIII) levels, especially in acute phases of the disease, and deposits of FXIII have been detected in both affected and macroscopically normal bowel mucosa[30,41,43-45]. Those results were confirmed by several other reports, comparing IBD patients to control subjects as well as patients affected by diverticulitis and rheumatoid subjects[46,47]. As anticipated above for other coagulation factors, this finding does not play an etiopathogenetic role in IBD but may represent only the result of chronic consumption of this enzyme associated to deposition of fibrin at the level of inflamed vessels in enteric mucosa.
The system of natural coagulation inhibitors
The system of coagulation inhibitors is composed of a family of proteins, globally referred to as serpins, a typical example of which is antithrombin (AT), and by the protein C pathway, in which a series of different proteins (such as protein C and protein S) and membrane receptors [thrombomodulin (TM), endothelial protein C receptor (EPCR)] are involved. AT is the physiological inhibitor of thrombin, factor Xa, FIXa, FXIa and FXIIa. There is a growing body of evidence that AT is not only an inhibitor of blood coagulation, but it is also able, when present at high concentrations, to reduce the inflammatory responses of endothelial and other cells[48-50]. Thus, AT was shown to reduce the mortality of patients with severe sepsis in a recent clinical trial[49]. AT-induced attenuation of inflammatory responses might be linked to endothelial production of prostacyclin and inhibition of leukocyte and endothelial cell expression of pro-inflammatory mediators via suppression of nuclear factor (NF)-κB activation[48,50,51]. Furthermore, AT prevents water-immersion restraint stress-induced gastric mucosal injury in rats by promoting the endothelial release of PGI2[52]. In addition, non-uniform information exists on the quantitative expression of these components in IBD. Overall, it seems that no differences in the levels of protein S[8], protein C[3,53] and ATIII[34,41] exist among IBD patients compared to controls. However, in studies comparing the levels of these molecules in the active vs inactive form of the disease or in controls, lower levels of protein C, ATIII and protein S were observed[21,30,34,53,54]. In a single study, higher levels of protein S and C in IBD patients compared to controls were also reported[30]. These conflicting results indicate that changes in systemic levels of these inhibitors do not necessarily reflect the local loss of inhibition of coagulation occurring within enteric mucosa in IBD. Hence, a new approach has tried to correlate an enhanced production of thrombin in IBD with a possible loss of function in natural anticoagulants mostly occurring on the endothelium of enteric mucosa. This issue will be addressed in the section below.
The fibrinolytic system
In normal hemostasis, the fibrinolytic system allows removal of a fibrin clot when the damaged vessel wall is restored. Activation and regulation of fibrinolysis occurs by multiple proteins and results in the generation of plasmin. Plasminogen may be activated by tissue plasminogen activator (tPA) or urokinase plasminogen activator (uPA). The latter binds to a cellular receptor [urokinase plasminogen activator receptor (uPAR)] resulting in enhanced activation of cell-bound plasminogen and its main role is the induction of pericellular proteolysis[55]. tPA is the most potent activator of plasminogen in plasma and the main regulator of fibrinolysis. After stimulation, tPA is locally released into the circulation from the endothelial cells where it is produced. tPA-mediated plasminogen activation is facilitated by a fibrin surface, which restricts fibrinolysis to the site of thrombus formation[56]. Moreover, once bound to fibrin, tPA is protected from inhibition by plasminogen activator inhibitor 1 (PAI-1), its principal inhibitor in plasma[57]. The level of PAI-1 in blood usually exceeds that of tPA; thus, in general, no active tPA circulates in plasma[58].
α2-Antiplasmin is the primary physiological inhibitor of plasmin, as it can very rapidly inhibit plasmin in plasma[59]. However, plasmin is partly protected from α2-antiplasmin inhibitory activity when the enzyme is bound to fibrin[60]. During thrombus formation, α2-antiplasmin is cross-linked to fibrin by factor XIIIa, facilitating local inhibition of fibrinolysis[61].
Another important player in the fibrinolytic system is thrombin-activatable fibrinolysis inhibitor (TAFI), which directly connects coagulation and fibrinolysis. It is activated by thrombin, but its activation is over 1000-fold enhanced by the thrombin-TM complex. Activated TAFI removes carboxyl-terminal lysine residues from partially degraded fibrin. Consequently, the binding of plasminogen and tPA to fibrin clots is decreased, which attenuates clot lysis[62].
Reduced activity of the fibrinolytic system has been described in IBD[63-65] Indeed, a reduction in activators (such as tPA) and an increase in inhibitors (such as PAI and TAFI) of the fibrinolytic system have been described in IBD patients[40,63-66]. This condition would favor pro-thrombotic mechanisms.
Cellular elements involved in the coagulation pathway
The haemostatic system is composed not only of soluble proteins and enzymes but also of different cell types. Platelets and endothelial cells play a central role in the maintenance of a physiological balance between pro- and anti-coagulant mechanisms. Moreover, accumulating evidence indicates that platelets and endothelial cells, besides their well-known haemostatic functions, play a role in inflammation and its resolution mechanisms[67].
