Published online Jan 28, 2011. doi: 10.3748/wjg.v17.i4.478
Revised: October 20, 2010
Accepted: October 27, 2010
Published online: January 28, 2011
AIM: To investigate the anti-tumor effects of nuclear factor-κB (NF-κB) inhibitor SN50 and related mechanisms of SGC7901 human gastric carcinoma cells.
METHODS: MTT assay was used to determine the cytotoxic effects of SN50 in gastric cancer cell line SGC7901. Hoechst 33258 staining was used to detect apoptosis morphological changes after SN50 treatment. Activation of autophagy was monitored with monodansylcadaverine (MDC) staining after SN50 treatment.Immunofluorescence staining was used to detect the expression of light chain 3 (LC3). Mitochondrial membrane potential was measured using the fluorescent probe JC-1. Western blotting analysis were used to determine the expression of proteins involved in apoptosis and autophagy including p53, p53 upregulated modulator of apoptosis (PUMA), damage-regulated autophagy modulator (DRAM), LC3 and Beclin 1. We detected the effects of p53-mediated autophagy activation on the apoptosis of SGC7901 cells with the p53 inhibitor pifithrin-α.
RESULTS: The viability of SGC7901 cells was inhibited after SN50 treatment. Inductions in the expression of apoptotic protein p53 and PUMA as well as autophagic protein DRAM, LC3 and Beclin 1 were detected with Western blotting analysis. SN50-treated cells exhibited punctuate microtubule-associated protein 1 LC3 in immunoreactivity and MDC-labeled vesicles increased after treatment of SN50 by MDC staining. Collapse of mitochondrial membrane potential Δψ were detected for 6 to 24 h after SN50 treatment. SN50-induced increases in PUMA, DRAM, LC3 and Beclin 1 and cell death were blocked by the p53 specific inhibitor pifithrin-α.
CONCLUSION: The anti-tumor activity of NF-κB inhibitors is associated with p53-mediated activation of autophagy.
- Citation: Zhu BS, Xing CG, Lin F, Fan XQ, Zhao K, Qin ZH. Blocking NF-κB nuclear translocation leads to p53-related autophagy activation and cell apoptosis. World J Gastroenterol 2011; 17(4): 478-487
- URL: https://www.wjgnet.com/1007-9327/full/v17/i4/478.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i4.478
Nuclear factor-κB (NF-κB) is an ubiquitously expressed family of Rel-related transcription factors[1]. Typically, in unstimulated cells, NF-κB is sequestered in the cytoplasm by binding to inhibitory κB proteins (IκB). In response to a variety of stimuli, such as inflammatory cytokines, oncogenes, and viruses, the proteasome-dependent degradation of IκB allows the translocation of NF-κB to the nucleus, where it binds to the promoter region of target genes involved in the control of different cellular responses, including apoptosis[2-4]. In many cancer cells, the constitutive activation of NF-κB activity lowers cell sensitivity to apoptotic stimuli and consequently favors neoplastic cell survival[5].
The mammalian NF-κB family contains 5 members: p50/p105 (NF-κB1), p52/p100 (NF-κB2), c-Rel, RelB, and p65 (RelA). These proteins are characterized by their Rel homology domains, which control DNA binding, dimerization and interactions with inhibitory factors known as IκB proteins[4,6]. NF-κB is first discovered and studied as a major activator of immune and inflammatory function via its ability to induce expression of genes encoding cytokines, cytokine receptors, and cell-adhesion molecules[4,7]. NF-κB recently has been found to be linked to the control of cell growth and oncogenesis. The role of NF-κB in cancer appears to be complex, but is likely to involve the ability of this transcription factor to control programmed cell death (PCD) and cell-cycle progression, and possibly cell differentiation, angiogenesis and cell migration. It has been reported that NF-κB is activated in cancer cells by several chemotherapies and by radiation, and that in many cases this response inhibits the radiotherapy- and chemotherapy-induced cell death[8].
