Published online Dec 28, 2011. doi: 10.3748/wjg.v17.i48.5305
Revised: June 28, 2011
Accepted: July 11, 2011
Published online: December 28, 2011
AIM: To investigate the existence and levels of sH2a, a soluble secreted form of the asialoglycoprotein receptor in human serum.
METHODS: Production of recombinant sH2a and development of a monoclonal antibody and an enzyme-linked immunosorbent assay (ELISA). This assay was used to determine the presence and concentration of sH2a in human sera of individuals of both sexes and a wide range of ages.
RESULTS: The recombinant protein was produced successfully and a specific ELISA assay was developed. The levels of sH2a in sera from 62 healthy individuals varied minimally (147 ± 19 ng/mL). In contrast, 5 hepatitis C patients with cirrhosis showed much decreased sH2a levels (50 ± 9 ng/mL).
CONCLUSION: Constant sH2a levels suggest constitutive secretion from hepatocytes in healthy individuals. This constant level and the decrease with cirrhosis suggest a diagnostic potential.
- Citation: Benyair R, Kondratyev M, Veselkin E, Tolchinsky S, Shenkman M, Lurie Y, Lederkremer GZ. Constant serum levels of secreted asialoglycoprotein receptor sH2a and decrease with cirrhosis. World J Gastroenterol 2011; 17(48): 5305-5309
- URL: https://www.wjgnet.com/1007-9327/full/v17/i48/5305.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i48.5305
The human asialoglycoprotein receptor (ASGPR) is expressed on the sinusoidal membrane of hepatocytes and serves in the clearance of asialoglycoproteins from the plasma[1]. As evaluated through whole-body scintigraphy, using a radioactive ASGPR ligand, a technetium-99m-labeled asialoglycoprotein analog, only the liver shows any significant expression of the ASGPR[2]. The levels of the ASGPR are much reduced upon hepatocyte dedifferentiation[3,4], upon chronic alcohol consumption[5] and in liver fibrosis and cirrhosis[6,7]. We had previously described a soluble form of the ASGPR, termed sH2a, secreted from the human hepatoma cell line HepG2. SH2a is formed by cleavage in the endoplasmic reticulum of its precursor[8], encoded by an alternatively spliced variant of the ASGPR H2 subunit mRNA[9], and does not arise by shedding at the cell surface. Here we show for a group of healthy individuals that ASGPR sH2a is secreted to the serum at surprisingly constant levels, and that these are much reduced in hepatitis C virus (HCV) patients with liver cirrhosis.
Immobilon-P paper was purchased from Millipore Corp (Bedford, MA). Protein A-sepharose was from Repligen (Cambridge, MA). N-glycosydase-F was obtained from Roche Applied Science. Succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate and dimethylpimelidate were from Pierce (Rockford, IL). A solution of 3,3’,5,5’-tetramethylbenzidine (TMB) was purchased from Kirkegaard and Perry Laboratories Inc. (Gaithersburg, MD). Alkaline phosphatase (ALP) substrate p-nitrophenylphosphate was from Chemicon International (Temecula, CA). Imperial protein stain was from Pierce. Common reagents were from Sigma.
Polyclonal antibodies specific for a peptide corresponding to the carboxyterminus of sH2a or to a peptide unique to sH2a (169 antibody) were described in earlier studies[8].
A monoclonal antibody was prepared by intraperitoneal immunization of BALB/c mice with a conjugate of keyhole limpet hemocyanin (KLH) with the carboxyterminal peptide of H2 and complete Freunds adjuvant. Conjugation was performed using succinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate. Hybridoma cells, resulting from fusion of the mouse splenocytes with NS/O myeloma cells, were screened by ELISA, selecting a clone (B9) that reacted strongly with the peptide but not with KLH. The isotype of B9 was IgG3, as analyzed using an Isostrip kit (Roche). Ascitic fluid, obtained by injection of hybridoma cells to mice was used in all experiments due to difficulties in IgG3 purification by standard methods. It did not show any significant background. Goat anti-mouse IgG conjugated to agarose was from Sigma. ALP- or HRP-conjugated goat anti-mouse or anti-rabbit IgG antibodies were from Jackson Laboratories (West Grove, PA).
