Published online Oct 28, 2011. doi: 10.3748/wjg.v17.i40.4542
Revised: April 3, 2011
Accepted: April 10, 2011
Published online: October 28, 2011
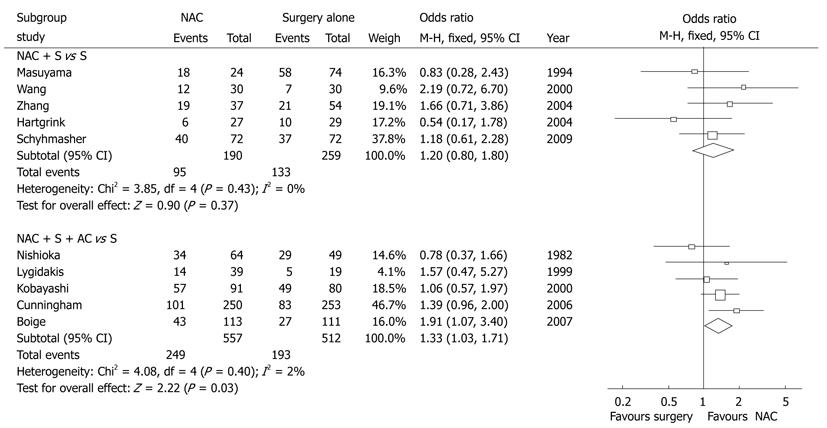
Neoadjuvant chemotherapy (NAC) has drawn more attention to the treatment of locally advanced gastric cancer (AGC) in the current multidisciplinary treatment model. EORTC trial 40954 has recently reported that NAC plus surgery without postoperative adjuvant chemotherapy could not benefit the locally AGC patients in their overall survival. We performed a meta-analysis of 10 studies including 1518 gastric cancer patients. Stratified subgroups were NAC plus surgery and NAC plus both surgery and adjuvant chemotherapy (AC), while control was surgery alone. The results showed that NAC plus surgery did not benefit the patients with locally AGC in their overall survival [odds ratio (OR) = 1.20, 95% CI 0.80-1.80, P = 0.37] and the number needed to treat (NNT) was 74. However, the NAC plus both surgery and AC had a slight overall survival benefit (OR = 1.33, 95% CI 1.03-1.71, P = 0.03) and NNT was 14, which is superior to the NAC plus surgery. Therefore, we recommend that combined NAC and AC should be used to improve the overall survival of the locally AGC patients.
- Citation: Chen XZ, Yang K, Liu J, Chen XL, Hu JK. Neoadjuvant plus adjuvant chemotherapy benefits overall survival of locally advanced gastric cancer. World J Gastroenterol 2011; 17(40): 4542-4544
- URL: https://www.wjgnet.com/1007-9327/full/v17/i40/4542.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i40.4542
We have read with great interest the excellent article by Li et al[1]. Gastric cancer is still one of the most common malignancies worldwide and about 80% patients with gastric cancer have advanced diseases[2]. Surgery is known as the only potentially curative treatment for this disease at resectable stages, while chemotherapy could play an important role in improving the prognosis of the patients[2,3]. Neoadjuvant chemotherapy (NAC) has drawn more attention to the treatment of locally advanced gastric cancer (AGC) in the current multidisciplinary treatment model. The EORTC trial 40954 observed the effect of the NAC without adjuvant chemotherapy (AC) following surgery for locally AGC (UICC stage III and IV-cM0). The protocol did not benefit the survival of the patients, but the R0 resection rate was significantly increased[4]. However, the fluorouracil-containing AC for AGC has shown significant survival benefit compared with surgery alone[5-7]. Therefore, we consider that combined NAC and AC may benefit locally AGC patients in overall survival.
The meta-analysis performed by Li et al[1], including 14 trials, compared the patients treated with NAC and those without NAC, showed an R0 resection rate of 75.2% vs 66.9% [odds ratio (OR) = 1.51, P = 0.0006, fixed model]. Therefore, this made us well understand the substantial effectiveness of NAC for locally AGC.