Recent studies have suggested that there are three types of PCD: apoptosis (PCD I), autophagic cell death (PCD II) and necrosis (PCD III)[9]. Autophagy is a genetically programmed, evolutionarily conserved process that degrades the long-living cellular proteins and organelles. Autophagy is important in normal development and response to changing environmental stimuli and, in addition to its role in cancer, and in numerous diseases, including bacterial and viral infections, neurodegenerative disorders, and cardiovascular diseases[10]. Autophagy involves the formation of a double-membrane vesicle, which encapsulates cytoplasm and organelles and fuses with lysosomes, thus degrading the contents of the vesicle. The formation of the double-membrane vesicle is a complex process involving 16 autophagy-related proteins (Atg proteins). Two ubiquitin-like conjugation systems are involved in autophagy. These systems produce modified complexes of autophagic regulators (Atg8-PE and Atg5-Atg12-Atg16) that may determine the formation and size of the autophagosome. Nucleation, expansion, uncoating, and completion of the autophagosome then occur, priming it to fuse with lysosomes[11].
The term “autophagic cell death” describes a form of programmed cell death morphologically distinct from apoptosis and presumed to result from excessive levels of cellular autophagy[12]. In classical apoptosis, or type I programmed cell death, there is early collapse of cytoskeletal elements but preservation of organelles until late in the process. In contrast, in autophagic, or type II, programmed cell death, there is early degradation of organelles but preservation of cytoskeletal elements until late stages. Whereas apoptotic cell death is caspase-dependent and characterized by internucleosomal DNA cleavage, caspase activation and DNA fragmentation occur very late (if at all) in autophagic cell death[13]. In contrast with necrosis, both apoptotic and autophagic cell death are characterized by the lack of a tissue inflammatory response. The mitochondrion may integrate cell death signals and autophagy activation. Mitochondria generate apoptotic signals but can be removed by autophagy when they are damaged; therefore, mitochondria represents a nexus at which autophagy may interact with apoptosis pathways[14].
The mutual regulation of NF-κB and autophagy has been reported[15]. Autophagy degrades nuclear shuttle protein-interacting kinase (NIK) and IκB kinase (IKK), and inhibits NF-κB activation, while NF-κB depresses autophagy[16]. We predict that activation of autophagy by blocking NF-κB may contribute to the anti-tumor actions of NF-κB inhibitors. We examined the effects of the nuclear import inhibitor SN50 on the activation of apoptosis and autophagy and the contribution of autophagy to the cytotoxic effects of SN50 in gastric cancer cell line SGC7901. The results showed that p53-dependent activation of apoptotic and autophagic pathways was induced by blocking the NF-κB nuclear transport, and autophagic activation contributed to SN50-induced death of cancer cells.
SGC7901 gastric cancer cells were purchased from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China); RPMI1640 medium from Gibco (Rockville, MD, USA); fetal bovine serum from Hangzhou Sijiqing Biological Engineering Material Co., Ltd., (Hangzhou, China); L-glutamine, MTT from Sigma (St Louis, MO, USA); antibodies against p53 (1:500), p65 (1:500), PUMA (1:500), LC3 (1:200), Beclin1 (1:700) from Cell Signaling Technology (Beverly, MA, USA); and antibodies against DRAM (1:700) from Santa Cruz Biotechnologies (Santa Cruz, CA, USA).
SN50 (BIOMOL, Plymouth Meeting, PA, USA) were diluted in distilled sterilization water to create a stock solution that was stored according to the manufacturer’s suggestions. Pifithrin-α (Pft-α, Cell Signaling Technology, Beverly, MA, USA) was diluted in DMSO to create a stock solution that was stored according to the manufacturer’s instructions. The final concentration of the SN50 solution used in the experiments was 18 μmol/L, and that of Pft-α was 30 μmol/L. This concentration of SN50 was selected on the basis of our pilot experiments on SGC7901 cells, and the concentration of Pft-α was selected following the manufacturer’s suggestions.
