INTRODUCTION
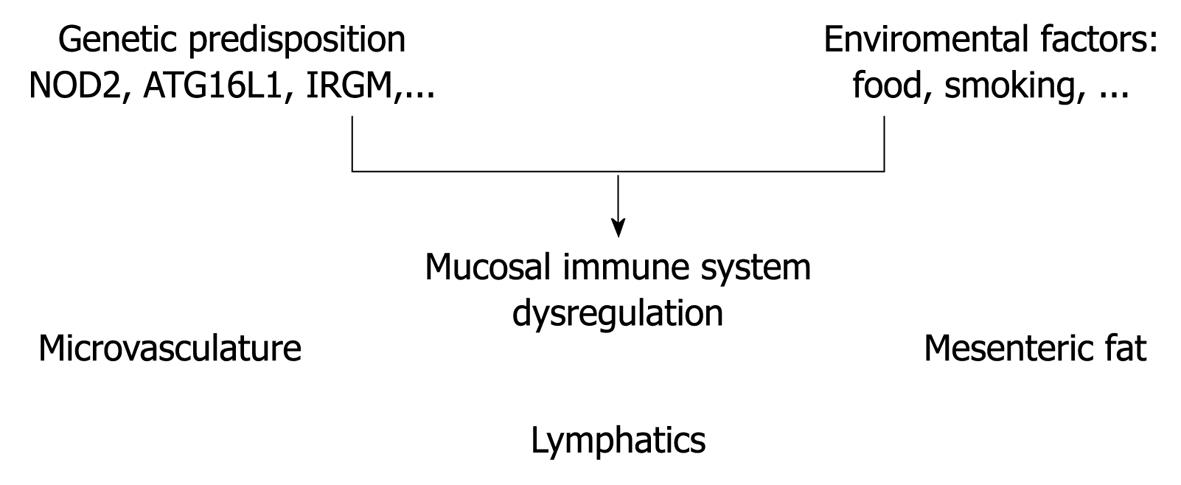
The genome-wide association studies in recent years have contributed significantly to the understanding of the pathogenesis of inflammatory bowel disease (IBD)[1]. The results obtained from these studies have not only confirmed the relevance of earlier characterized pathways, but equally have opened novel avenues. One possible hypothesis for the etiology of IBD is that the mucosal immune system is hyper-responsive to luminal antigens (e.g. dietary factors, commensal bacteria) in genetically predisposed individuals[2]. This hypothesis is limited to the intestinal lumen and wall. Focusing on Crohn’s disease, the inflammation is not restricted to the luminal side of the intestinal wall. Rather, transmural inflammation presents as the dominant phenotype, which leads to the question of whether extraintestinal/extraluminal structures contribute to the inflammatory process. In the present overview, three extraluminal structures are discussed, which have been demonstrated to play a role in the regulation of intestinal inflammation, namely the mesenteric fat tissue, microvasculature and lymphatics (Figure 1).
Figure 1 Extraluminal structures contributing to Crohn’s disease.
The figure illustrates the potential contribution of the extraluminal structures of mesenteric fat tissue, lymphatics and microvasculature to the dysregulation of the mucosal immune system.
MESENTERIC FAT TISSUE
Historic view
Crohn BB himself provided the first evidence that the mesenteric fat tissue might play a role in the pathogenesis of Crohn’s disease, by describing local hypertrophy of the mesenteric fat adjacent to inflamed intestinal segments[3]. This phenomenon, which is also called “creeping fat” or “fat wrapping” is restricted to Crohn’s disease, and is not observed in ulcerative colitis or other forms of chronic intestinal inflammation.
Anatomical view
The characteristic ‘‘fat wrapping’’ seen only in Crohn’s disease represents fat hypertrophy that results in partial cover of the intestinal circumference, which is defined as > 50% coverage of the intestinal surface by adipose tissue and occurs in both the large and small bowel. The localization of this ‘‘creeping fat’’ correlates with transmural inflammation, ulceration, and stricture formation[3]. These observational results have been underlined by magnetic resonance imaging that quantified the amount of intra-abdominal fat in relation to total body fat, which indicates that the intra-abdominal fat but not total body fat increases[4]. Adipocytes within this hypertrophied fat are significantly smaller, but a fourfold increase in the total number of adipocytes is present in the mesentery of Crohn’s disease patients as compared to controls[5]. How can we explain this observation and what might be the possible contribution to disease?