Platelets
Platelets can release a number of mediators of the inflammatory response, including cytokines, chemokines, nitric oxide (NO) and eicosanoids. Furthermore, they interact with polymorphonuclear cells (PMN) and monocytes, regulating their extravasation and recruitment at sites of inflammation. Along these lines, platelets have the enzymatic machinery to synthesize both pro- and anti-inflammatory eicosanoids. As an example, platelets contain 2-lipoxygenase (12-LO), a key enzyme in the biosynthesis of the lipoxins (LXs), arachidonic acid metabolites with potent anti-inflammatory properties[68]. LX formation during platelet/PMN interactions occurs in vivo and represents a main stop signal of inflammation[69]. Thus, a sustained inflammatory response, as it occurs in IBD may originate by both increased formation of pro-inflammatory mediators, and reduction in counter-regulatory signals.
Platelet integrin GPIbα is a ligand of P-selectin, a transmembrane adhesion molecule present on endothelial cells, and supports platelet rolling on activated endothelium. P-Selectin binding to P-Selectin Glycoprotein Ligand 1 (PSGL-1) stimulates the release of microparticles bearing tissue factor on their surface by leukocytes[70]. P-selectin is split as a soluble form (sP-selectin) that stimulates expression and exposition on the monocyte surface of further tissue factor[71]. Microparticles, together with sPselectin, are considered the main factors responsible for the high procoagulant status of blood in inflammation[72]. During adhesion to endothelium, platelets release pro-inflammatory cytokine CD40-ligand (CD40L) (CD154, gp39) that can stimulate the endothelium, stabilize platelet aggregates, their binding to blood cells and vascular wall cells and promote stable thrombus formation[73]. CD40L is expressed on activated platelets and also on immune system cells activated during inflammation (activated CD4+ T cells, basophils, and mast cells)[74]. This factor is a transmembrane protein related to tumor necrosis factor (TNF)-α. The inducible CD40L on platelets binds to the CD40 receptor on endothelial cells and on monocytes, macrophages, and smooth muscle cells (SMC)[74]. The CD40/CD40L interaction plays an important role in inflammation and atherothrombosis[73]. Through binding to the ligand, CD40 induces the inflammatory response independent of cytokines. The pro-inflammatory activity of CD40L also occurs on platelets and other cells by stimulation of the expression of chemokines [monocyte chemotactic protein 1 (MCP-1)], interleukins (IL-6, IL-8), pro-inflammatory adhesive molecules [vascular cell adhesive molecule-1 (VCAM-1)], intracellular adhesive molecules (ICAM-1, CD54), and P-/E-selectins.
Notably, the activity of CD40L induces the expression of tissue factor, which, as mentioned above, is the major inducer of blood coagulation, and suppresses the expression of TM, which is a thrombin cofactor in activation of the protein C anticoagulant system[75]. Upon binding of CD40L to the CD40 receptor, intracellular signaling results in activation of the transcriptional factor NF-κB and its translocation into the nucleus. This event induces the expression of new molecules of CD40L and CD40. The interaction of CD40 expressed by endothelial cells with CD40L exposed on activated platelets stimulates the synthesis of a powerful pro-inflammatory mediator, platelet activating factor (PAF), which induces platelet aggregation with leukocytes and also contributes to remodeling of vessels, stimulating neo-angiogenesis[76].
Platelet activation is associated with the metalloprotease-mediated split of a soluble fragment of the CD40-ligand (sCD40L)[76]. Soluble CD40L was shown to promote blood coagulation by two mechanisms: induction of tissue factor expression on monocytes and activation of platelets via interaction with integrin αIIb/β3. The sCD40L binding to integrin αIIb/β3 activates platelets at high shear stress and stabilizes arterial thrombi[74]. Increased levels of CD40L on platelets and sCD40L in circulating blood were found in clinical settings characterized by thrombosis associated with inflammation, such as unstable angina, myocardial infarction and other cardiovascular diseases[77,78].
Thus, a realistic scenario shows that platelet activation during inflammation and expression of adhesive proteins, P-selectin and integrins, leads to their aggregation with leukocytes and the release of contents of intracellular granules. In conclusion, platelet-platelet and platelet-leukocyte aggregates produce a cell surface, which provides activation of both blood coagulation and inflammation.
The association between active IBD and thrombocytosis was first recognized in 1968 and it became clear that patients with IBD also have increased numbers of circulating platelet aggregates and activated platelets compared with healthy controls[79-81]. More recently, it was demonstrated in studies from different groups that supranormal platelet-leukocyte aggregates are frequently present in patients with IBD compared with both healthy and inflammatory controls[82]. In other studies, significant changes in platelet volume were also observed[83,84]. In particular, an increase in platelet count[83] and a statistically significant decrease in MPV was noted in patients with colitis compared with healthy controls. Moreover, MPV of active colitis patients was significantly lower than that of the inactive phase of the disease. It is, however, difficult to correlate this finding with the functional alterations that could be responsible for the platelet-mediated thrombotic mechanisms summarized above. It may be hypothesized that in IBD the reduction in MPV may be associated with a peripheral platelet activation responsible for an exalted formation of platelet-platelet, platelet-PMN and platelet-endothelium adducts. This process would mainly involve younger platelets which have a bigger volume. Thus, the overall reduction of MPV could reflect the relative prevalence in circulating blood of less reactive platelets, which are older and smaller[85]. In a very recent study, an enhanced expression of CD40/CD40L in intestinal epithelial cells was demonstrated[86]. In particular, endoscopy biopsies taken from CD and UC patients showed a positive immunofluorescence staining for CD40 in intestinal epithelial cells of inflamed ileal or colonic mucosa, while no staining was observed in uninvolved intestinal segments[86]. These findings provide, for the first time, direct evidence for the epithelial expression and modulation of CD40 in IBD-affected mucosa and indicate its involvement in the pro-inflammatory and platelet-activating function of inflamed intestinal cells.