SGC7901 cells were maintained in RPMI1640 medium containing 10% heat-inactivated fetal bovine serum, 0.03% L-glutamine and incubated in a 5% CO2 atmosphere at 37°C. Cells in a mid-log phase were used in experiments. Cell viability was assessed by MTT assay. To determine the time-course of response of SGC7901 cells to SN50, SGC7901 cells were plated into 96-well microplates (7 × 104 cells/well), and SN50 (18 μmol/L) was added to the culture medium and cell viability was assessed with MTT assay 24, 48 and 72 h after drug treatment. MTT (Sigma, St Louis, MO, USA) solution was added to the culture medium (500 mg/L as a final concentration) for 4 h before the end of treatment, and the reaction was stopped by addition of 10% acided SDS 100 μL. The absorbance value (A) at 570 nm was read using an automatic multiwell spectrophotometer (Bio-Rad, Richmond, CA, USA). The percentage of cell death was calculated as follows: cell death (%) = (1-A of experiment well/A of positive control well) × 100%.
Mitochondrial Δψ was determined using the KeyGEN Mitochondrial Membrane Sensor Kit (KeyGEN, Nanjing, China). The mitosensor dye aggregates in the mitochondria of healthy cells and emits red fluorescence against a green monomeric cytoplasmic background staining. However, in cells with a collapsed mitochondrial Δψ, the dye cannot accumulate in the mitochondria and remains as monomers throughout the cells with green fluorescence[17]. Briefly, SGC7901 cells were incubated with SN50 in 24-well plates for indicated times and then pelleted, washed with PBS, and resuspended in 0.5 mL of diluted mitosensor reagent (1 μmol/mL in incubation buffer). After the cells were incubated with mitosensor reagent for 20 min, 0.2 mL incubation buffer was added and the cells were centrifuged and resuspended in 40 μL incubation buffer. Finally, the cells were washed and resuspended in 1 mL phosphate buffered solution (PBS) for flow cytometric analysis.
Exponentially growing cells were plated onto 24-chamber culture slides, cultured for 24 h and incubated with the drug in 10% FCS/RPMI1640 for 6, 12 and 24 h. Autophagic vacuoles were labeled with monodansylcadaverine (MDC)[18] (Sigma, St Louis, MO, USA) by incubating cells with 0.001 mmol/L MDC in RPMI1640 at 37°C for 10 min. After incubation, cells were washed three times with phosphate buffered solution (PBS) and immediately analyzed with a fluorescence microscope (Nikon Eclipse TE 300, Japan) equipped with a filter system (V-2A excitation filter: 380-420 nm, barrier filter: 450 nm). Images were captured with a CCD camera and imported into Photoshop.
SGC7901 cells were seeded onto 24-chamber culture slides and treated with SN50 (18 μmol/L). After fixation in methanol for 10 min and blocked with a buffer containing 1% BSA 0.1% Triton X-100 for 1 h, cells were incubated with either primary antibody against LC3 from Cell Signaling Technology (Beverly, MA, USA) diluted at 1:200 with PBS containing 1% bovine serum albumin (BSA) at 4°C overnight, and then incubated for 1 h with 1:500 secondary fluorescence conjugated antibodies (Sigma) to visualize the binding sites of the primary antibody under laser confocal microscope (Leisa, Germany).
After treatment, cell cultures were washed twice with PBS and incubated with 2 μmol/L Hoechst 33258 (Beyotime, Nantong, China) for 1 h in dark at 37°C. After washing thrice with PBS, the cells were viewed under a fluorescence microscope (Nikon, Tokyo, Japan) equipped with a UV filter. The images were recorded on a computer with a digital camera (DXM 1200, Nikon) attached to the microscope, and the images were processed by computer. The Hoechst reagent was taken up by the nuclei of the cells, and apoptotic cells exhibited a bright blue fluorescence.