Recombinant sH2a was generated by PCR using the primers GAACCATCAGGAGGATCCCAAAGTGAGGGTC and GGAATTCTCAGGCCACCTCGCC and cloned into pET28a (Novagen), which adds an N-terminal 6XHis tag, using BamHI and EcoRI. Expression was in Escherichia coli Rosetta DE3 pLysS at 37 °C until A600 = 0.6 and then with isopropyl β-D-1-thiogalactopyranosid induction for 2 h at 32 °C. After cell sonication and addition of 6 mol guanidinium-hydrochloride, tagged sH2a was purified on Ni2+-NTA-agarose (Qiagen) followed by dialysis. Protein concentration was determined by the bicinchoninic acid assay (Pierce).
NIH 3T3 cells and a stable transfectant expressing H2a (2-18 cell line)[8] were grown in Dulbecco's modification of Eagle's medium (DMEM) supplemented with 10% newborn calf serum. HepG2 cells were grown in MEM plus 10% fetal calf serum.
Immunoprecipitations from cell supernatants (1.2-1.5 mL from 90 mm plates), using rabbit anti-H2a carboxyterminal or 169 antibodies were done as described before[8]. Immunoprecipitations from serum samples were done in a similar manner or using anti-H2a antibody crosslinked to protein A-sepharose with dimethylpimelidate where indicated. Immunoprecipitation with B9 was followed by goat anti-mouse IgG bound to agarose. Treatment of immunoprecipitates with N-glycosidase-F and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) were done as described before[8].
Immunoblotting was done as described before[10] using anti-H2a carboxyterminal antibody and detection was performed with TMB solution or using the electrochemiluminescence procedure and a Bio-Rad ChemiDoc XRS system.
Corning ELISA plate wells were coated with the carboxyterminal peptide of sH2a (5 μg/mL) and blocked with 3% bovine serum albumin in Tris-buffered saline (TBS), pH 7.5. B9-containing ascitic fluid (1:1500) was preincubated overnight at 4 °C with serial dilutions of the serum sample and then incubated on the coated ELISA plate wells for 1 h at room temperature (RT). After TBS wash wells were reacted with goat anti-mouse IgG conjugated to ALP for 1 h at RT. P-nitrophenyl phosphate was added and A quantified at 405 nm.
Retrospective samples were from a group of healthy blood donors and a group of HCV-infected patients with cirrhosis, at the Liver Unit, Tel Aviv Sourasky Medical Center. Cirrhosis was determined by percutaneous liver biopsy, performed using a Tru-Core II [R] biopsy instrument under ultrasound guidance. The study had a priori approval by the hospital ethical committee according to the Helsinki Declaration and written informed consent was obtained from all participants.
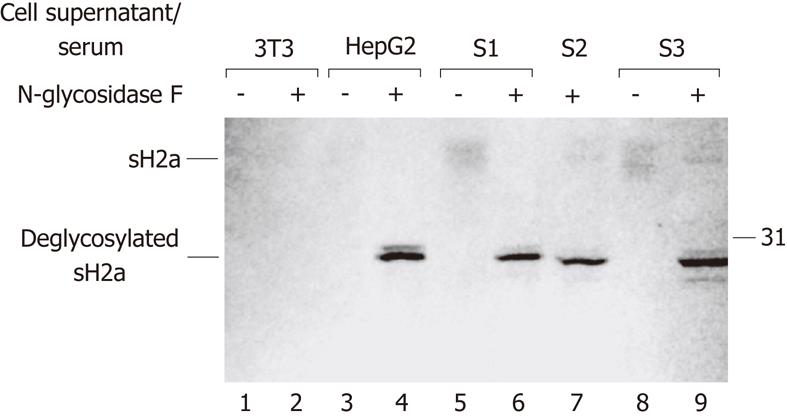
We had seen that sH2a is normally secreted from the human hepatoma cell line HepG2[8]. To analyze whether sH2a is present in human serum, we subjected samples of normal human sera to immunoprecipitation with anti-H2a carboxyterminal antibody, treatment with N-glycosidase-F, SDS-PAGE and western blotting with the same antibody (Figure 1, lanes 6, 7 and 9). We detected a band of about 28 kDa, similar in size to the one observed for sH2a in media from HepG2 cells (Figure 1, lane 4). Media from a control cell line that does not express sH2a (NIH 3T3 cells) showed no signal. Without the N-glycosidase-F treatment a disperse band of glycosylated sH2a of about 40 kDa is seen (lanes 3, 5 and 8), which probably represents heterogeneously glycosylated species. We also analyzed for the presence of ASGPR H1 in normal human serum and none was detected (data not shown).