Finally, another paper from our group showed that in vitro activated platelets directly increase CD40L expression by intestinal endothelial cells, leading them to interact with other immune cells and sustain intestinal chronic inflammation. This pathway has been proposed as a new mechanism of chronic inflammation, as a result of the complex interplay among different cell types in the intestinal mucosa[87].
Endothelium
In a normal artery, endothelium creates a non-thrombogenic surface that acts as a selectively permeable barrier. Endothelium plays a key role in response to vascular injury, regulating leukocyte adhesion, platelet activation and adhesion and blood coagulation. Endothelium expresses and responds to multiple active substances, including cell adhesive molecules, cytokines and chemokines, to accomplish these functions[88,89]. Injury to a vessel wall results in the triggering and propagation of inflammatory and coagulation events. The cell adhesion molecules (CAM) are expressed onto the surface of activated endothelial cells and attach leukocytes and platelets. Adhesive proteins provide for the binding and spreading of leukocytes, their rolling, and their further transmigration across endothelium. There are three major classes of CAM: selectins, the immunoglobulin superfamily CAM and integrins. Some integrins in turn can be receptors of CAM and the endothelial adhesion molecule, von Willebrand factor (VWF), which binds platelets.
Weibel-Palade bodies in endothelial cells and platelet α-granules contain and release platelet P-selectin (CD62P, GMP140) responsible for adhesion of leukocytes, their rolling, and for stabilization of platelet aggregates[90,91]. The lectin-containing N-terminal domain of P-selectin binds to PSGL-1 on monocytes, neutrophils and platelets[91,92].
E-selectin (CD62E) is another molecule exposed on the endothelial cell surface that can bind to PSGL-1 in response to mechanical injury and inflammatory mediators as IL-1β, TNF-α, bacterial toxins and oxidants[89]. P- and E-selectin mediate rolling of activated and quiescent platelets on activated endothelium similar to the mechanism of leukocyte rolling[93].
The immunoglobulin superfamily CAM includes ICAM-1 (CD54), ICAM-2 and VCAM-1, which are expressed by many cell types including endothelial and SMC. In response to vascular injury, these cells upregulate expression of ICAM-1 and VCAM-1[89], engaged in leukocyte adhesion. Adhesion of platelets to injured endothelium is controlled by VWF, a multimeric protein, whose molecular weight ranges from 0.5 to 20 million Da[94] and is stored and released from Weibel-Palade bodies in endothelial cells[95]. Hence, VWF is considered a marker of endothelial injury. The VWF molecule contains domains responsible for binding blood coagulation factor VIII and platelet integrins such as glycoprotein transmembrane complexes GPIb/IX/V and integrin αIIb3 (GPIIb/IIIa), as well as collagen[94]. VWF binds subendothelial collagens and after immobilization attaches to platelets via the membrane complex GPIbα-IX-V[96]. VWF may be involved in the pathogenesis of acute thrombotic occlusion of stenosed arteries, where high shear stress promotes the formation of “stretched” VWF conformers, which are suitable for binding to platelets and subendothelial components[88]. P-Selectin could serve as an anchor site for the ultra large VWF multimers on the surface of activated endothelium, to facilitate their cleavage at the Tyr1605-Met1606 peptide bond by the disintegrin and metalloproteinase with thrombospondin motif-13 (ADAMTS-13)[97]. Microvascular dysfunction has been clearly demonstrated in IBD patients and involved several aspects of endothelium biochemical physiology[98,99]. In particular, such dysfunction involves an alteration in nitrogen and reactive oxygen species balance, where the microvascular endothelium fails to generate ·NO, a potent vasodilator and anti-aggregating agent, forming instead elevated levels of superoxide anion[54]. However, the mechanism responsible for the loss of endothelial nitrogen oxide in IBD gut microvessels also involves additional biochemical pathways. Previous studies showed an acquired deficient transcription of nitric oxide synthase 2 (NOS2) in chronically inflamed IBD endothelium[100]. Furthermore, more recently, it was demonstrated that decreased production of nitrogen oxide in IBD endothelial cells can also arise from the induction by many inflammatory cytokines (IL2, TNF-α) of the enzyme arginase (isoform I and II)[101]. This enzyme converts L-Arg into urea and L-ornithine, precursors for polyamines and L-proline compounds, which are vital to tissue homeostasis and wound repair[102]. Arginase I and II compete with inducible NOS (iNOS, NOS2), the most relevant inducible pathway for the production of ·NO, for L-Arg, which is their common substrate in endothelial cells[103]. Thus, an increased arginase activity in IBD may contribute to inhibit the production of a potent antithrombotic agent such as nitric oxide. The increased production of reactive oxygen species in inflamed endothelium may also contribute to oxidative stress in VWF molecules, which become unresponsive to proteolysis by ADAMTS-13 and the accumulation of ultra large VWF multimers[104]. The latter are the most haemostatically active forms of VWF and, favoring platelet adhesion and aggregation, may contribute to microvascular thrombosis in IBD.