For extraction of total cell proteins, cells were washed with pre-cooled PBS and subsequently lysed in pre-cooled RIPA lysis buffer [50 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 1 mmol/L dithiothreitol (DTT), 0.25% sodium deoxycholate, 0.1% NP-40] containing 1 mmol/L phenylmethysulfonyl fluoride (PMSF), 50 mmol/L sodium pyrophosphate, 1 mmol/L Na3VO4, 1 mmol/L NaF, 5 mmol/L EDTA, 5 mmol/L EGTA, and protease inhibitors cocktail. Cell lysis was performed on ice for 30 min. Clear protein extracts were obtained by centrifugation for 30 min at 4°C. For nuclear protein extraction, the Nuclear Protein Extraction Kit (KeyGEN, Nanjing, China) was used according to the manufacturer’s instructions. Protein extraction from SGC7901 gastric cancer cells was performed as previously described. Protein concentration was determined with a Bradford protein assay kit. Proteins were resolved on 8.5% polyacrylamide gels and subsequently transferred onto nitrocellulose membranes. For immunoblotting, nitrocellulose membranes were incubated with specific antibodies recognizing target proteins overnight at 4°C. The membranes were then incubated with HRP-conjugated secondary antibody (1:3000) for 1 h at room temperature and subsequently analyzed by enhanced chemiluminescence (ECL) detection system (Amersham Pharmacia Biotech) and visualized by autoradiography. Protein β-actin (1:5000; Sigma) was used as loading controls.
All data were presented as mean ± SD. Statistical analysis was carried out by ANOVA followed by a Dennett’s test, and P < 0.05 was considered statistically significant.
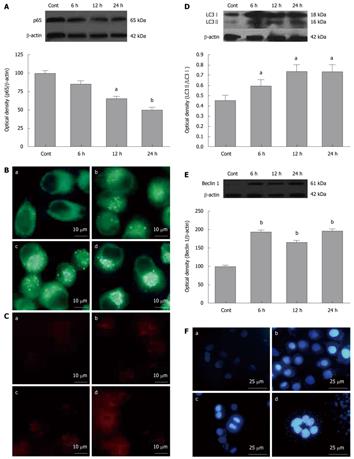
To confirm the blockade of NF-κB p65 nuclear translocation by SN50, we prepared nuclear proteins from in SGC7901 cells treated with SN50 (18 μmol/L) for 6-24 h and the nuclear p65 protein levels were measured with Western blotting analysis. The results showed that SN50 lowered the nuclear p65 levels in SGC7901 cells, suggesting that NF-κB activity can be inhibited by SN50 (Figure 1A). The autofluorescence substance MDC has been recently shown to be a marker for late autophagic vacuoles (L-AVs) but not endosomes. To determine if SN50 treatment increases the formation of autophagosomes, SGC7901 cells were treated with SN50 and then incubated with MDC. MDC was trapped in acidic, membrane-rich organelles and also exhibited increased fluorescence quantum yield in response to the compacted lipid bilayers present in L-AVS[19]. When cells are viewed under a fluorescence microscope, autophagic vacuoles (AVs) stained with MDC appeared as distinct dot-like structures distributed in the cytoplasm or localizing in the perinuclear regions. This study found that there was an increase in the number of MDC-labeled vesicles after treatment of SN50 for 6-24 h (Figure 1B).
Microtubule-associated protein 1 light chain 3 (LC3), the mammalian ontology of Atg8, targets to the autophagosomal membranes in an Atg5-dependent manner and remains there even after Atg12-Atg5 dissociates. LC3 is considered to be the only credible marker of the autophagosome in mammalian cells[20]. The present study used immunofluorescence to detect the expression and localization of LC3. The results showed that SN50 induced punctuate distribution of LC3 immunoreactivity, and the formation of autophagosomes was enhanced by SN50 (Figure 1C). There are two forms of LC3, LC3-I and LC3-II. During formation of autophagosomes, cytoplasmic form LC3-I is cleaved and liquefied to give rise to membranous form LC3-II. To determine if SN50 increases the production of LC3-II, Western blotting analysis was used to detect the protein levels of LC3-I and LC3-II. The results showed that the levels of LC3, particularly LC3-II, increased, leading to an increased ratio of LC3-II/LC3-I after SN50 treatment (Figure 1D). Beclin 1 is an autophagy regulator and plays an important role in tumorigenesis and autophagic activation. Similar increases in Beclin 1 proteins were also detected after SN50 treatment (Figure 1E). Treatment with 18 μmol/L SN50 for 6, 12 and 24 h in SGC7901 cells produced intense Hoechst-positive staining for condensed nuclei indicative of apoptosis. Significant increase in Hoechst staining was observed along with apoptosis when cells were treated with 18μmol/L SN50. The result indicated that SN50 activated autophagy and induced cell apoptosis (Figure 1F).