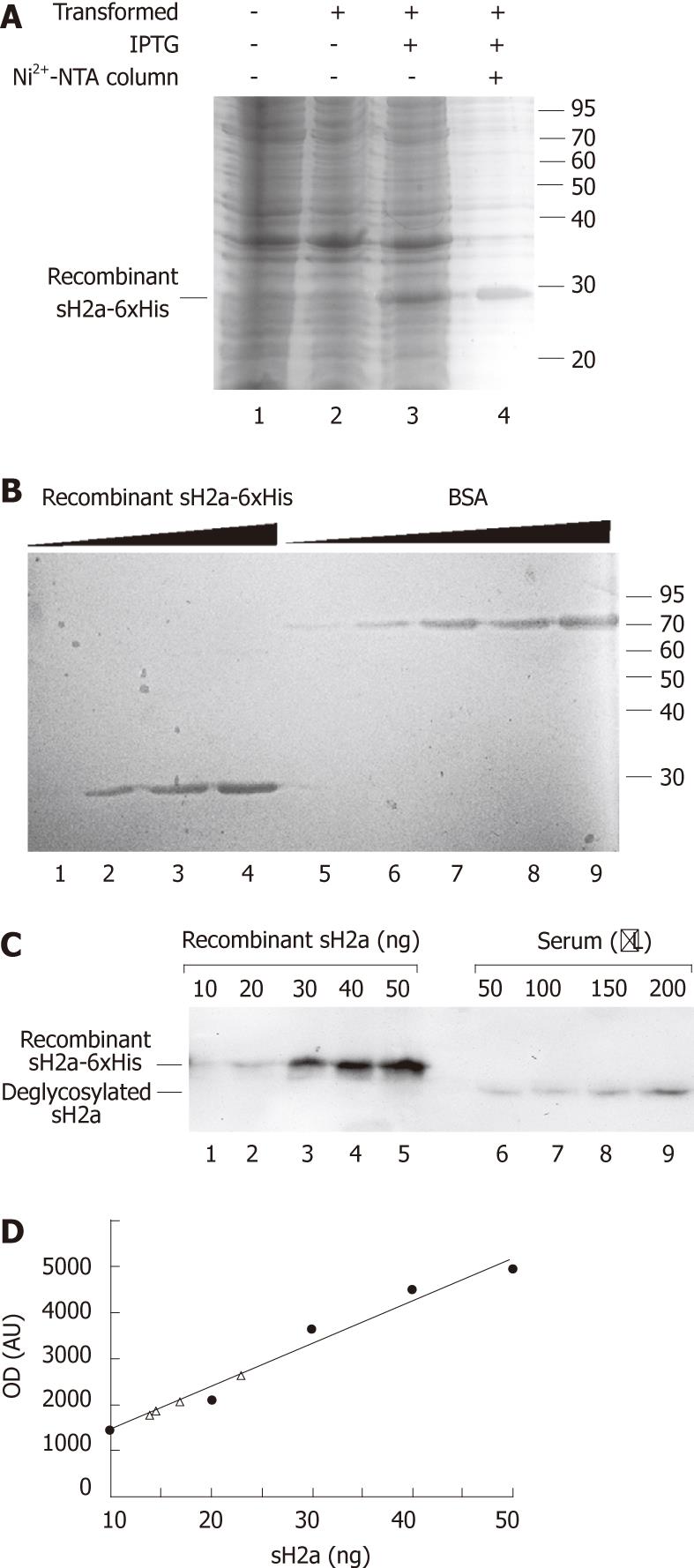
We produced a recombinant 6xHis-tagged sH2a (Figure 2A and B), which allowed us to estimate the level of sH2a in serum. sH2a contained in a sample of normal human serum was compared with recombinant sH2a by immunoprecipitation followed by immunoblot, giving an estimated sH2a concentration of 148 ± 22 ng/mL of serum (Figure 2C and D).