Based on the original meta-analysis, we performed another subgroup analysis by classifying the intervention arms into NAC plus surgery or NAC plus both surgery and AC, while the trials contaminated with AC in the control arm were excluded[1]. In each subgroup, 5 prospective trials were included for repooled analysis (1518 patients). The meta-analysis showed that the NAC plus surgery did not bring overall survival benefit to the patients with locally AGC (OR = 1.20, 95% CI 0.80-1.80, P = 0.37) and the number needed to treat (NNT) was 74 (Figure 1). However, NAC plus both surgery and AC had a slight overall survival benefit (OR = 1.33, 95% CI 1.03-1.71, P = 0.03) and NNT was 14, which was superior to the NAC plus surgery (Figure 1). It implies that one out of 14 locally advanced patients treated by NAC plus both surgery and AC was benefited in survival, while the remainings might be at risk of recurrence or even death.
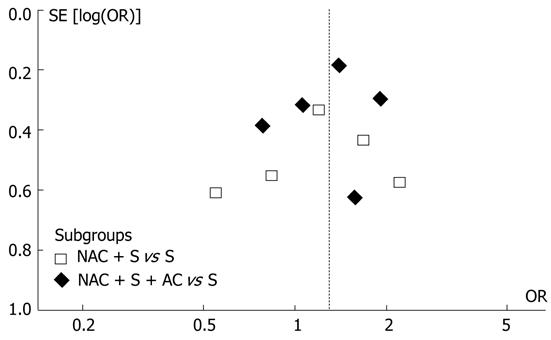
In addition, funnel plot observation did not indicate obvious publication bias in the two subgroups (Figure 2). The sensitivity analysis showed similar results by excluding the trials with Jadad score less than 3[1]. The original meta-analysis noted that the effect of NAC is more pronounced in Western countries and in doublet or triplet chemotherapy regimens[1]. In our subgroup analysis, the subgroups involved both Western and Asian studies as well as both single-agent and multi-agent regimens. These factors might not confound the present analysis.
Interestingly, EORTC trial 40 954 did not show any survival benefit by NAC for the locally advanced diseases (UICC stage III and IV-cM0)[4], but the meta-analysis by Li et al[1] found that more advanced diseases (pT3-4) were benefited by NAC (OR = 1.91, P < 0.05) than pT1-2 diseases (P > 0.05). Based on the original data from Li et al[1], it is impossible to perform subgroup analysis of stage III and IV-cM0 diseases, but it would be meaningful to figure out which sub-population might actually benefit from NAC plus both surgery and AC.
In summary, we think NAC is an effective therapy to increase the R0 resection rate for locally AGC, however, combined NAC and AC would improve the overall survival of the patients. Whether the increased R0 resection rate is associated with the improved overall survival and which sub-population might benefit from the combined treatment requires further studies.
Peer reviewer: Robert C Moesinger, MD/FACS, Northern Utah Surgeons, Adjunct Assistant Professor, Department of Surgery, University of Utah, 4403 Harrison Blvd No. 1635, Ogden, UT 84403, United States
S- Editor Tian L L- Editor Ma JY E- Editor Li JY
| 1. | Li W, Qin J, Sun YH, Liu TS. Neoadjuvant chemotherapy for advanced gastric cancer: a meta-analysis. World J Gastroenterol. 2010;16:5621-5628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 75] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Chen XZ, Jiang K, Hu JK, Zhang B, Gou HF, Yang K, Chen ZX, Chen JP. Cost-effectiveness analysis of chemotherapy for advanced gastric cancer in China. World J Gastroenterol. 2008;14:2715-2722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Chen XZ, Hu JK, Zhou ZG, Rui YY, Yang K, Wang L, Zhang B, Chen ZX, Chen JP. Meta-analysis of effectiveness and safety of D2 plus para-aortic lymphadenectomy for resectable gastric cancer. J Am Coll Surg. 2010;210:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210-5218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 533] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 5. | Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 604] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 6. | Hu JK, Li CM, Chen XZ, Chen ZX, Zhou ZG, Zhang B, Chen JP. The effectiveness of intravenous 5-fluorouracil-containing chemotherapy after curative resection for gastric carcinoma: A systematic review of published randomized controlled trials. J Chemother. 2007;19:359-375. [PubMed] |
| 7. | Hu JK, Chen ZX, Zhou ZG, Zhang B, Tian J, Chen JP, Wang L, Wang CH, Chen HY, Li YP. Intravenous chemotherapy for resected gastric cancer: meta-analysis of randomized controlled trials. World J Gastroenterol. 2002;8:1023-1028. [PubMed] |