To conclude, endothelium plays an essential role in inflammation due to its central “gatekeeper” function, which controls the quality and quantity of leukocytes that transmigrate from the vasculature into the interstitial space, regulates vascular tone and promotes platelet adhesion and aggregation. The latter function directly affects the haemostatic system and may clearly favor thrombotic phenomena. Several papers reviewed over the last few years suggest an activated status of endothelium in the course of IBD[98,99].
A COHERENT SCENARIO FOR UNBALANCED HAEMOSTASIS IN IBD
At this point a question arises as to whether the haemostatic and inflammatory alterations briefly described in the above paragraphs could be functionally linked in a coherent framework.
Globally, the coagulation system in IBD patients seems to sustain pro-thrombotic mechanisms, involving both soluble factors and cells, such as platelets, endothelium and leukocytes. This conclusion is supported by results obtained from new laboratory assays. The conventional and global coagulation tests such as PT and APTT both have low sensitivity and specificity and do not contain sufficient amounts of TM or glycosaminoglycans. Thus, these assays do not automatically reflect the coagulation reactions and their inhibition as they occur in vivo[105,106]. In contrast, the latest generation of methods that monitor the tissue factor induced thrombin generation in the presence of TM are credited as better laboratory tools to represent the balance of pro- and anti-coagulant forces operating in plasma[105-108]. In a recent study from Saibeni et al[105] endogenous thrombin potential, a parameter of the thrombin generation curve, was significantly higher in IBD patients than controls only when the test was performed in the presence of TM. This new assay strongly suggests that in IBD, as anticipated above, the increased generation of thrombin is mainly linked to a partial loss of function of natural anticoagulant pathways, and particularly of the TM-PC system.
Thus, systemic coagulation alterations in IBD may be recognized using more sophisticated techniques, which better reflect the in vivo setting. Furthermore, pathogenic considerations suggest that the coagulation imbalance in IBD could be particularly relevant in the vasculature of enteric mucosa, where inflammation shows the majority of destroying effects.
THE INFLAMMATION-COAGULATION INTERPLAY WITHIN THE INTESTINAL MILIEU
In addition to the demonstration that coagulation abnormalities and thromboembolic complications are clinically relevant events in IBD, they have been shown to exert effects at the mucosal level, where a coagulative imbalance exists[3].
In fact, one of the earliest abnormalities in CD mucosa is the presence of platelet thrombi cross-linked with fibrin in the mucosal microvasculature[109]. This feature, however, is not specific for CD and can be found in other idiopathic IBD[110]. The involvement of the microcirculation in IBD pathogenesis is underlined by the analysis of a segment of the small and/or large bowel during active IBD which reveals vasodilatation, venocongestion, edema, infiltration of large inflammatory cells and ulcerations[111]. This picture is the result of an unregulated intestinal inflammation with a consequent abnormal immune response and production of inflammatory cytokines, which, in turn, sustain the activation of the microvascular endothelium and subsequent recruitment of more leukocytes into the intestinal wall. This uncontrolled inflammatory response produces dramatic alterations in gut microvascular function which contributes greatly to perpetuating the inflammatory damage observed in IBD[98,112].
Coagulation factors mainly interact with local endothelium, although this interaction is potently conditioned by many features of the mucosal immunity.
The principal link between endothelium and the coagulation cascade is determined by Protease-activated receptors (PARs)[113]. PARs, and in particular PAR-1 and PAR-2, are cellular receptors activated after proteolytic cleavage by enzymes[114], mainly thrombin and activated factor X. Only a single study reports over-expression of PAR1 in patients with IBD[115], and there are no data available on animal models addressing its role in IBD. The expression of PAR2, which is greatly increased in patients with UC and CD[116-118], and the functional consequences of its activation in animal models are more widely documented.
Next to expression in the intestine, PAR expression in enteric neurons might be highly relevant for IBD because PARs can mediate gut inflammation via neurogenic mechanisms. Interestingly, PAR activation on submucosal and myenteric neurons causes severe edema in rat models. Moreover, the local activation of PAR2 but not PAR1 in the gut causes colitis through a neurogenic mechanism[116]. Taken together, these results point towards PAR2 expression/cleavage as a cardinal factor in IBD.
The APC-TM system is the natural pathway by which the pro-inflammatory activity of PAR-1 and 2 signaling is contra-balanced. In the following section major findings on how thrombin, factor X and APC contribute to IBD, will be shown and briefly discussed.