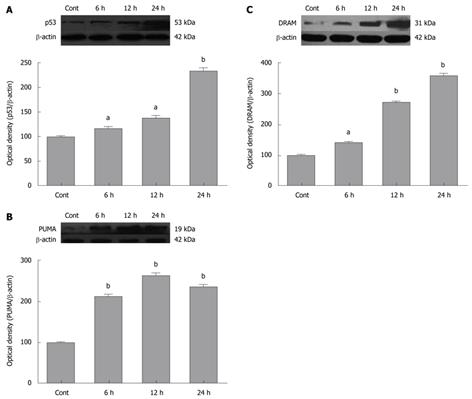
Inhibition of NF-κB has anti-tumor effects. To confirm if SN50 induced expression of pro-apoptotic proteins in SGC7901 cells, Western blotting analysis were used to detect the expression of p53 and its target proteins. The analyses revealed a robust increase in p53 protein levels in SGC7901 cells 6 h after SN50 treatment (Figure 2A).
PUMA is a p53 target protein involved in p53-mediated apoptosis. To determine if SN50 increases the expression of PUMA, protein levels of PUMA in SGC7901 cells were detected with Western blotting analysis. The results showed that PUMA was significantly increased after the treatment with SN50 (Figure 2B). Crighton et al[21] recently identified a new p53 target gene damage-regulated autophagic modulator (DRAM). DRAM is a lysosomal protein with six membrane-spanning regions. Its exogenous expression leads to the accumulation of autophagosomes. The previous studies found that DRAM is required for p53-induced apoptosis and DRAM-dependent autophagy acts upstream of cytochrome c release from mitochondria[21,22]. The present study indicated that SN50 treatment also resulted in a significant increase in DRAM protein levels in SGC7901 cells (Figure 2C).
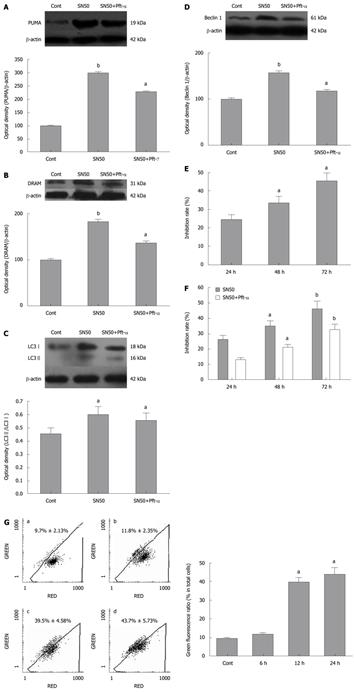
Pifithrin-α (Pft-α) was used as a specific inhibitor of signaling by the tumor suppressor protein p53[23]. To determine the contribution of p53 to SN50-induced expression of pro-apoptotic and autophagic proteins and apoptosis of SGC7901 cells, the cells were pre-treated with the p53 specific inhibitor Pft-α 6 h before the addition of SN50. The results showed that when p53 was inhibited, the induction of PUMA and DRAM was inhibited (Figure 3A and B). Similarly, pft-α significantly decreased the SN50-induced upregulation of LC3-II and Beclin 1 in SGC7901 cells (Figure 3C and D).