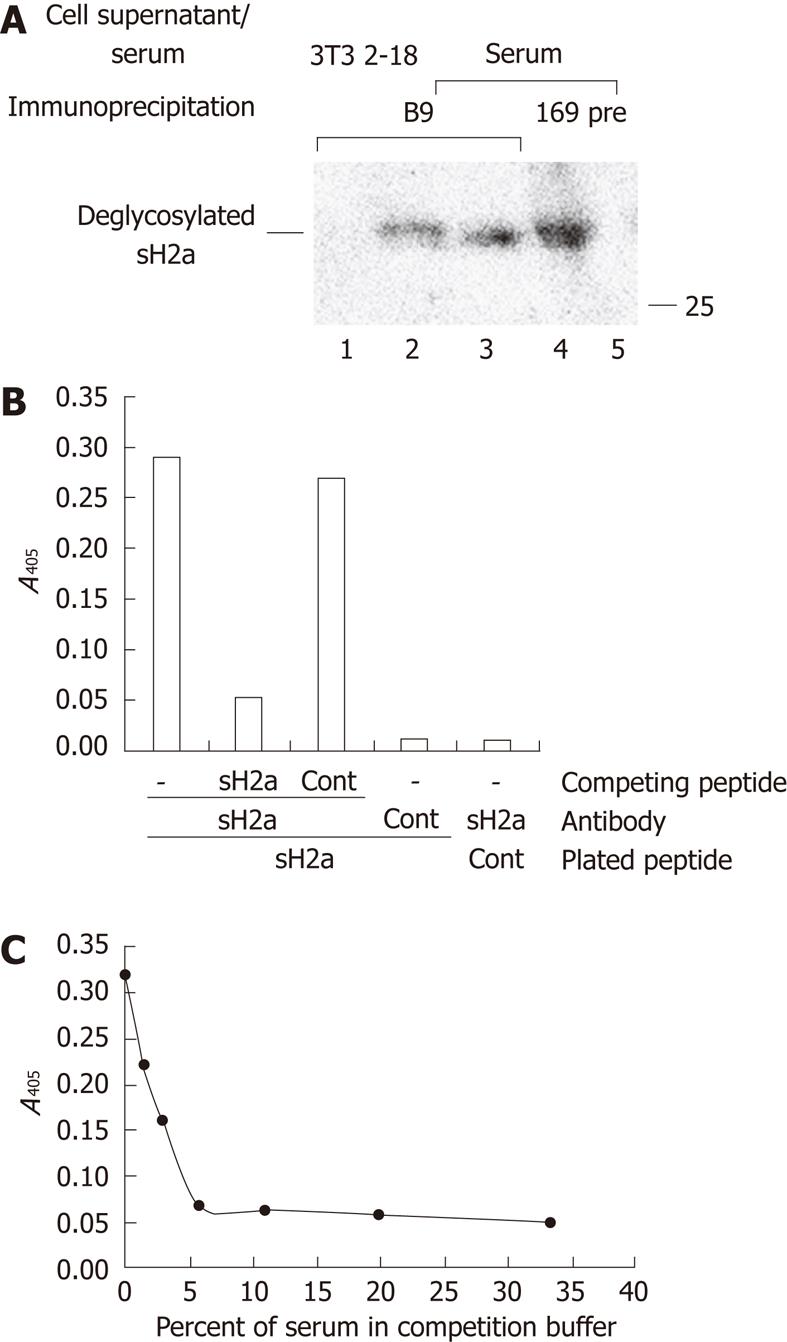
For comparative analysis of the concentration of sH2a in serum we developed a new specific mouse monoclonal anti-peptide H2a antibody. The B9 hybridoma produced a monoclonal antibody that recognized specifically sH2a secreted from transfected NIH 3T3 cells (2-18 cell line) or present in human serum (Figure 3A). The B9 antibody was used to develop an ELISA assay based on the binding of this antibody to its peptide. The binding could be competed by 81% after preincubation of the antibody with a solution containing 0.5 μg/mL of the same peptide, but not of a control peptide (Figure 3B). The binding could also be competed in a concentration-dependent manner by preincubation of the antibody with normal human serum; a 1:32 dilution of serum resulted in 50% reduction in the binding (Figure 3C).