Thrombin
Once thrombin is sequentially activated through the intrinsic and extrinsic pathway, it not only amplifies the coagulant process but it can also favor inflammation induced by other stimuli, either through ischemia (consequent upon thrombosis), indirectly through the generation of downstream mediators or directly via signals through protease-activated receptors (PAR)[119].
Thrombin activates PARs, thereby establishing a link between activation of coagulation and pathophysiology of IBD. Indeed, thrombin signals through PAR1, PAR3 (in mouse) and PAR4, while tissue factor (TF)/FVIIa activates PAR2, and FXa activates PAR1 and PAR2[116,120].
In addition to promoting platelet activation, thrombin exerts influence over monocytes, macrophages[121] and neutrophils in processes related to tissue repair at the site of injury[122,123]. Thrombin also interacts through an equilibrium high affinity binding with the N-terminus of GpIbα of platelets and endothelial cells[124,125]. Notably, on platelet membrane, binding of thrombin to GpIb, accelerates cleavage of PAR1 by the enzyme[126]. Thrombin also affects endothelial cells through various pathways including NF-κB, early growth response factor-1 and GATA binding proteins[127]. Thrombin signaling might result in post-transcriptional changes, including calcium influx, cytoskeletal reorganization, and release of soluble mediators, growth factors, and matrix metalloproteinases. In addition, thrombin signaling results in changes in downstream gene transcription, for example increasing the expression of genes involved in cell proliferation, inflammation, leukocyte adhesion, vasomotor tone, and hemostasis[128-130].
Factor Xa
Borensztajn et al[116] suggested that Factor Xa signaling through PAR2 contributes to the progression of IBD and fibro-proliferative responses. Because FXa is a well-known PAR2 agonist, FXa-induced PAR2 activation is gaining attention in intestinal pathology. Accordingly, in a variety of endothelial in vitro systems, FXa induces an array of pro-inflammatory responses and the deposition of connective tissue growth factor[116,131,132]. It also leads to the activation of NF-kB, and the release of IL-6, IL-8, and MCP-1 on endothelial cells as well as fibroblasts[120,133]. Moreover, on endothelial cells, FXa induces the expression of E-selectin and both intracellular ICAM-1 and VCAM-1, resulting in leukocyte adhesion[116,134,135]. In synergy with tumor necrosis factor, FXa induces TF expression via inhibition of its negative regulators IkBa and A20[116,136]. Most of these responses are mediated via PAR2 activation, although some studies showed minor involvement of PAR1[136,137].
Although the potential pro-inflammatory role of FXa on epithelial cells of the gastrointestinal tract is not fully investigated, studies on Hela cells showing that FXa induces activation of the pro-inflammatory transcription factor NF-κB, suggest that it plays an important role[116,138]. Finally, FXa also affects immune cells inducing the production of IL-2 by lymphocytes[139]. Evidence that FXa may mediate inflammatory responses in vivo has come from several studies. In particular, Cirino et al[140] demonstrated that FXa induces the formation of edema when injected subcutaneously in a rat paw inflammation model, via local recruitment of mast cells.
The PC pathway
Traditionally described as a major anti-coagulant system, the protein C (PC) pathway, consisting of TM, the EPCR and activated PC (APC), is gaining increasing attention as an important regulator of microvascular inflammation, and in particular intestinal inflammation observed in IBD[141]. The anticoagulant function of the PC pathway has been reviewed extensively[142-144]. The main components of the PC system are the cell membrane receptor for PC, referred to as EPCR, the integral membrane glycoprotein TM, and two vitamin K-dependent plasma proteins, the zymogen PC, and the cofactor protein S. Upon cleavage of a dodecapeptide from the N-terminus of the light chain of PC by the thrombin-TM complex, the zymogen PC is activated to PC. Protein C per se is a poor substrate for thrombin. Allosteric binding of free thrombin to TM enhances, by several orders of magnitude, the thrombin-PC interaction and subsequent conversion of PC zymogen into its proteolytically active form, APC. The rate of PC activation by the TM-thrombin complex is further enhanced when the substrate PC zymogen is bound to its receptor, EPCR, which is able to reduce the Km value of the catalytic interaction with thrombin. The extent of in vivo PC activation is therefore greatly linked to the bioavailability of PC, thrombin and, critically, by the density of TM and EPCR molecules expressed on endothelial cells. With the exception of disseminated intravascular coagulation, consumptive coagulopathy and defective biosynthesis, thrombin bioavailability in first approximation reflects the intensity of coagulation activation. Within such limits, it was shown that thrombin formation and generation of APC are strongly correlated[145]. APC is formed mainly within the microcirculation, where endothelial cells express high levels of TM. As a consequence, due to the very small intravascular volume, the concentration of TM may be > 100 nmol/L, greatly exceeding the Kd value of the thrombin-TM interaction. Under these conditions, any amount of formed thrombin is rapidly and completely bound to TM. At variance with this situation, expression of TM is much lower in the endothelium of larger arteries and veins. Notably, EPCR expression is inversely related to that of TM, as it is more abundant in large vessel endothelium than in microcapillary beds. Thus, the efficiency and extent of APC formation differs considerably between different organs. The anticoagulant activity of the PC pathway includes the limited proteolysis by APC of the activated forms of coagulation factors V and VIII (FVa and FVIIIa), thereby limiting active thrombin generation. Protein S, in turn, cooperates with APC in inactivating FVa and FVIIIa, exerting an accelerating effect[146]. The current model of APC suppression of excessive thrombin generation assumes that the EPCR-bound pool of endothelial cell-associated APC plays a more important role for FV inactivation than the circulating plasma pool of APC. In part, this may be explained by the fact that binding of APC to cell surfaces is mediated only by EPCR, implying that the site of EPCR expression largely dictates the site of the anticoagulant function of APC. This model is fully consistent with the finding that FVa is highly susceptible to proteolytic degradation by APC when it is associated with the endothelial cell surface. FVa is instead refractory to APC cleavage in the platelet-associated prothrombinase complex[147]. Other mechanisms can potentially limit the anticoagulant activity of APC on, or close to platelets, i.e. inhibition of APC by the vitronectin-plasminogen activator inhibitor-1 complex, secondary to local release of plasminogen activator inhibitor-1 from activated platelets, and the inhibition of protein S activity by platelet factor 4 released from platelet α-granules[148-150]. Notably, platelet factor 4 can inhibit the anticoagulant function of APC alone, but not its ability to cleave and activate PAR-1. Thus, the interaction of platelet factor 4 may potentially redirect APC function toward anti-inflammatory and cytoprotective signaling pathways.