Adipocytes and chronic inflammation
Each lymph node in our body is in close proximity to adipose tissue. Once the lymph nodes are activated, the number of adipocytes increases, which allows for the supply of sufficient energy for a functional immune system[6-8]. However, is the role of the mesenteric fat tissue restricted to energy supply? In the first studies to analyze the expression of pro-inflammatory mediators in fat tissue, an increase of tumor necrosis factor (TNF)-α and the adipokine leptin was demonstrated in the fat tissue of Crohn’s disease patients, in comparison to non-inflammatory controls[4]. In addition, adipocytes express C-reactive protein, and there is a significant correlation between serum C-reactive protein levels and increased mesenteric fat density in Crohn’s disease[9]. What is the relevance of these mediators released by the adipose tissue?
Adipokines
Various adipokines are released by adipose tissue. The relevance of adipokines in IBD has been summarized recently in broad detail[10]. The adipokines characterized best with regard to intestinal inflammation are leptin and adiponectin, respectively.
Leptin is a 16-kDa peptide predominantly produced by adipocytes, which signals the status of satiety to the hypothalamus[11]. Leptin deficiency or non-function of the long isoform of the leptin receptor (OB-Rb) is associated with massive obesity in mice and humans. From a structural point of view, leptin can be classified as a helical cytokine[12]. Thus, the structure of leptin suggests a regulatory function within the immune system. In humans, leptin deficiency is rare, but results in impaired T-cell proliferation and is associated with increased mortality in childhood due to infection[13,14]. In mice, leptin deficiency has been associated with protection from dextran sodium sulfate (DSS)-, oxazolone- and trinitrobenzene sulfonic acid (TNBS)-induced colitis. In addition, results from the transfer model of colitis indicate that leptin serves as crucial T-cell stimulator in intestinal inflammation[15-17]. In addition, leptin stimulates the proliferation of naive CD4+ T cells and affects T-cell polarization[15,18,19]. In Crohn’s disease, increased expression of leptin mRNA as well as protein in the hypertrophic mesenteric fat has been reported[20,21]. Together with data from animal studies, a pro-inflammatory role for leptin in Crohn’s disease has been suggested.
Adiponectin, a 30-kDa polypeptide, contributes 5-10 μg/mL to 0.01% of plasma proteins, and hence is the most abundant adipokine in the circulation[22]. Adiponectin has a high affinity to form trimers that can further multimerize to polymers, which results in various high and low molecular isoforms. The biological significance of the different high and low molecular forms is not finally understood. In Crohn’s disease patients, adiponectin mRNA and protein release is upregulated in hypertrophied adipose tissue, as compared to normal adipose tissue from the same subjects, or mesenteric adipose tissue from ulcerative colitis patients and controls[23]. Data concerning the effects on disease severity in experimental models of colitis are conflicting. Whereas one group has observed increased susceptibility to the chemically induced model of DSS colitis[24], another has reported protection against DSS- as well as TNBS-induced colitis in adiponectin-deficient mice[25]. To confuse the issue even more, a third study has reported that adiponectin deficiency does not affect the outcome of disease in interleukin (IL)-10-deficient mice that develop colitis spontaneously[26]. In the model of chronic TNBS-induced colitis in rats, the size of mesenteric adipocytes is decreased, and production of adiponectin, besides other mediators, is increased in perinodal mesenteric fat[27]. As a result of the conflicting effects mediated by adiponectin on immune cells, both pro- and anti-inflammatory consequences of altered adiponectin production in IBD are possible. However, adiponectin does seem to modulate immune responses, and abnormal production could thus be involved in the altered responsiveness of immune cells that occurs in IBD.
Recent data from genetic studies have added independent support for such dysregulated production of adiponectin and leptin in Crohn’s disease. In mice, deficiency of the autophagy gene Atg16l1 results in upregulation of leptin as well as adiponectin mRNA expression[28]. In humans, the ATG16L1 risk allele is associated with an increased risk of developing Crohn’s disease[28,29]. Further cross-population studies are needed to ascertain whether this mutation is the cause of the altered leptin and adiponectin production in the hypertrophic fat of Crohn’s disease patients. However, so far, it is tempting to speculate that the ATG16L1 risk allele and the subsequent altered production of adipokines might contribute to the predisposition to Crohn’s disease.