SN50 inhibited SGC7901 viability in a time-dependent fashion. MTT assays revealed that after 24 h of treatment, the rate of inhibition had reached 25.31% ± 4.13% at the highest dose of 18 μmol/L used. When the incubation time was prolonged to 72 h, the inhibition rate rose up to 44.79% ± 1.65% and after 48 h of treatment the rate was about 34.19% ± 2.06% (Figure 3E). To evaluate the contribution of p53 to SN50-induced death of SGC7901 cells, the cells were pre-treated with the p53 specific inhibitor Pft-α 6 h before SN50. As shown in Figure 3F, Pft-α significantly attenuated the inhibitory effects of SN50 in a time-dependent manner. Mitochondria plays a central role in regulating cell death and survival. Diverse proapoptotic stimuli act on mitochondria, triggering mitochondrial membrane potential collapse, cytochrome c release and caspase activation. We detected collapse of mitochondrial membrane potential Δψ as early as 6 h after SN50 treatment. This change reached a peak 24 h after SN50 treatment (Figure 3G).
NF-κB signaling pathways play critical roles in a variety of physiological and pathological processes. One function of NF-κB is to promote cell survival through induction of target genes, whose products inhibit components of the apoptotic machinery in normal and cancerous cells. NF-κB can also prevent programmed necrosis by inducing genes encoding antioxidant proteins. Regardless of mechanism, many cancer cells, of either epithelial or hematopoietic origin, use NF-κB to achieve resistance to anticancer drugs and radiation. Hence, inhibition of NF-κB activation offers a strategy for treatment of different malignancies and can induce apoptosis in gastric cancer SGC7901 cells.
NF-κB plays an important role in proliferation and survival of tumor cells. NF-κB binding sites have been identified in the promoter region of cyclin D1. NF-κB promotes cyclin D1 expression and cell cycle progression[24,25]. NF-κB activity has been shown to inhibit activation of caspase-8, thus inhibiting the apoptosis initiation[26]. Activation of NF-κB confers resistance of tumor cells to radiochemotherapy-induced cytotoxicity[27,28]. In contrast, various NF-κB inhibitors inhibit tumor cell growth and induce cell death through apoptotic mechanisms[29].
The tumor suppressor p53 plays a central role in sensing various genotoxic stresses. Our results showed that blocking NF-κB with SN50 induced expression of p53 remarkably. P53 downstream point such as the apoptosis gene PUMA was up-regulated after the treatment of SN50. P53 is known to play an important role in apoptosis by regulating expression of pro-apoptotic proteins. PUMA is one of p53 target genes involved in apoptosis. The activation of PUMA by DNA damage is dependent on p53 and is mediated by the direct binding of p53 to the PUMA promoter region[30]. PUMA plays an essential role in p53-dependent and -independent apoptosis induced by a variety of stimuli[31,32]. Here, we demonstrated that the inhibitor of NF-κB p65 nuclear import, SN50, significantly up-regulated the levels of PUMA, indicating that apoptosis may be triggered by SN50. In supporting this notion, we found that mitochondria membrane potential was collapsed after SN50 treatment. Mitochondria plays a central role in regulating cell death and survival. Diverse proapoptotic stimuli act on mitochondria, triggering mitochondrial membrane potential collapse, cytochrome c release and caspase activation. The mitochondrial permeability transition (MPT) represents an important event initiating apoptotic cell death.
Increasing evidence suggests that autophagy plays an important role in tumor cell growth, differentiation and response to anti-tumor drugs[33]. Many classical anti-tumor drugs have been found to exert their cytotoxic actions by autophagic mechanisms[34-36]. It has been suggested that autophagic death may play a role in both physiological and pathological cell death. This issue has been addressed by some recent reviews[32,37]. In the present study, inhibitor of NF-κB p65 nuclear import with SN50 resulted in a significant increase in the levels of DRAM, a newly identified p53 target gene involved in autophagy activation and cell death[21]. We also found that SN50 increased the expression of LC3, Beclin 1, particularly the production of LC3-II. LC3 is an autophagosomal ortholog of yeast Atg8. LC3 has been best characterized as an autophagosomal marker in mammalian autophagy, and the levels of LC3 may also reflect the levels of autophagy[38]. Beclin 1 is the mammalian orthologue of the yeast ATG6-Vps30 gene. It can complement the defect in autophagy present in ATG6-/- yeast strains and stimulate autophagy when overexpressed in mammalian cells[13]. Beclin 1 is monoallelically deleted in human breast and ovarian cancers and is expressed at reduced levels in those tumors[13,39]. The present results suggest that autophagy is induced by SN50 and its activation may contribute to anti-tumor effects of NF-κB inhibitors.