Overall, the relatively poor ability of APC to suppress thrombin generation in forming platelet aggregates might support effective and localized platelet-dependent hemostasis while sustaining the systemic anticoagulant potential. Thus, the APC activity in the presence of platelets may be considered another example of the compartmentalized haemostatic system.
Recently, it was demonstrated that surface-immobilized PC supports in a GPIbα- and apolipoprotein E receptor 2-dependent manner the adhesion and aggregation of platelets under flow conditions[151]. Thus, the ability of zymogen to engage these receptors raises the question as to whether PC immobilization occurs in vivo and whether changes in PC plasma levels are associated with altered platelet adhesion and aggregation.
Protein S circulates in blood in complex with a carrier protein, C4b-binding protein[152]. The level of C4b-binding protein increases in clinical settings characterized by inflammation. Hence, the amount of bound protein S increases, causing a decrease of free protein S concentration[152]. It is known that the anticoagulant function of protein S is exerted by its free form. Thus, systemic inflammatory conditions may represent a risk factor for protein-S dependent thrombotic disorders[152]. Finally, protein S exerts both APC-dependent and aPC-independent anticoagulant effects. The APC-dependent mechanism, involving the cofactor function of protein S for the acceleration of APC-mediated degradation of factors VIII and V, is likely the physiologically dominant pathway. The APC-independent anticoagulant activity of protein S is attained by stimulating the inhibition of tissue factor (TF) by tissue factor pathway inhibitor (TFPI)[153]. The latter blocks the intermediate complex of TF-FVIIa-FXa, thereby preventing substrate exchange of already activated FXa for new FX. Protein S enhances the inhibitory interaction of TFPI with the TF initiation complex, and thereby limits the extent of thrombin generation in plasma. This APC-independent anticoagulant activity of protein S is most pronounced at low TF levels. Due to the anticoagulant effects described above, severe protein S deficiency is associated with severe thrombotic disorders.
The relevance of the PC system for the prevention of atherothrombotic diseases is further corroborated by studies in animals. Some interesting aspects of in vivo PC activation were unraveled by analyzing the role of EPCR in the response of mice to an inflammatory challenge with lipopolysaccharide (LPS)[154]. In these studies, mice lacking EPCR showed substantially enhanced activation of coagulation, concomitantly with reduced APC formation attributable to the absence of EPCR. Yet, the plasma APC levels in LPS-challenged EPCR-deficient mice were almost identical to that measured in wild-type animals. The authors of this study then showed that in wild-type mice a large fraction (approximately 40%) of APC did not enter the systemic circulation but remained bound to endothelial cell-associated EPCR at its site of activation. It is this sequestered APC pool that is completely missing in EPCR deficient mice, and its absence apparently accounts for all the pro-coagulant and pro-inflammatory effects of EPCR deficiency in mice[154].
Recent studies have shown that the anti-inflammatory effect of APC, at least in part, is mediated through the EPCR-dependent proteolysis of PAR-1 in endothelial cells[155,156]. This finding seems paradoxical, as it is known that the cleavage of PAR-1 by thrombin elicits potent pro-thrombotic and pro-inflammatory responses[157]. It is well known that thrombin is mainly responsible for the activation of PC in the presence of TM and that the enzyme also cleaves PAR-1 with a high catalytic efficiency, which is 3-4 orders of magnitude higher than that of APC. This finding raises the question as to how APC in the presence of thrombin is able to produce physiologically significant cleavage of PAR1 associated with protective signaling events[158], as is presented in Figure 1. Notably, both APC and PC bind to EPCR with a similar equilibrium constant, so that it can be hypothesized that thrombin can increase the local concentrations of EPCR-bound APC[159]. This phenomenon may induce channeling of the protease directly into the signaling pathway.