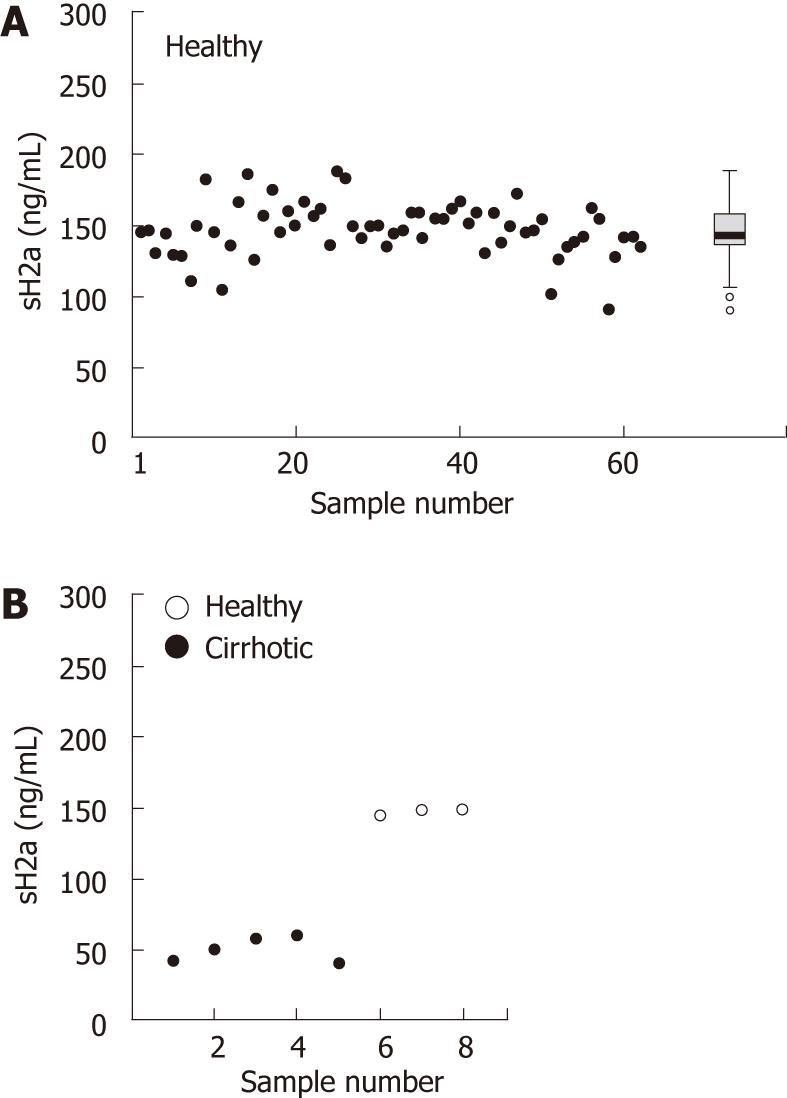
Using the ELISA assay described above we analyzed samples of serum obtained from 62 healthy individuals, with both male and female individuals with a wide range of ages. The levels of sH2a were very constant, 147 ± 19 ng/mL, giving a median of 146.5, with an interquartile range of 137-158 ng/mL (Figure 4A).