The evidence has shown that p53 is not only involved in apoptosis but also in autophagy. The present study investigated if p53 signaling plays an essential role in autophagy activation and cell death induced by NF-κB inhibition. P53 signaling was blocked with a specific inhibitor pft-α, the compound has been shown to selectively inhibit p53 activity and p53-mediated apoptosis in vitro and in vivo[40]. The results demonstrated that pft-α blocked SN50-induced increases in PUMA, LC3-II and Beclin 1. Moreover, SN50-induced cell death was significantly attenuated by pft-α. These data suggest that p53 mediates activation of apoptosis and autophagy and cell death following blockade of NF-κB. This study shed new lights on elucidating molecular mechanisms of anti-tumor actions of NF-κB inhibitors.
In summary, the present study revealed that a new mechanism associated with NF-κB inhibition triggered impairment of cell proliferation and induction of apoptosis of cancer cells. Blocking NF-κB increases expression of p53, induces pro-apoptotic and autophagic proteins. P53 contributes to NF-κB inhibitor-induced apoptosis of cancer cells by activating autophagic mechanisms. Further investigation of the relationship between autophagy activation and anti-tumor effects of NF-κB inhibitors will unveil new strategies for tumor therapy.
Nuclear factor-κB (NF-κB) signaling pathways play critical roles in a variety of physiological and pathological processes. The authors predicted that activation of autophagy by blocking NF-κB may contribute to the anti-tumor actions of NF-κB inhibitors.
SN50 is a specific inhibitor of NF-κB p65. The anti-tumor activity of SN50 might be related to the induction of apoptosis of tumor cells, but the precise mechanism of its anti-tumor activity is not well understood.
Blocking NF-κB increases the expression of p53, and induces pro-apoptotic and autophagic proteins. P53 contributes to the NF-κB inhibitor-induced apoptosis of cancer cells through both apoptotic and autophagic mechanisms. Further investigation of the relationship between autophagy activation and anti-tumor effects of NF-κB inhibitors will unveil new strategies for tumor therapy.
Blocking NF-κB increases expression of p53, induces pro-apoptotic and autophagic proteins. P53 contributes to NF-κB inhibitor-induced apoptosis of cancer cells by activating autophagic mechanisms. And it will provide new idea for tumor treatment.
NF-κB: The NF-κB comprises a family of transcription factors involved in the regulation of a wide variety of biological responses. SN50:SN50 is a kind of NF-κB p65 nuclear translocation inhibitor. Autophagy: Autophagy is a general term for the degradation of cytoplasmic components within lysosomes. There are three types of autophagy: macroautophagy, microautophagy, and chaperone-mediated autophagy, and the term “autophagy” usually indicates macroautophagy.
The authors examined the effects of the nuclear import inhibitor SN50 on activation of apoptosis and autophagy and the contribution of autophagy to cytotoxic effects of SN50 in gastric cancer cell line SGC7901. The results showed that blocking NF-κB nuclear transport leads to p53-dependent activation of apoptotic and autophagic pathways, and autophagy activation contributes to SN50-induced death of cancer cells.