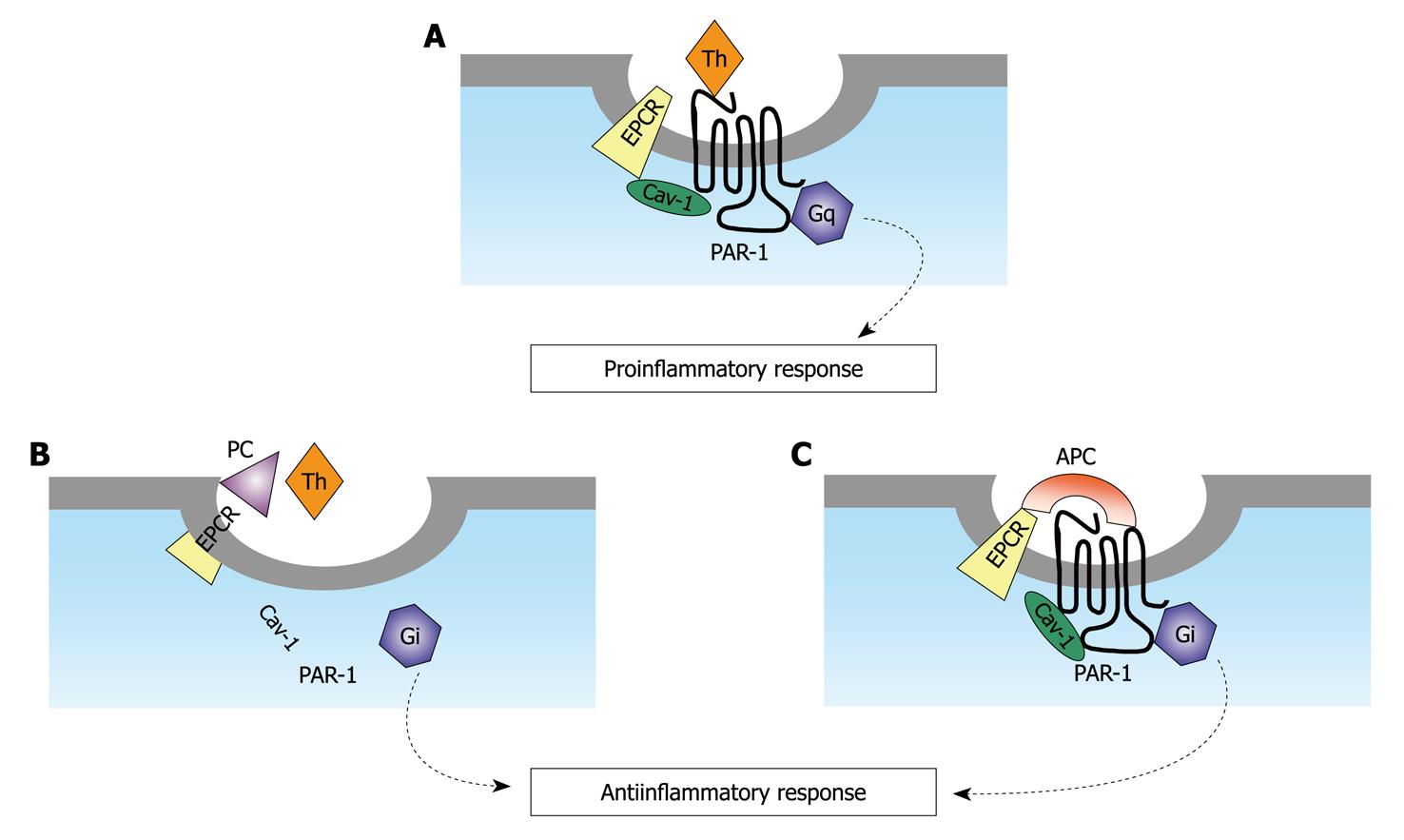
Figure 1 Models of protease-activated receptor-1 cleavage and activation by either activated protein C or thrombin when endothelial protein C receptor is occupied by its ligand protein C.
A: The unoccupied endothelial protein C receptor (EPCR) is associated with caveolin-1 (Cav-1) within lipid rafts of endothelial cells. Upon thrombin cleavage of protease-activated receptor (PAR)-1, a pro-inflammatory signal is generated through G12/13 and Gq under these conditions; B: The occupancy of EPCR by protein C (PC) results in dissociation of EPCR from Cav-1. This process is linked with the coupling of PAR-1 to Gi. Thrombin cleavage of PAR-1 initiates an antiinflammatory response under these conditions; C: The same as (B) except that the EPCR and PAR-1 dependent protective signaling response is mediated by activated protein C (APC) (adapted from[159]).
In a very elegant study, Rezaie and coworkers demonstrated that the critical receptors required for both protein C activation (TM and EPCR) and APC cellular signaling (EPCR and PAR-1) pathways colocalize in the membrane lipid rafts of endothelial cells. The co-localization of EPCR and PAR-1 in lipid rafts of endothelial cells is a fundamental requirement for the cellular signaling activity of APC, which leads to anti-inflammatory and anti-apoptotic cellular effects, such as phosphorylation of mitogen-activated protein kinase[160] and suppression of NFkB expression[161].