The data described above indicate that adipokines are able to regulate the acquired immune response and that the production of some is altered in mesenteric fat of IBD patients. What kind of stimulus is required to modify the production of these adipokines in patients with Crohn’s disease? Translocation of luminal antigens (e.g. bacteria) from the intestinal lumen to the adipose tissue could offer this stimulus, presuming that adipocytes and pre-adipocytes express innate receptors.
Adipocytes as cells of the innate immune system
The release of free fatty acids by adipocytes following lipopolysaccharide (LPS) stimulation, and hence responsiveness of fat cells to bacterial components, was first detected over 30 years ago[30]. In line with these historic data, the expression of toll-like receptor (TLR)4 and TLR2 was described in adipocytes generated from the 3T3-L1 cell line[31]. Furthermore, our group and others have demonstrated that adipocytes and their precursors from mice and humans express TLR1-TLR11 and that specific stimulation induces secretion of immune regulatory mediators[32-34]. In addition, data from our group indicate expression of functional nucleotide oligomerization domain (NOD) proteins-1 and -2 on pre-adipocytes[35]. Expression of these NOD proteins in adipocytes and pre-adipocytes is further regulated by TNF-α or LPS (NOD2), respectively, IFNγ (NOD1)[35]. This observation is of particular interest, since mutations in the NOD2 gene have been associated with an increased risk of developing Crohn’s disease[36-38]. Thus, adipocytes and pre-adipocytes share functional properties of immune cells, which suggest an active role in defense against bacterial and viral antigens in vivo. Hence, adipocytes and pre-adipocytes could represent a yet ignored population of innate immune cells.
A working model could be that primary increased production of pro-inflammatory mediators in the mesenteric fat due to genetic predisposition might contribute to the development of Crohn’s disease. Additionally, the massive cytokine production in the inflamed colon, in addition to translocalizing bacteria, could further induce the production of pro-inflammatory mediators in the adjacent adipose tissue, thus inducing a vicious cycle, in which inflammatory conditions in the intestine and the mesenteric fat support each other.
LYMPHATICS
The lymphatic system is closely connected to and within the intestine, and is a neglected structure. In 2008, Van Kruiningen et al[39] reminded us of their presence in a concise review. They reviewed the pathological descriptions of Crohn’s disease in the era before antibiotic, corticosteroid, immunomodulatory and biological therapy. These pathologists described lesions in the basal portion of the lamina propria, in the superficial and deep submucosa, and in the subserosa, which suggested lymphatic disease. These lesions comprised lymphocytic thrombi within the lymphatics and multiple large aggregates of lymphocytes with or without multi-nucleated giant cells, a picture consistent with chronic lymphangitis[39]. The granulomas of Crohn’s disease appear to be in and around the very thin-walled lymphatics that are found adjacent to small vessels[40]. This further supports the idea that lymphatics might directly contribute to the pathogenesis of this disease.
Almost more intriguing are the rat and pig models in which regional lymphatics of the small intestine were obstructed with sclerosing agents[41,42]. These animals subsequently developed segmental intestinal disease that was characterized by many of the alterations that occur in Crohn’s disease, including lymphocytic and granulomatous changes. Remarkably, enteroenteric as well as enterocutaneous fistulas developed in these models, which are not seen in animal models routinely used today[41,42]. Additional observations have pointed out that the distribution and character of these lesions represent obstructive lymphocytic lymphangitis[43]. In these older studies, the connection between the shorter segments of Crohn’s disease in the jejunum and the longer segments in the ileum, with the shorter vasa recta of the jejunum and the longer lymphatic collecting ducts of the ileum, was emphasized[39,43].
Very recent work by Vetrano et al[44] has provided further experimental data underlining the relevance of lymphatics in IBD[44]. They have concentrated on the expression of D6, a promiscuous decoy receptor and scavenger for CC chemokines that plays a non-redundant role in the control of the inflammatory response in various organs. Vetrano et al[44] have demonstrated upregulation of D6 in human colitis. The expression could be localized to lymphatic vessels and leukocytes in the mucosa. D6-deficient mice showed an increased susceptibility to experimental colitis when compared to wild-type mice. Via bone-marrow chimeras, the regulatory function of D6 in colitis has been tracked to the stromal/lymphatic compartment, and a contribution of hematopoietic cells could be excluded. Thus, these data further emphasize the regulatory role of the lymphatic system in intestinal inflammation.