We then analyzed sH2a levels blindly in sera from a group that included 3 healthy subjects and 5 hepatitis C patients with cirrhosis, as determined by biopsy. All patients had a very low level of sH2a, 50 ± 9 ng/mL, about a third of that in the healthy individuals (Figure 4B).
Our results show for the first time the presence of ASGPR sH2a in human serum, with surprisingly constant levels in healthy individuals and a striking decrease in cirrhosis. Although the number of samples from cirrhotic patients that were available to us is very small, the fact that in healthy individuals sH2a is so constant and in all patients analyzed it is dramatically reduced suggests that this might be a general phenomenon.
Hepatocytes are targets for most insults to the liver, including hepatitis viruses and alcohol[11]. The damage caused abrogates the function of the hepatocytes and leads to a fibrogenic process that further impairs liver function and develops to cirrhosis[12,13]. In this context we suggest that sH2a, secreted to constant levels in healthy individuals, is a marker of liver function, correlating to the mass of functional hepatocytes. Reduction of the levels of sH2a could reflect early events in the fibrogenic process, affecting hepatocyte function.
Release of soluble ASGPR fragments to human serum had been described previously[14,15]. These fragments were recognized by polyclonal anti-ASGPR antibodies or by a mixture of monoclonal ones, for which it was not elucidated if they detect H1, H2a or H2b. They probably recognize mainly the H1 subunit (the most abundant) after release from damaged hepatocytes. Indeed it was shown in mice that ASGPR H1 subunit is released increasingly upon hepatocyte damage[16]. This is different from the case of sH2a, which arises from an mRNA that is made specifically for the production of a constitutively secreted protein, the levels of which are reduced by impaired function of the hepatocytes that secrete it. Because of the presence and constancy of sH2a in serum of healthy individuals, it is probably not a favored antigen for autoantibodies that appear in autoimmune hepatitis against the membrane ASGPR, which were found to recognize mainly the H1 subunit[17,18].
The levels of sH2a in serum are reduced in a manner that resembles the reduction in hepatocyte membrane ASGPR in cirrhosis[6,7]. Therefore, sH2a could have a potential for noninvasive diagnosis of liver disease.
We would like to thank Shimon Reif for help in the writing of the Helsinki protocol.
A specific and sensitive non-invasive indicator of liver function has long been sought after, as the classical markers like albumin levels and prothrombin time can reveal only severe liver disease. The asialoglycoprotein receptor is specifically expressed in the liver and sH2a is a soluble secreted form.
Several proposed experimental markers or combinations of routine tests are being evaluated, to measure the degree of liver fibrosis in the pathway to cirrhosis. Some of these markers are already commercially available. Nevertheless, the gold standard continues to be the invasive and risky procedure of biopsy.
This study describes for the first time that sH2a is present in human serum. The levels of sH2a were found to be surprisingly constant in a healthy group and dramatically reduced in liver cirrhosis.
SH2a could be a potential non-invasive diagnostic reporter that reflects the functional mass of the major liver cell type, the hepatocytes.
The asialoglycoprotein receptor serves in the clearance of asialoglycoproteins from the plasma. Asialoglycoproteins derive from many glycoproteins in circulation (hormones, growth factors, etc.) that at some point have lost the terminal sialic acids in their glycans, by the action of sialidases. Cirrhosis is the end stage of liver disease caused by a wide range of factors like infectious hepatitis, alcoholism, obesity, etc.
The authors present an important step in the potential development of more sensitive and accurate non-invasive markers to assess functional liver mass.