TM co-localization with these receptors on the same membrane microdomain can also recruit thrombin to activate the EPCR-bound protein C, therefore eliciting PAR-1 signaling events that are involved in the APC protective pathways[159] linked to dissociation of caveolin subunits (Figure 1). These findings explain how thrombin effectively channels endogenous APC to the protective signaling pathways, through cleaving the same receptor of thrombin.
The APC pathway in IBD
TM and EPCR expression is diminished in the colonic mucosal microvasculature of IBD patients[3,162-164], but is increased in their sera, suggesting increased shedding of TM and EPCR from cells. Inflammatory cytokines also down-regulate TM and EPCR by inhibiting transcription on cultured intestinal endothelial cells (HIMEC)[162]. These changes in TM and EPCR expression would be expected to affect the conversion of protein C in its activated form, which, in addition to its anticoagulant properties, also has potent anti-inflammatory activity[165], as described above.
Restoring the function of the PC pathway has anti-inflammatory effects on HIMEC, by decreasing pro-inflammatory cytokines secretion as well as adhesion molecules induced by TNF-α stimulation[144,162,166]. Furthermore, restoration of APC by supplementation reduces stress-induced gastric mucosal injury in rats by inhibiting the decrease in gastric mucosal blood flow through attenuation of the activated neutrophil-induced endothelial cell injury via inhibition of TNF-α production[167].
Overall, it can be concluded that a homeostatic balance exists between thrombin and APC in coagulation and inflammation. In particular, activated thrombin promotes the generation of APC and the two molecules influence the extent of both fibrin (clot) formation and the inflammatory response. This mechanism is mediated mainly by the cleavage of PAR-1 by either APC or thrombin on endothelial cells. Through binding to EPCR, APC would reverse the pro-inflammatory effects of thrombin on the same PAR[127,168].
THERAPEUTIC PERSPECTIVES FOR COAGULATION ABNORMALITIES IN IBD
Based on the reported findings from several studies, one can conclude that the PC pathway is strategically located at the crossroads between coagulation and inflammation, where it exerts entirely unexpected roles in the damage that occurs in chronic inflammatory conditions[169]. Unraveling the pathogenic role of the PC pathway offers a very promising tool in the therapeutic arsenal against IBD as well as many other chronic inflammatory diseases. Inflammation most likely mediates systemic hypercoagulability through various cytokines, which can affect the coagulation cascade at numerous points as well as platelet quantity and function. Unfortunately, perhaps due to this diversity of prothrombotic abnormalities that can exist in IBD patients and their likely multifactorial etiology, no specific therapy has ever been proposed in any clinical randomized trial to correct the cytokine-linked pro-inflammatory unbalance and pro-thrombotic phenomena occurring in IBD. Notably, unfractioned (UFH) and low-molecular-weight heparins (LMWHs), apart from their known anticoagulant/antithrombotic activities, display a broad spectrum of immune modulating and anti-inflammatory properties, such as modulation of cytokine production, T-lymphocyte cytotoxic activity[170] and inhibition of leukocyte adhesion, activation and trafficking[171]. Based on these features, these molecules have been proposed for the treatment of IBD. Some open studies suggested the efficacy of UFH[172,173] and LMWHs[174] for the treatment of active UC. Conversely, large controlled studies using UFH and LMWHs did not show a clear efficacy[175-178]. Moreover, a recent meta-analysis by Shen et al[179] indicated no significant additive benefit for heparins in the treatment of active UC. However, all studies included in the meta-analysis were very heterogeneous about their clinical, methodological and pharmacological features.
These studies, not only considered different definitions for response and remission, but also used different heparins, with theoretically very different anti-inflammatory activities[179]. For these reasons, it is still difficult to set the real value of this therapeutic approach in IBD.
Recently, experimental data on animal models of IBD suggested efficacy of LMWHs, when selectively delivered in the site of disease, compared to the other route of administration. The multimatrix oral formulation MMX releasing parnaparin sodium at three different doses was evaluated in a clinical trial in patients with mild-to-moderate UC activity[180]. This study, carried out on ten UC patients, showed no relevant side effects, including either interference with haemostatic parameters or increased bleeding. After treatment, seven patients were in clinical remission and only one achieved endoscopic healing. However, in a recent meta-analysis it was found that there is no evidence to support the use of UFH or LMWH for the treatment of active UC. In this study no further trials examining these drugs in patients with UC were warranted, except perhaps a trial of UFH in patients with mild disease[181]. Furthermore, it has to be outlined that any benefit found using heparins in this clinical setting should be weighed against a possible increased risk of rectal bleeding, especially in patients with active UC.
In conclusion, a direct therapeutic approach for controlling inflammation-driven imbalances in the coagulation system in IBD patients is not yet available. The growing body of evidence concerning the molecular and cellular perturbations in this setting should be unraveled to promote a more efficacious, pathogenesis-oriented therapy for these disorders.