In line with these observations, Van Kruiningen et al[39] have suggested recently to focus again on the lymphatic damage in Crohn’s disease, and the identification of possible harmful agents that cause lymphangitis and lesions in the lymphatic endothelium. Although the lymphatics are not completely separated from the intestine, they represent the second structure that should be reevaluated in Crohn’s disease.
MICROVASCULATURE
In similar close proximity to the intestinal wall is the microvasculature that is embedded in the mesenteric fat tissue, which thus provides an additional link as outlined below.
Whether increased vascularization as assessed by mesenteric angiography or Doppler ultrasound reflects Crohn’s disease activity is disputed[45]. Recent evidence for angiogenesis playing a role in IBD pathogenesis has prompted interest in anti-angiogenic therapies for IBD[46,47]. Remarkably, angiogenesis plays a crucial role in various chronic inflammatory disorders such as artherosclerosis, rheumatoid arthritis, peptic ulcer, IBD, psoriasis, and Alzheimer’s disease[48]. Growth of new blood vessels is intrinsic to inflammation and is associated with structural changes, including activation and proliferation of endothelial cells and capillary and venule remodeling, all of which result in an expansion of the tissue microvascular bed[49-51]. As inflammation evolves, vessels expand to supply nutrients that sustain the accumulation of activated immune cells, and in the chronic phase, local immune cells overproduce endothelial cell growth factors[49].
This expansion of the vascular network facilitates several mechanisms. The influx of inflammatory cells increases the nutrient supply that allows the metabolically active immune response to take place, and the activated endothelium contributes to the local production of cytokines, chemokines, and matrix metalloproteinases[52,53]. In chronic inflammatory disorders, infiltration by macrophages and lymphocytes, tissue damage and repair occur concurrently, and the newly formed vessels become permanent[51,54]. The anatomical expansion and increased activation of the remodeled microvascular bed foster further influx of immune cells, and angiogenesis and inflammation become co-dependent processes[55]. Both innate as well as adaptive immune responses promote angiogenesis.
Thus, the endothelium, and more specifically, the endothelial cells of the microvasculature seem to assume a central function, because they are not only capable of generating a range of mediators, but also display distinct adhesive molecule patterns, to activate a unique sets of genes and form capillaries[56-58]. In addition, endothelial cells act depending on the body compartment heterogeneity[56,59-61]. An example is the expression of mucosal addressin cellular adhesion molecule-1 by Peyer’s patches and high endothelial venules to recruit α4β7 homing receptor-positive naïve lymphocytes[62]. Similarly, endothelial cells from brain, liver, and other organs express distinct surface markers, protein transporters, and intracellular enzymes[61-63]. This heterogeneity becomes of particular interest when considering the regulation of organ-specific inflammation; in our case, intestinal inflammation. The distribution and infiltration of leukocytes is tightly regulated by numerous homing and adhesion molecules on the surface of microvascular and immune cells[64]. At inflamed sites, endothelial cells still control the type and number of immune cells that extravasate into the interstitium in a dysregulated fashion[65,66].
An additional cell population has been identified to play a crucial role in the process of cell infiltration via the endothelium in areas of inflammation, namely platelets. They normally circulate without attaching to the endothelium, but do so when the endothelial cells become activated, and platelet adherence triggers inflammation[67]. Activated platelets produce massive amounts of pro-inflammatory mediators and interact with various other cell populations[68,69]. In inflamed areas, the microvasculature can recruit leukocytes through a platelet-dependent mechanism, but at the same time, platelet recruitment is leukocyte dependent[70]. The details of this crucial interaction have been summarized by other reviews[71].
A number of animal studies have proven that the process of angiogenesis can be taken advantage of as a therapeutic approach. Hence, in models of DSS-induced inflammation, as well as spontaneous colitis in IL-10-deficient mice, angiogenesis occurs. However, when this angiogenesis is inhibited, clinical severity and the signs of histological inflammation decrease significantly[47]. Furthermore, vascular endothelial growth factor A that induces angiogenesis has been recently shown to be upregulated in samples from patients with IBD, and in mice with colitis. The overexpression of VEGF-A in mice exposed to DSS was followed by deterioration of disease and an additional increase in angiogenesis when compared to DSS-exposed wild-type mice[72].