Peer reviewer: Jeff Butterworth, MB, FRCP, Department of Gastroenterology, Shrewsbury and Telford Hospital NHS Trust, Mytton Oak Road, Shrewsbury, Shropshire SY3 8XQ, United Kingdom
S- Editor Tian L L- Editor Webster JR E- Editor Xiong L
| 1. | Drickamer K. Clearing up glycoprotein hormones. Cell. 1991;67:1029-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Kudo M, Vera DR, Trudeau WL, Stadalnik RC. Validation of in vivo receptor measurements via in vitro radioassay: technetium-99m-galactosyl-neoglycoalbumin as prototype model. J Nucl Med. 1991;32:1177-1182. [PubMed] |
| 3. | Stockert RJ. The asialoglycoprotein receptor: relationships between structure, function, and expression. Physiol Rev. 1995;75:591-609. [PubMed] |
| 4. | Trerè D, Fiume L, De Giorgi LB, Di Stefano G, Migaldi M, Derenzini M. The asialoglycoprotein receptor in human hepatocellular carcinomas: its expression on proliferating cells. Br J Cancer. 1999;81:404-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Ishii H. Mechanisms of ethanol-induced impairment in asialoglycoprotein receptor expression and function. J Gastroenterol. 1998;33:920-921. [PubMed] |
| 6. | Burgess JB, Baenziger JU, Brown WR. Abnormal surface distribution of the human asialoglycoprotein receptor in cirrhosis. Hepatology. 1992;15:702-706. [PubMed] |
| 7. | Pimstone NR, Stadalnik RC, Vera DR, Hutak DP, Trudeau WL. Evaluation of hepatocellular function by way of receptor-mediated uptake of a technetium-99m-labeled asialoglycoprotein analog. Hepatology. 1994;20:917-923. [PubMed] |
| 8. | Tolchinsky S, Yuk MH, Ayalon M, Lodish HF, Lederkremer GZ. Membrane-bound versus secreted forms of human asialoglycoprotein receptor subunits. Role of a juxtamembrane pentapeptide. J Biol Chem. 1996;271:14496-14503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Lederkremer GZ, Lodish HF. An alternatively spliced miniexon alters the subcellular fate of the human asialoglycoprotein receptor H2 subunit. Endoplasmic reticulum retention and degradation or cell surface expression. J Biol Chem. 1991;266:1237-1244. [PubMed] |
| 10. | Kamhi-Nesher S, Shenkman M, Tolchinsky S, Fromm SV, Ehrlich R, Lederkremer GZ. A novel quality control compartment derived from the endoplasmic reticulum. Mol Biol Cell. 2001;12:1711-1723. [PubMed] |
| 11. | Higuchi H, Gores GJ. Mechanisms of liver injury: an overview. Curr Mol Med. 2003;3:483-490. [PubMed] |
| 12. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 13. | Gressner OA, Weiskirchen R, Gressner AM. Biomarkers of liver fibrosis: clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clin Chim Acta. 2007;381:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Hilgard P, Schreiter T, Stockert RJ, Gerken G, Treichel U. Asialoglycoprotein receptor facilitates hemolysis in patients with alcoholic liver cirrhosis. Hepatology. 2004;39:1398-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Yago H, Kohgo Y, Kato J, Watanabe N, Sakamaki S, Niitsu Y. Detection and quantification of soluble asialoglycoprotein receptor in human serum. Hepatology. 1995;21:383-388. [PubMed] |
| 16. | Kakegawa T, Ise H, Sugihara N, Nikaido T, Negishi N, Akaike T, Tanaka E. Soluble asialoglycoprotein receptors reflect the apoptosis of hepatocytes. Cell Transplant. 2002;11:407-415. [PubMed] |
| 17. | Schreiter T, Liu C, Gerken G, Treichel U. Detection of circulating autoantibodies directed against the asialoglycoprotein receptor using recombinant receptor subunit H1. J Immunol Methods. 2005;301:1-10. [PubMed] |
| 18. | Treichel U, McFarlane BM, Seki T, Krawitt EL, Alessi N, Stickel F, McFarlane IG, Kiyosawa K, Furuta S, Freni MA. Demographics of anti-asialoglycoprotein receptor autoantibodies in autoimmune hepatitis. Gastroenterology. 1994;107:799-804. [PubMed] |