Peer reviewer: Dr. Hui-Kang Liu, PhD, Assistant Research Fellow, National Research Institute of Chinese Medicine, 155-1, Li-nung street section 2, Taipei 112, Taiwan, China
S- Editor Sun H L- Editor Ma JY E- Editor Ma WH
| 2. | Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221-227. |
| 3. | Barkett M, Gilmore TD. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6910-6924. |
| 4. | Baldwin AS Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649-683. |
| 5. | Bours V, Dejardin E, Goujon-Letawe F, Merville MP, Castronovo V. The NF-kappa B transcription factor and cancer: high expression of NF-kappa B- and I kappa B-related proteins in tumor cell lines. Biochem Pharmacol. 1994;47:145-149. |
| 6. | Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225-260. |
| 7. | Gilmore TD. The Rel/NF-kappaB signal transduction pathway: introduction. Oncogene. 1999;18:6842-6844. |
| 8. | Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001;107:241-246. |
| 9. | Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663-669. |
| 10. | Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990-995. |
| 11. | Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12 Suppl 2:1542-1552. |
| 12. | Schweichel JU, Merker HJ. The morphology of various types of cell death in prenatal tissues. Teratology. 1973;7:253-266. |
| 13. | Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439-448. |
| 14. | Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679-2688. |
| 15. | Djavaheri-Mergny M, Amelotti M, Mathieu J, Besançon F, Bauvy C, Souquère S, Pierron G, Codogno P. NF-kappaB activation represses tumor necrosis factor-alpha-induced autophagy. J Biol Chem. 2006;281:30373-30382. |
| 16. | Xiao G. Autophagy and NF-kappaB: fight for fate. Cytokine Growth Factor Rev. 2007;18:233-243. |
| 17. | Rashid SF, Moore JS, Walker E, Driver PM, Engel J, Edwards CE, Brown G, Uskokovic MR, Campbell MJ. Synergistic growth inhibition of prostate cancer cells by 1 alpha,25 Dihydroxyvitamin D(3) and its 19-nor-hexafluoride analogs in combination with either sodium butyrate or trichostatin A. Oncogene. 2001;20:1860-1872. |
| 18. | Biederbick A, Kern HF, Elsässer HP. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur J Cell Biol. 1995;66:3-14. |
| 19. | Niemann A, Takatsuki A, Elsässer HP. The lysosomotropic agent monodansylcadaverine also acts as a solvent polarity probe. J Histochem Cytochem. 2000;48:251-258. |
| 20. | Yoshimori T. Autophagy: a regulated bulk degradation process inside cells. Biochem Biophys Res Commun. 2004;313:453-458. |
| 21. | Crighton D, Wilkinson S, O’Prey J, Syed N, Smith P, Harrison PR, Gasco M, Garrone O, Crook T, Ryan KM. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126:121-134. |
| 23. | Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733-1737. |
| 24. | Westwick JK, Lee RJ, Lambert QT, Symons M, Pestell RG, Der CJ, Whitehead IP. Transforming potential of Dbl family proteins correlates with transcription from the cyclin D1 promoter but not with activation of Jun NH2-terminal kinase, p38/Mpk2, serum response factor, or c-Jun. J Biol Chem. 1998;273:16739-16747. |
| 25. | Westwick JK, Lambert QT, Clark GJ, Symons M, Van Aelst L, Pestell RG, Der CJ. Rac regulation of transformation, gene expression, and actin organization by multiple, PAK-independent pathways. Mol Cell Biol. 1997;17:1324-1335. |
| 26. | Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Richardson PG, Hideshima T, Munshi N, Treon SP, Anderson KC. Biologic sequelae of nuclear factor-kappaB blockade in multiple myeloma: therapeutic applications. Blood. 2002;99:4079-4086. |
| 27. | Wang CY, Guttridge DC, Mayo MW, Baldwin AS Jr. NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol. 1999;19:5923-5929. |
| 28. | Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS Jr. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680-1683. |
| 29. | Mitsiades N, Mitsiades CS, Poulaki V, Anderson KC, Treon SP. Intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human multiple myeloma cells. Blood. 2002;99:2162-2171. |
| 30. | Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673-682. |
| 31. | Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683-694. |
| 32. | Yu J, Zhang L. No PUMA, no death: implications for p53-dependent apoptosis. Cancer Cell. 2003;4:248-249. |
| 33. | Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891-2906. |
| 34. | Bursch W, Ellinger A, Kienzl H, Török L, Pandey S, Sikorska M, Walker R, Hermann RS. Active cell death induced by the anti-estrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: the role of autophagy. Carcinogenesis. 1996;17:1595-1607. |
| 35. | Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11:448-457. |
| 36. | Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, Domingo D, Yahalom J. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439-444. |
| 37. | Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463-477. |
| 38. | Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720-5728. |